








生物技术通报 ›› 2022, Vol. 38 ›› Issue (10): 243-253.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1550
沈俊强1( ), 张莉萍2, 于瑞明2, 王永录2, 潘丽2, 刘霞1(
), 张莉萍2, 于瑞明2, 王永录2, 潘丽2, 刘霞1( ), 刘新生2(
), 刘新生2( )
)
收稿日期:2021-12-13
出版日期:2022-10-26
发布日期:2022-11-11
作者简介:沈俊强,男,硕士研究生,研究方向:猪肠道病毒病相关病原的流行病学;E-mail:基金资助:
SHEN Jun-qiang1( ), ZHANG Li-ping2, YU Rui-ming2, WANG Yong-lu2, PAN Li2, LIU Xia1(
), ZHANG Li-ping2, YU Rui-ming2, WANG Yong-lu2, PAN Li2, LIU Xia1( ), LIU Xin-sheng2(
), LIU Xin-sheng2( )
)
Received:2021-12-13
Published:2022-10-26
Online:2022-11-11
摘要:
分别建立基于猪嵴病毒(PKV)结构蛋白VP0与VP1的间接ELISA检测方法。对PKV结构蛋白VP0与VP1的基因进行合成并连接至原核表达载体pET-32a后,转入感受态细胞BL21中,用IPTG诱导表达的重组蛋白经Ni柱纯化后,分别以重组蛋白pET-32a-VP0与pET-32a-VP1作为包被抗原,采用棋盘滴定法建立两种间接ELISA检测方法,并进行重复性、敏感性、特异性实验和临床检测。结果显示重组蛋白pET-32a-VP0与pET-32a-VP1抗原的最佳包被浓度分别为2 mg/mL和2.5 mg/mL,反应条件均为37℃、1 h;封闭液最佳条件为5%脱脂乳,37℃、2 h;血清最佳孵育条件为1∶200,37℃、1 h;二抗最佳孵育条件分别为1∶20 000和1∶15 000,37℃、1 h;最佳显色时间为10 min,两种间接ELISA检测方法临界值分别为0.306和0.277。试验结果表明本研究成功建立了能够有效检测PKV血清特异性抗体的间接ELISA方法,且方法具有重复性好、敏感性高、特异性强、稳定性好等特点,对今后PKV的临床诊断及抗体检测试剂盒的开发奠定了重要基础。
沈俊强, 张莉萍, 于瑞明, 王永录, 潘丽, 刘霞, 刘新生. 猪嵴病毒结构蛋白VP0与VP1原核表达及间接ELISA方法的建立[J]. 生物技术通报, 2022, 38(10): 243-253.
SHEN Jun-qiang, ZHANG Li-ping, YU Rui-ming, WANG Yong-lu, PAN Li, LIU Xia, LIU Xin-sheng. Porcine Kobuvirus Structural Proteins VP0 and VP1 Prokaryotic Expression and Establishment of Indirect ELISA Method[J]. Biotechnology Bulletin, 2022, 38(10): 243-253.
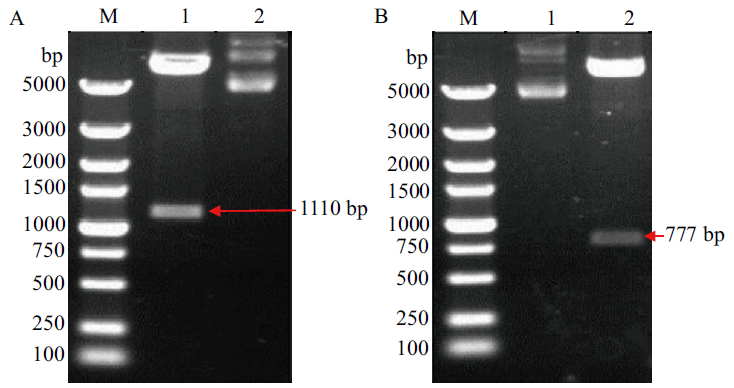
图1 重组质粒pET-32a-VP0与pET-32a-VP1双酶切鉴定 M:5000bp DNA marker;A2、B1:重组质粒DNA;A1:重组质粒pET-32a-VP0双酶切;B2:重组质粒pET-32a-VP1双酶切
Fig.1 Double digestion identification of recombinant plas-mid pET-32a-VP0 and pET-32a-VP1 M:5000bp DNA marker. A2、B1:Recombinant plasmid DNA. A1:Recombi-nant plasmid pET-32a-VP0 double digestion. B2:Recombinant plasmid pET-32a-VP1 double digestion

图2 重组蛋白pET-32a-VP0与pET-32a-VP1诱导表达 M:Marker;A1-4:重组蛋白pET-32a-VP0菌液超声;A5、B4:空载体pET-32a;B1-3:重组蛋白pET-32a-VP1菌液超声
Fig. 2 Induced expression of recombinant protein pET-32a-VP0 and pET-32a-VP1 M:Marker. A1-4:Recombinant protein pET-32a-VP0 bacteriophage sonication. A5 and B4:Empty vector pET-32a. B1-3:Recombinant protein pET-32a-VP1 bacteriophage sonication
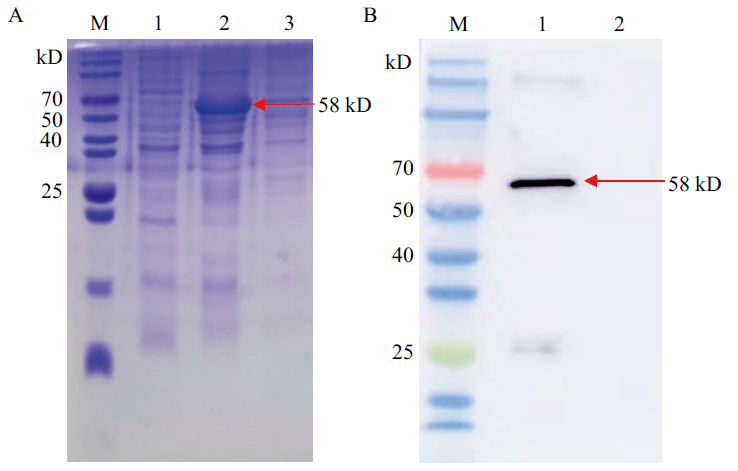
图3 重组蛋白pET-32a-VP0可溶性分析及Western blot-ting验证 M:Marker;A1:重组蛋白pET-32a-VP0超声上清;A2:重组蛋白pET-32a-VP0超声沉淀;A3、B2:空载体pET-32a;B1:纯化的重组蛋白pET-32a-VP0
Fig. 3 Solubility analysis and Western blotting validation of recombinant protein pET-32a-VP0 M:Marker. A1:Recombinant protein pET-32a-VP0 sonication supernatant. A2:Recombinant protein pET-32a-VP0 sonication precipitation. A3 and B2:Empty vector pET-32a. B1:Purified recombinant protein pET-32a-VP0

图4 重组蛋白pET-32a-VP1可溶性分析及Western blot-ting验证 M:Marker;A1:重组蛋白pET-32a-VP1超声上清;A2:重组蛋白pET-32a-VP1超声沉淀;A3、B2:空载体pET-32a;B1:纯化的重组蛋白pET-32a-VP1
Fig.4 Solubility analysis and Western blotting validation of recombinant protein pET-32a-VP1 M:Marker.A1:Recombinant protein pET-32a-VP1 sonication supernatant. A2:Recombinant protein pET-32a-VP1 sonication precipitation. A3 and B2:Empty vector pET-32a. B1:Purified recombinant protein pET-32a-VP1
| 血清稀释度Serum dilution | PN值 PN value | 抗原包被浓度Antigen encapsulation concentration/(mg·mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 2.5 | 2 | 1 | 0.5 | 0.25 | 0.1 | |||
| 1∶100 | N | 0.594 | 0.418 | 0.353 | 0.279 | 0.148 | 0.240 | 0.163 | |
| P | 2.477 | 2.234 | 2.167 | 2.234 | 2.273 | 2.265 | 1.991 | ||
| P/N | 4.170 | 5.344 | 6.136 | 8.001 | 15.368 | 9.456 | 12.185 | ||
| 1∶150 | N | 0.466 | 0.244 | 0.305 | 0.237 | 0.178 | 0.155 | 0.126 | |
| P | 2.307 | 2.080 | 2.049 | 1.956 | 2.039 | 1.964 | 1.883 | ||
| P/N | 4.953 | 8.531 | 6.728 | 8.267 | 11.483 | 12.636 | 14.910 | ||
| 1∶200 | N | 0.374 | 0.230 | 0.194 | 0.179 | 0.169 | 0.164 | 0.132 | |
| P | 2.212 | 2.141 | 2.040 | 1.896 | 1.960 | 1.930 | 1.699 | ||
| P/N | 5.907 | 8.547 | 10.515 | 10.614 | 11.616 | 11.791 | 12.852 | ||
| 1∶300 | N | 0.242 | 0.205 | 0.257 | 0.211 | 0.132 | 0.126 | 0.101 | |
| P | 2.144 | 1.856 | 1.669 | 1.609 | 1.732 | 1.604 | 1.212 | ||
| P/N | 8.868 | 9.050 | 6.504 | 7.644 | 13.134 | 12.746 | 12.019 | ||
| 1∶400 | N | 0.191 | 0.133 | 0.188 | 0.134 | 0.120 | 0.107 | 0.090 | |
| P | 2.023 | 1.640 | 1.521 | 1.401 | 1.545 | 1.249 | 1.075 | ||
| P/N | 10.574 | 12.321 | 8.075 | 10.497 | 12.929 | 11.706 | 11.976 | ||
| 1∶500 | N | 0.144 | 0.105 | 0.107 | 0.111 | 0.099 | 0.096 | 0.079 | |
| P | 2.111 | 1.708 | 1.591 | 1.367 | 1.337 | 1.148 | 0.791 | ||
| P/N | 14.642 | 16.249 | 14.824 | 12.318 | 13.474 | 11.906 | 9.982 | ||
表1 棋盘法测定重组蛋白pET-32a-VP0抗原最佳包被浓度与血清稀释度
Table 1 Optimal encapsulation concentration of recombinant protein pET-32a-VP0 antigen with serum dilution determined by checkerboard method
| 血清稀释度Serum dilution | PN值 PN value | 抗原包被浓度Antigen encapsulation concentration/(mg·mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 2.5 | 2 | 1 | 0.5 | 0.25 | 0.1 | |||
| 1∶100 | N | 0.594 | 0.418 | 0.353 | 0.279 | 0.148 | 0.240 | 0.163 | |
| P | 2.477 | 2.234 | 2.167 | 2.234 | 2.273 | 2.265 | 1.991 | ||
| P/N | 4.170 | 5.344 | 6.136 | 8.001 | 15.368 | 9.456 | 12.185 | ||
| 1∶150 | N | 0.466 | 0.244 | 0.305 | 0.237 | 0.178 | 0.155 | 0.126 | |
| P | 2.307 | 2.080 | 2.049 | 1.956 | 2.039 | 1.964 | 1.883 | ||
| P/N | 4.953 | 8.531 | 6.728 | 8.267 | 11.483 | 12.636 | 14.910 | ||
| 1∶200 | N | 0.374 | 0.230 | 0.194 | 0.179 | 0.169 | 0.164 | 0.132 | |
| P | 2.212 | 2.141 | 2.040 | 1.896 | 1.960 | 1.930 | 1.699 | ||
| P/N | 5.907 | 8.547 | 10.515 | 10.614 | 11.616 | 11.791 | 12.852 | ||
| 1∶300 | N | 0.242 | 0.205 | 0.257 | 0.211 | 0.132 | 0.126 | 0.101 | |
| P | 2.144 | 1.856 | 1.669 | 1.609 | 1.732 | 1.604 | 1.212 | ||
| P/N | 8.868 | 9.050 | 6.504 | 7.644 | 13.134 | 12.746 | 12.019 | ||
| 1∶400 | N | 0.191 | 0.133 | 0.188 | 0.134 | 0.120 | 0.107 | 0.090 | |
| P | 2.023 | 1.640 | 1.521 | 1.401 | 1.545 | 1.249 | 1.075 | ||
| P/N | 10.574 | 12.321 | 8.075 | 10.497 | 12.929 | 11.706 | 11.976 | ||
| 1∶500 | N | 0.144 | 0.105 | 0.107 | 0.111 | 0.099 | 0.096 | 0.079 | |
| P | 2.111 | 1.708 | 1.591 | 1.367 | 1.337 | 1.148 | 0.791 | ||
| P/N | 14.642 | 16.249 | 14.824 | 12.318 | 13.474 | 11.906 | 9.982 | ||
| 血清稀释度Serum dilution | PN值 PN value | 抗原包被浓度Antigen encapsulation concentration/(mg·mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 2.5 | 2 | 1 | 0.5 | 0.25 | 0.1 | |||
| 1∶100 | N | 0.348 | 0.299 | 0.288 | 0.220 | 0.142 | 0.146 | 0.106 | |
| P | 2.309 | 2.238 | 2.234 | 2.047 | 2.109 | 1.904 | 1.798 | ||
| P/N | 6.634 | 7.497 | 7.751 | 9.291 | 14.897 | 13.012 | 16.946 | ||
| 1∶150 | N | 0.258 | 0.354 | 0.209 | 0.160 | 0.134 | 0.136 | 0.118 | |
| P | 2.246 | 2.153 | 2.050 | 1.849 | 1.937 | 1.516 | 1.543 | ||
| P/N | 8.706 | 6.082 | 9.799 | 11.527 | 14.414 | 11.169 | 13.105 | ||
| 1∶200 | N | 0.230 | 0.199 | 0.188 | 0.164 | 0.141 | 0.164 | 0.104 | |
| P | 2.221 | 2.087 | 1.986 | 1.719 | 1.716 | 1.426 | 1.328 | ||
| P/N | 9.649 | 10.503 | 10.562 | 10.479 | 12.216 | 8.672 | 12.769 | ||
| 1∶300 | N | 0.159 | 0.174 | 0.136 | 0.112 | 0.136 | 0.197 | 0.256 | |
| P | 2.134 | 2.127 | 2.169 | 1.655 | 1.465 | 1.154 | 1.089 | ||
| P/N | 13.461 | 12.239 | 15.898 | 14.832 | 10.807 | 5.864 | 4.260 | ||
| 1∶400 | N | 0.137 | 0.151 | 0.139 | 0.098 | 0.094 | 0.090 | 0.090 | |
| P | 1.970 | 1.890 | 1.632 | 1.312 | 1.304 | 1.155 | 1.021 | ||
| P/N | 14.380 | 12.517 | 11.741 | 13.388 | 13.872 | 12.833 | 11.344 | ||
| 1∶500 | N | 0.120 | 0.165 | 0.131 | 0.092 | 0.081 | 0.085 | 0.074 | |
| P | 1.971 | 1.846 | 1.588 | 1.225 | 1.197 | 1.107 | 1.014 | ||
| P/N | 0.419 | 4.099 | 6.638 | 14.336 | 20.273 | 21.851 | 26.822 | ||
表2 棋盘法测定重组蛋白pET-32a-VP1抗原最佳包被浓度与血清稀释度
Table 2 Optimal encapsulation concentration of recombinant protein pET-32a-VP1 antigen with serum dilution determined by checkerboard method
| 血清稀释度Serum dilution | PN值 PN value | 抗原包被浓度Antigen encapsulation concentration/(mg·mL-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 2.5 | 2 | 1 | 0.5 | 0.25 | 0.1 | |||
| 1∶100 | N | 0.348 | 0.299 | 0.288 | 0.220 | 0.142 | 0.146 | 0.106 | |
| P | 2.309 | 2.238 | 2.234 | 2.047 | 2.109 | 1.904 | 1.798 | ||
| P/N | 6.634 | 7.497 | 7.751 | 9.291 | 14.897 | 13.012 | 16.946 | ||
| 1∶150 | N | 0.258 | 0.354 | 0.209 | 0.160 | 0.134 | 0.136 | 0.118 | |
| P | 2.246 | 2.153 | 2.050 | 1.849 | 1.937 | 1.516 | 1.543 | ||
| P/N | 8.706 | 6.082 | 9.799 | 11.527 | 14.414 | 11.169 | 13.105 | ||
| 1∶200 | N | 0.230 | 0.199 | 0.188 | 0.164 | 0.141 | 0.164 | 0.104 | |
| P | 2.221 | 2.087 | 1.986 | 1.719 | 1.716 | 1.426 | 1.328 | ||
| P/N | 9.649 | 10.503 | 10.562 | 10.479 | 12.216 | 8.672 | 12.769 | ||
| 1∶300 | N | 0.159 | 0.174 | 0.136 | 0.112 | 0.136 | 0.197 | 0.256 | |
| P | 2.134 | 2.127 | 2.169 | 1.655 | 1.465 | 1.154 | 1.089 | ||
| P/N | 13.461 | 12.239 | 15.898 | 14.832 | 10.807 | 5.864 | 4.260 | ||
| 1∶400 | N | 0.137 | 0.151 | 0.139 | 0.098 | 0.094 | 0.090 | 0.090 | |
| P | 1.970 | 1.890 | 1.632 | 1.312 | 1.304 | 1.155 | 1.021 | ||
| P/N | 14.380 | 12.517 | 11.741 | 13.388 | 13.872 | 12.833 | 11.344 | ||
| 1∶500 | N | 0.120 | 0.165 | 0.131 | 0.092 | 0.081 | 0.085 | 0.074 | |
| P | 1.971 | 1.846 | 1.588 | 1.225 | 1.197 | 1.107 | 1.014 | ||
| P/N | 0.419 | 4.099 | 6.638 | 14.336 | 20.273 | 21.851 | 26.822 | ||
| 优化条件 Optimization condition | 反应条件优化Optimization of reaction conditions | |||||
|---|---|---|---|---|---|---|
| 抗原包被 Antigen-coated | 封闭液 Sealing fluid | 一抗孵育 Primary antibody incubation | 二抗孵育 Secondary antibody incubation | 底物显色 Substrate chromogenesis | ||
| 最佳稀释度 Optimal dilution | 2 mg/mL 2.5 mg/mL | 5%脱脂乳 5% Skimmed milk powder | 1∶200 | 1∶20 000 1∶15 000 | ||
| 反应条件 Reaction condition | 37℃,1 h | 37℃,2 h | 37℃,1 h | 37℃,1 h | 避光10 min Avoid light 10 min | |
表3 反应条件优化结果
Table 3 Optimization results of reaction conditions
| 优化条件 Optimization condition | 反应条件优化Optimization of reaction conditions | |||||
|---|---|---|---|---|---|---|
| 抗原包被 Antigen-coated | 封闭液 Sealing fluid | 一抗孵育 Primary antibody incubation | 二抗孵育 Secondary antibody incubation | 底物显色 Substrate chromogenesis | ||
| 最佳稀释度 Optimal dilution | 2 mg/mL 2.5 mg/mL | 5%脱脂乳 5% Skimmed milk powder | 1∶200 | 1∶20 000 1∶15 000 | ||
| 反应条件 Reaction condition | 37℃,1 h | 37℃,2 h | 37℃,1 h | 37℃,1 h | 避光10 min Avoid light 10 min | |
| 样品Sample | 批内变异系数Variation intra-batch coefficient | 批间变异系数Variation inter-batch coefficient | ||||||
|---|---|---|---|---|---|---|---|---|
| 平均值Arithmetic mean | 标准差Standard deviation | CV/% | 平均值Arithmetic mean | 标准差Standard deviation | CV/% | |||
| 1 | 0.195 | 0.009 | 4.6 | 0.189 | 0.011 | 5.8 | ||
| 2 | 0.188 | 0.011 | 5.9 | 0.203 | 0.007 | 3.4 | ||
| 3 | 0.202 | 0.008 | 4.0 | 0.201 | 0.014 | 7.0 | ||
| 4 | 2.223 | 0.073 | 3.3 | 2.131 | 0.061 | 2.9 | ||
| 5 | 2.371 | 0.062 | 2.6 | 2.169 | 0.081 | 3.7 | ||
| 6 | 2.189 | 0.089 | 4.1 | 2.438 | 0.092 | 3.8 | ||
表4 重组蛋白pET-32a-VP0重复性实验
Table 4 Reproducibility experiments of recombinant protein pET-32a-VP0
| 样品Sample | 批内变异系数Variation intra-batch coefficient | 批间变异系数Variation inter-batch coefficient | ||||||
|---|---|---|---|---|---|---|---|---|
| 平均值Arithmetic mean | 标准差Standard deviation | CV/% | 平均值Arithmetic mean | 标准差Standard deviation | CV/% | |||
| 1 | 0.195 | 0.009 | 4.6 | 0.189 | 0.011 | 5.8 | ||
| 2 | 0.188 | 0.011 | 5.9 | 0.203 | 0.007 | 3.4 | ||
| 3 | 0.202 | 0.008 | 4.0 | 0.201 | 0.014 | 7.0 | ||
| 4 | 2.223 | 0.073 | 3.3 | 2.131 | 0.061 | 2.9 | ||
| 5 | 2.371 | 0.062 | 2.6 | 2.169 | 0.081 | 3.7 | ||
| 6 | 2.189 | 0.089 | 4.1 | 2.438 | 0.092 | 3.8 | ||
| 样品Sample | 批内变异系数Variation intra-batch coefficient | 批间变异系数Variation inter-batch coefficient | ||||||
|---|---|---|---|---|---|---|---|---|
| 平均值Arithmetic mean | 标准差Standard deviation | CV/% | 平均值Arithmetic mean | 标准差Standard deviation | CV/% | |||
| 1 | 0.161 | 0.008 | 5.0 | 0.169 | 0.011 | 6.5 | ||
| 2 | 0.183 | 0.011 | 6.0 | 0.192 | 0.007 | 3.6 | ||
| 3 | 0.166 | 0.012 | 7.2 | 0.159 | 0.013 | 8.2 | ||
| 4 | 1.882 | 0.058 | 3.1 | 1.803 | 0.061 | 3.4 | ||
| 5 | 1.764 | 0.074 | 4.2 | 1.922 | 0.081 | 4.2 | ||
| 6 | 1.777 | 0.092 | 5.2 | 2.004 | 0.092 | 4.6 | ||
表5 重组蛋白pET-32a-VP1重复性实验
Table 5 Reproducibility experiments of recombinant protein pET-32a-VP1
| 样品Sample | 批内变异系数Variation intra-batch coefficient | 批间变异系数Variation inter-batch coefficient | ||||||
|---|---|---|---|---|---|---|---|---|
| 平均值Arithmetic mean | 标准差Standard deviation | CV/% | 平均值Arithmetic mean | 标准差Standard deviation | CV/% | |||
| 1 | 0.161 | 0.008 | 5.0 | 0.169 | 0.011 | 6.5 | ||
| 2 | 0.183 | 0.011 | 6.0 | 0.192 | 0.007 | 3.6 | ||
| 3 | 0.166 | 0.012 | 7.2 | 0.159 | 0.013 | 8.2 | ||
| 4 | 1.882 | 0.058 | 3.1 | 1.803 | 0.061 | 3.4 | ||
| 5 | 1.764 | 0.074 | 4.2 | 1.922 | 0.081 | 4.2 | ||
| 6 | 1.777 | 0.092 | 5.2 | 2.004 | 0.092 | 4.6 | ||

图5 重组蛋白pET-32a-VP0与pET-32a-VP1特异性实验 A:重组蛋白pET-32a-VP0;B:重组蛋白pET-32a-VP1
Fig. 5 Specificity assay of recombinant protein pET-32a-VP0 and pET-32a-VP1 A:Recombinant protein pET-32a-VP0. B:Recombinant protein pET-32a-VP1

图6 重组蛋白pET-32a-VP0与pET-32a-VP1敏感性实验 A:重组蛋白pET-32a-VP0;B:重组蛋白pET-32a-VP1
Fig.6 Sensitivity assay of recombinant protein pET-32a-VP0 and pET-32a-VP1 A:Recombinant protein pET-32a-VP0. B:Recombinant protein pET-32a-VP1
| [1] |
Reuter G, Boldizsár A, Kiss I, et al. Candidate new species of kobuvirus in porcine hosts[J]. Emerg Infect Dis, 2008, 14(12):1968-1970.
doi: 10.3201/eid1412.080797 pmid: 19046542 |
| [2] |
Yu JM, Jin M, Zhang Q, et al. Candidate porcine kobuvirus, China[J]. Emerg Infect Dis, 2009, 15(5):823-825.
doi: 10.3201/eid1505.081518 URL |
| [3] |
Khamrin P, Maneekarn N, Hidaka S, et al. Molecular detection of kobuvirus sequences in stool samples collected from healthy pigs in Japan[J]. Infect Genet Evol, 2010, 10(7):950-954.
doi: 10.1016/j.meegid.2010.06.001 pmid: 20547246 |
| [4] |
Park SJ, Kim HK, Moon HJ, et al. Molecular detection of porcine kobuviruses in pigs in Korea and their association with diarrhea[J]. Arch Virol, 2010, 155(11):1803-1811.
doi: 10.1007/s00705-010-0774-1 URL |
| [5] |
Sisay Z, Wang QH, Oka T, et al. Prevalence and molecular characterization of porcine enteric caliciviruses and first detection of porcine kobuviruses in US swine[J]. Arch Virol, 2013, 158(7):1583-1588.
doi: 10.1007/s00705-013-1619-5 pmid: 23456421 |
| [6] |
Ribeiro J, Arruda Leme R, Alfieri AF, et al. High frequency of Aichivirus C(porcine kobuvirus)infection in piglets from different geographic regions of Brazil[J]. Trop Animal Heal Prod, 2013, 45(8):1757-1762.
doi: 10.1007/s11250-013-0428-x URL |
| [7] | 孟丽, 陶洁, 李本强, 等. 猪7种腹泻相关病毒的临床检测及猪嵴病毒的遗传进化分析[J]. 生物工程学报, 2017, 33(8):1292-1303. |
| Meng L, Tao J, Li BQ, et al. Clinical detection of seven porcine diarrhea-associated viruses and evolution analysis of porcine kobuvirus[J]. Chin J Biotechnol, 2017, 33(8):1292-1303. | |
| [8] |
Yang F, Liu XW, Zhou YC, et al. Histopathology of porcine Kobuvirus in Chinese piglets[J]. Virol Sin, 2015, 30(5):396-399.
doi: 10.1007/s12250-015-3608-1 pmid: 26475611 |
| [9] |
Theuns S, Vanmechelen B, Bernaert Q, et al. Nanopore sequencing as a revolutionary diagnostic tool for porcine viral enteric disease complexes identifies porcine kobuvirus as an important enteric virus[J]. Sci Rep, 2018, 8(1):9830.
doi: 10.1038/s41598-018-28180-9 pmid: 29959349 |
| [10] | 张莎. 猪嵴病毒流行病学调查及CH/HZ/2011株全基因序列分析[D]. 哈尔滨: 东北农业大学, 2013. |
| Zhang S. Epidemiological investigation of porcine kobuvirus and genome sequence analysis of CH/HZ/2011Strain[D]. Harbin: Northeast Agricultural University, 2013. | |
| [11] | 胡军勇, 汤细彪, 胡睿铭, 等. 猪库布病毒RT-PCR检测方法的建立及湖北省流行病学初步调查[J]. 华中农业大学学报, 2012, 31(4):485-489. |
| Hu JY, Tang XB, Hu RM, et al. Porcine kobuvirus RT-PCR detection assay establishment and application in primary epidemiology investigation in Hubei Province[J]. J Huazhong Agric Univ, 2012, 31(4):485-489. | |
| [12] | 王玮, 刘海源, 李鹏飞, 等. 2014年-2016年山东省猪嵴病毒流行病学调查[J]. 中国预防兽医学报, 2018, 40(8):670-674. |
| Wang W, Liu HY, Li PF, et al. Epidemiological investigation of porcine Kobuvirus in Shandong Province from 2014 to 2016[J]. Chin J Prev Vet Med, 2018, 40(8):670-674. | |
| [13] | 沈素芳, 汤赛冬, 孙泉云. 猪Kobu病毒感染概况[J]. 动物医学进展, 2012, 33(1):115-116. |
| Shen SF, Tang SD, Sun QY. Introduction to porcine Kobuvirus infection[J]. Prog Vet Med, 2012, 33(1):115-116. | |
| [14] |
Yamashita T, Kobayashi S, Sakac K, et al. Isolation of cytopathic small round viruses with BS-C-l cells from patients with gastroenteritis[J]. J Infect Dis, 1991, 164(5):954-957.
pmid: 1658159 |
| [15] |
Yamashita T, Ito M, Kabashima Y, et al. Isolation and characterization of a new species of Kobuvirus associated with cattle[J]. J Gen Virol, 2003, 84(Pt 11):3069-3077.
doi: 10.1099/vir.0.19266-0 pmid: 14573811 |
| [16] |
Reuter G, Boldizsár A, Pankovics P. Complete nucleotide and amino acid sequences and genetic organization of porcine Kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae[J]. Arch Virol, 2009, 154(1):101-108.
doi: 10.1007/s00705-008-0288-2 pmid: 19096904 |
| [17] |
Sasaki J, Kusuhara Y, Maeno Y, et al. Construction of an infectious cDNA clone of Aichi virus(a new member of the family Picornaviridae)and mutational analysis of a stem-loop structure at the 5' end of the genome[J]. J Virol, 2001, 75(17):8021-8030.
pmid: 11483747 |
| [18] |
Wang EL, Yang B, Liu W, et al. Complete sequencing and phylogenetic analysis of porcine kobuvirus in domestic pigs in Northwest China[J]. Arch Virol, 2014, 159(9):2533-2535.
doi: 10.1007/s00705-014-2087-2 pmid: 24777826 |
| [19] |
Sasaki J, Nagashima S, Taniguchi K. Aichi virus leader protein is involved in viral RNA replication and encapsidation[J]. J Virol, 2003, 77(20):10799-10807.
pmid: 14512530 |
| [20] |
Rossmann MG, Arnold E, Erickson JW, et al. Structure of a human common cold virus and functional relationship to other picornaviruses[J]. Nature, 1985, 317(6033):145-153.
doi: 10.1038/317145a0 URL |
| [21] |
Liu PJ, Li P, Lyu WT, et al. Epidemiological study and variation analysis of the porcine kobuvirus 3D gene in Sichuan Province, China[J]. Virol Sin, 2015, 30(6):460-463.
doi: 10.1007/s12250-015-3632-1 pmid: 26637336 |
| [22] |
Okitsu S, Khamrin P, Thongprachum A, et al. Sequence analysis of porcine Kobuvirus VP1 region detected in pigs in Japan and Thailand[J]. Virus Genes, 2012, 44(2):253-257.
doi: 10.1007/s11262-011-0692-7 pmid: 22109708 |
| [23] | 谢荣辉, 刘霞, 赵灵燕, 等. 猪嵴病毒荧光定量RT-PCR检测方法的建立和应用[J]. 中国兽医科学, 2018, 48(4):438-442. |
| Xie RH, Liu X, Zhao LY, et al. Establishment and application of the real-time reverse transcription polymerase chain reaction for detection of porcine kobuvirus[J]. Chin Vet Sci, 2018, 48(4):438-442. | |
| [24] | 李淞, 朱玲, 周远成, 等. 猪嵴病毒RT-PCR检测方法的建立及应用[J]. 中国兽医学报, 2013, 33(8):1150-1153. |
| Li S, Zhu L, Zhou YC, et al. Development and application of RT-PCR assay for detection of porcine kobuvirus[J]. Chin J Vet Sci, 2013, 33(8):1150-1153. | |
| [25] | 韩磊, 彭志豪, 刘莹, 等. 猪嵴病毒VP0基因的原核表达及间接ELISA检测方法的建立[J]. 中国动物传染病学报, 2021.1-7. |
| Han L, Peng ZH, Liu Y, et al. Prokaryotic expression of porcine Kobuvirus VP0 gene and establishment of indirect elisa detection method[J]. Chin J Animal Infect Dis, 2021. 1-7. | |
| [26] | 田野. 猪嵴病毒VP1基因克隆与表达及抗体检测ELISA方法的建立[D]. 扬州: 扬州大学, 2013. |
| Tian Y. Gene clone and prokaryotic expression of structural protein VP1 of porcine Kobuvirus and development of ELISA for detection of antibody to VP1[D]. Yangzhou: Yangzhou University, 2013. | |
| [27] | 祝俊鹏. 猪嵴病毒CH441株VP3基因的原核表达及间接ELISA方法的建立[D]. 兰州: 甘肃农业大学, 2015. |
| Zhu JP. Prokaryotic expression of the VP3 gene of porcine kobuvirus CH441 and development of indirect ELISA[D]. Lanzhou: Gansu Agricultural University, 2015. | |
| [28] | 朱庆贺, 苗艳, 李文辉, 等. 猪嵴病毒重组VP3蛋白间接ELISA检测方法的建立[J]. 中国兽医科学, 2017, 47(9):1150-1153. |
| Zhu QH, Miao Y, Li WH, et al. Establishment of an indirect ELISA based on VP3 protein for the detection of the antibodies against porcine kobuvirus[J]. Chin Vet Sci, 2017, 47(9):1150-1153. | |
| [29] |
Reuter G, Kecskeméti S, Pankovics P. Evolution of porcine kobuvirus infection, Hungary[J]. Emerg Infect Dis, 2010, 16(4):696-698.
doi: 10.3201/eid1604.090937 pmid: 20350391 |
| [30] | 梁丹洁. 广西猪嵴病毒的感染状况调查与基因序列分析[D]. 南宁: 广西大学, 2014. |
| Liang DJ. The epidemic situation research and genome sequence analysis of porcine kobuvirus in Guangxi Province[D]. Nanning: Guangxi University, 2014. | |
| [31] | 毛亚萍, 卞大伟. SARS-CoV-2毒株S蛋白突变及其影响的生物信息学分析[J]. 病毒学报, 2020, 36(6):1020-1027. |
| Mao YP, Bian DW. Bioinformatics analysis of mutations in the spike protein of SARS-CoV-2 and their effects[J]. Chin J Virol, 2020, 36(6):1020-1027. |
| [1] | 梅欢, 李玥, 刘可蒙, 刘吉华. 小檗碱桥酶高效原核表达及生物合成l-SLR的研究[J]. 生物技术通报, 2023, 39(7): 277-287. |
| [2] | 索青青, 吴楠, 杨慧, 李莉, 王锡锋. 水稻咖啡酰辅酶A-O-甲基转移酶基因的原核表达、抗体制备和应用[J]. 生物技术通报, 2022, 38(8): 135-141. |
| [3] | 覃雪晶, 王雨涵, 曹一博, 张凌云. 青杄PwHAP5基因原核表达及多克隆抗体制备[J]. 生物技术通报, 2022, 38(8): 142-149. |
| [4] | 王光丽, 范婵, 王辉, 卢惠芳, 夏灵尹, 黄健, 闵迅. 霍乱弧菌溶血素HlyA的原核表达、纯化及多克隆抗体制备与鉴定[J]. 生物技术通报, 2022, 38(7): 269-277. |
| [5] | 汪巧菊, 胡雨萌, 温亚亚, 宋丽, 孟闯, 潘志明, 焦新安. 新型冠状病毒S1蛋白的表达及活性鉴定[J]. 生物技术通报, 2022, 38(3): 157-163. |
| [6] | 山草梅, 叶蕾, 张连虎, 况卫刚, 孙晓棠, 马建, 崔汝强. 水稻抗潜根线虫基因OsRAI1的克隆及功能分析[J]. 生物技术通报, 2021, 37(7): 146-155. |
| [7] | 曾福源, 苏泽辉, 周诗慧, 谢妙, 庞欢瑛. 溶藻弧菌PEPCK蛋白原核表达及其乙酰化、琥珀酰化修饰的鉴定[J]. 生物技术通报, 2021, 37(5): 84-91. |
| [8] | 张西西, 张怡青, 李玉林, 韩笑, 王国强, 王晓军, 王旭东, 王云龙. 新型冠状病毒(SARS-CoV-2)N蛋白C端重组蛋白的原核表达、纯化及应用[J]. 生物技术通报, 2021, 37(5): 92-97. |
| [9] | 白福美, 李至敏, 王小琴, 胡紫微, 鲍玲玲, 李志敏. 集胞藻PCC6803中N-乙酰鸟氨酸转氨酶的生化表征及结构分析[J]. 生物技术通报, 2021, 37(5): 98-107. |
| [10] | 瞿欢, 李成, 陈汭, 廖艺杰, 曹三杰, 文翼平, 颜其贵, 黄小波. 猪δ冠状病毒S1-CTD的截短表达及间接ELISA抗体方法的建立[J]. 生物技术通报, 2021, 37(5): 273-280. |
| [11] | 彭利忠, 张鹏, 周雯雯, 曾旭辉, 张小宁. 精子特异性蛋白Cabs1多克隆抗体的制备及多用途验证[J]. 生物技术通报, 2021, 37(3): 261-270. |
| [12] | 贺扬, 余巧玲, 王均, 覃川杰, 李华涛. 罗非鱼原核表达基因研究进展[J]. 生物技术通报, 2021, 37(2): 195-202. |
| [13] | 唐禄, 董丽平, 尹茉莉, 刘磊, 董媛, 王会岩. 成纤维细胞生长因子20单克隆抗体的制备及鉴定[J]. 生物技术通报, 2021, 37(10): 179-185. |
| [14] | 段应策, 胡姿仪, 杨帆, 李金涛, 邬向丽, 张瑞颖. 香菇草酰乙酸水解酶基因LeOAH1克隆及表达分析[J]. 生物技术通报, 2020, 36(9): 227-234. |
| [15] | 陈汭, 付嘉钰, 刘浩宇, 李成, 赵玉佳, 胡靖飞, 瞿欢, 曹三杰, 文心田, 文翼平, 赵勤, 伍锐, 黄小波. 猪δ冠状病毒(PDCoV)N蛋白的原核表达及多克隆抗体制备[J]. 生物技术通报, 2020, 36(8): 104-110. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||