








生物技术通报 ›› 2022, Vol. 38 ›› Issue (11): 227-237.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0280
收稿日期:2022-03-06
出版日期:2022-11-26
发布日期:2022-12-01
作者简介:郭宇飞,男,硕士研究生,研究方向:工业微生物;E-mail:基金资助:
GUO Yu-fei( ), YAN Rong-mei, ZHANG Xiao-ru, CAO Wei, LIU Hao(
), YAN Rong-mei, ZHANG Xiao-ru, CAO Wei, LIU Hao( )
)
Received:2022-03-06
Published:2022-11-26
Online:2022-12-01
摘要:
葡萄糖二酸是葡萄糖的一种二元羧酸衍生物,是重要的平台化合物,被应用于医药、化工等领域。本研究以黑曲霉为底盘细胞,通过表达来自恶臭假单胞菌Pseudomonas putida KT2440的糖醛酸脱氢酶基因ppudh,成功在黑曲霉中实现了葡萄糖二酸的合成,产量为18.74 mg/L;在此基础上通过共过表达黑曲霉自身来源的肌醇加氧酶(anmioxA)和肌醇-1-磷酸合酶(aninoA)、酿酒酵母Saccharomyces cerevisiae S288C来源的羧酸转运蛋白(scJEN1),强化了合成通路和外泌途径,将产量提高至102.10 mg/L;通过表达来自乳酸乳球菌Lactococcus lactis subsp. cremoris MG1363的NADH氧化酶(llnox),建立NAD+辅因子循环系统,使产量进一步提高至115.65 mg/L;利用RNA干扰技术对竞争支路中的关键酶磷酸果糖激酶(pfkA)和葡萄糖-6-磷酸脱氢酶(zwf)进行弱化表达,葡萄糖二酸最终的产量达到313.65 mg/L。本研究为微生物高效生产葡萄糖二酸和下游相关产品生产奠定基础。
郭宇飞, 闫荣媚, 张小茹, 曹威, 刘浩. 代谢工程改造黑曲霉生产葡萄糖二酸[J]. 生物技术通报, 2022, 38(11): 227-237.
GUO Yu-fei, YAN Rong-mei, ZHANG Xiao-ru, CAO Wei, LIU Hao. Metabolic Engineering Modification of Aspergillus niger for the Production of D-glucaric Acid[J]. Biotechnology Bulletin, 2022, 38(11): 227-237.
| 年份Year | 菌株Strain | 基因改造Genes madified | 碳源Carbon source | 产量Yield/(g·L-1) | 引用文献Reference |
|---|---|---|---|---|---|
| 2009 | E. coli BL21 Star(DE3) | ino1,MIOX,udh | Glucose | 1.13 | [ |
| 2010 | E. coli BL21 Star(DE3) | ino1,MIOX,udh | Glucose | 2.50 | [ |
| 2014 | E. coli MG1655 | ino1,SUMO-MIOX,udh | Myo-inositol | 4.85 | [ |
| 2016 | S. cerevisiae CEN. PK2-1D | ino1,inm,MIOX,udh | Glucose | 1.60 | [ |
| 2017 | E. coli L19S | ino1,MIOX,udh | Glucose | 0.80 | [ |
| 2017 | S. cerevisiae CEN. PK2-1C | MIOX,udh,ino1,pfk1 | Glucose | 0.23 | [ |
| 2018 | E. coli BL21 Star(DE3) | cscB,cscA,cscK,ino1, MIOX,udh,suhB | Sucrose | 1.42 | [ |
| 2018 | Pichia pastoris GS115 | MIOX,udh | Glucose | 2.60 | [ |
| 2018 | S. cerevisiae BY4741 | ino1,MIOX,udh | Glucose,myo-inositol | 6.00 | [ |
| 2020 | E. coli BL21 Star(DE3) | ino1,MIOX,udh,suhB | Glucose | 5.35 | [ |
| 2020 | S. cerevisiae | ino1,inm,MIOX,udh,vgb | Glucose | 6.38 | [ |
表1 微生物法发酵生产葡萄糖二酸的研究进展
Table 1 Research progress of producing gluconic acid by microbial fermentation
| 年份Year | 菌株Strain | 基因改造Genes madified | 碳源Carbon source | 产量Yield/(g·L-1) | 引用文献Reference |
|---|---|---|---|---|---|
| 2009 | E. coli BL21 Star(DE3) | ino1,MIOX,udh | Glucose | 1.13 | [ |
| 2010 | E. coli BL21 Star(DE3) | ino1,MIOX,udh | Glucose | 2.50 | [ |
| 2014 | E. coli MG1655 | ino1,SUMO-MIOX,udh | Myo-inositol | 4.85 | [ |
| 2016 | S. cerevisiae CEN. PK2-1D | ino1,inm,MIOX,udh | Glucose | 1.60 | [ |
| 2017 | E. coli L19S | ino1,MIOX,udh | Glucose | 0.80 | [ |
| 2017 | S. cerevisiae CEN. PK2-1C | MIOX,udh,ino1,pfk1 | Glucose | 0.23 | [ |
| 2018 | E. coli BL21 Star(DE3) | cscB,cscA,cscK,ino1, MIOX,udh,suhB | Sucrose | 1.42 | [ |
| 2018 | Pichia pastoris GS115 | MIOX,udh | Glucose | 2.60 | [ |
| 2018 | S. cerevisiae BY4741 | ino1,MIOX,udh | Glucose,myo-inositol | 6.00 | [ |
| 2020 | E. coli BL21 Star(DE3) | ino1,MIOX,udh,suhB | Glucose | 5.35 | [ |
| 2020 | S. cerevisiae | ino1,inm,MIOX,udh,vgb | Glucose | 6.38 | [ |
| 菌株Strain | 特征描述Characteristics | 来源Source |
|---|---|---|
| Escherichia coli JM109 | - | 本实验室保存 |
| Agrobacterium tumerfaciens AGL-1 | - | 本实验室保存 |
| S834 | A.niger ATCC1015,Tet-on::cre,ΔoahA,ΔcexA | 本实验室保存 |
| S985 | ΔoahA,ΔcexA,OEppudh | 本研究 |
| S1223 | ΔoahA,ΔcexA,OEppudh,OEanmioxA | 本研究 |
| S1877 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1 | 本研究 |
| S2086 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA | 本研究 |
| S2390 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA,OEllnox | 本研究 |
| S2603 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA,OEllnox,RNAipfkA | 本研究 |
| S2635 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA,OEllnox,RNAipfkA,RNAizwf | 本研究 |
表2 本研究使用的菌株
Table 2 Strains used in this study
| 菌株Strain | 特征描述Characteristics | 来源Source |
|---|---|---|
| Escherichia coli JM109 | - | 本实验室保存 |
| Agrobacterium tumerfaciens AGL-1 | - | 本实验室保存 |
| S834 | A.niger ATCC1015,Tet-on::cre,ΔoahA,ΔcexA | 本实验室保存 |
| S985 | ΔoahA,ΔcexA,OEppudh | 本研究 |
| S1223 | ΔoahA,ΔcexA,OEppudh,OEanmioxA | 本研究 |
| S1877 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1 | 本研究 |
| S2086 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA | 本研究 |
| S2390 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA,OEllnox | 本研究 |
| S2603 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA,OEllnox,RNAipfkA | 本研究 |
| S2635 | ΔoahA,ΔcexA,OEppudh,OEanmioxA,OEscJEN1,OEaninoA,OEllnox,RNAipfkA,RNAizwf | 本研究 |
| 质粒Plasmid | 特征描述Characteristics | 来源Source |
|---|---|---|
| pLH454 | loxP-hph-loxP,gpdA promoter,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH509 | loxP-hph-loxP,pkiA promoter,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH756 | loxP-hph-loxP,mbfA promoter,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH1418 | loxP-hph-loxP,glaA promoter,gfp loop,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH1453 | loxP-hph-loxP,pkiA promoter,gfp loop,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH690 | loxP-hph-loxP,gpdA promoter,ppudh gene,trpC terminator,hygr,kanr | 本研究 |
| pLH737 | loxP-hph-loxP,pkiA promoter,anmioxA gene,trpC terminator,hygr,kanr | 本研究 |
| pLH782 | loxP-hph-loxP,gpdA promoter,scJEN1 gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1020 | loxP-hph-loxP,gpdA promoter,aninoA gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1317 | loxP-hph-loxP,mbfA promoter,llnox gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1421 | loxP-hph-loxP,glaA promoter,sense sequence of zwf gene,gfp loop,antisense sequence of zwf gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1454 | loxP-hph-loxP,pkiA promoter,sense sequence of pfkA gene,gfp loop,antisense sequence of pfkA gene,trpC terminator,hygr,kanr | 本研究 |
表3 本研究使用的质粒
Table 3 Plasmids used in this study
| 质粒Plasmid | 特征描述Characteristics | 来源Source |
|---|---|---|
| pLH454 | loxP-hph-loxP,gpdA promoter,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH509 | loxP-hph-loxP,pkiA promoter,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH756 | loxP-hph-loxP,mbfA promoter,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH1418 | loxP-hph-loxP,glaA promoter,gfp loop,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH1453 | loxP-hph-loxP,pkiA promoter,gfp loop,trpC terminator,hygr,kanr | 本实验室保存 |
| pLH690 | loxP-hph-loxP,gpdA promoter,ppudh gene,trpC terminator,hygr,kanr | 本研究 |
| pLH737 | loxP-hph-loxP,pkiA promoter,anmioxA gene,trpC terminator,hygr,kanr | 本研究 |
| pLH782 | loxP-hph-loxP,gpdA promoter,scJEN1 gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1020 | loxP-hph-loxP,gpdA promoter,aninoA gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1317 | loxP-hph-loxP,mbfA promoter,llnox gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1421 | loxP-hph-loxP,glaA promoter,sense sequence of zwf gene,gfp loop,antisense sequence of zwf gene,trpC terminator,hygr,kanr | 本研究 |
| pLH1454 | loxP-hph-loxP,pkiA promoter,sense sequence of pfkA gene,gfp loop,antisense sequence of pfkA gene,trpC terminator,hygr,kanr | 本研究 |
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| Primers related to ppudh gene | |
| YZ-ppudh-F | ACCACCACCCCCTTCAAT |
| YZ-ppudh-R | TCAGTGATGGTGGTGATGGT |
| qPCR-ppudh-F | GCAACCACACCATCGGCTTCTAC |
| qPCR-ppudh-R | CCATAGCGGTCGAAGTAGAAGGAGG |
| Primers related to anmioxA gene | |
| anmioxA-F | ATCAATCATCCGTCAAGATGGAATTCGCACCAGTCGCTGTGTCTCCT |
| anmioxA-R | CCAGATCTCTGCAGGGTACCGAGCTCCTACCACTTGATCACCTTGTTGG |
| qPCR-anmioxA-F | GAAGCTCAACACCCTCATCGACG |
| qPCR-anmioxA-R | ATGAGACCGGTCAGCTGCATC |
| Primers related to scJEN1 gene | |
| YZ-scJEN1-F | ATGTCCTCCAGCATTACCGAT |
| YZ-scJEN1-R | GACCTTGGCGTAATCTTCCTT |
| qPCR-scJEN1-F | GACCACGAGAAGCTGTACCATAACCC |
| qPCR-scJEN1-R | GGGCTTATCTTCTTCGTCCTCCTCG |
| Primers related to aninoA gene | |
| aninoA-F | CACATCTAAACAATGGAATTCATGGCTCCCCACGCAAGC |
| aninoA-R | AGTGGATCCCTGCAGGGTACCCTAGAACAGCTTGTGCTCCAG |
| qPCR-aninoA-F | GGCCACCAACTACCACTTCAAGG |
| qPCR-aninoA-R | CCACAGAGCCGTAGTAGTTGGAGG |
| Primers related to llnox gene | |
| YZ-llnox-F | TCATCGGCACCAACCACG |
| YZ-llnox-R | ACGGTCATGTAGTTGTAGGGG |
| qPCR-llnox-F | ATCTGGTCATCAACTGCATCGG |
| qPCR-llnox-R | CCAGGGCGATGTAGGTGAAATC |
| Primers related to zwf gene | |
| zwf-S-F | TACACCTCAGCAATGGAGCTCAGAAGGAGCAGAACAGAATCT |
| zwf-S-R | GCTCCTGGAAGATCTGAGCTCATCCAGAGACTTTCCGTACT |
| zwf-A-F | CGCATCGAGCTGAAGGGTACCATCCAGAGACTTTCCGTACT |
| zwf-A-R | AAGTGGATCGCATGCGGTACCAGAAGGAGCAGAACAGAATCT |
| YZ-PglaA-F | ACCTGCGTTATAGCTTCCCG |
| YZ-gfp-F | AGGAGCGCACCATCTTCTTC |
| YZ-gfp-R | CTCGATGCGGTTCACCAGG |
| YZ-TtrpC-R | CTCCGGAGCTGACATCGAC |
| qPCR-zwf-F | GAACGAGAGGTGGGACGGTG |
| qPCR-zwf-R | CAGGCAGCTTGGAGTTCATCTTG |
| Primers related to pfkA gene | |
| pfkA-S-F | ATCAATCATCCGTCAAGATGGAATTCACTATGATGACACCCCGATCT |
| pfkA-S-R | GGTGCGCTCCTGGAGGTACCGAGCTCCAAAGTTGTCACGCAGGAAGT |
| pfkA-A-F | TGAACCGCATCGAGCTGAAGCTGCAGCAAAGTTGTCACGCAGGAAGT |
| pfkA-A-R | TGATTTCAGTAACGTTAAGTCTGCAGACTATGATGACACCCCGATCT |
| YZ-PpkiA-F | GCGAGGAGAAAATTCAGCACC |
| YZ-gfp-F | AGGAGCGCACCATCTTCTTC |
| YZ-gfp-R | CTCGATGCGGTTCACCAGG |
| YZ-TtrpC-R | CTCCGGAGCTGACATCGAC |
| qPCR-pfkA-F | ACCATTTCCAACAACGTGCCG |
| qPCR-pfkA-R | TGTCACGCAGGAAGTCAATGTCG |
| Primers related to actA gene | |
| qPCR-actA-F | TCCTCACCCTCAGATACCC |
| qPCR-actA-R | CACCGTCACCAGAGTCCA |
表4 本研究使用的引物
Table 4 Primers used in this study
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| Primers related to ppudh gene | |
| YZ-ppudh-F | ACCACCACCCCCTTCAAT |
| YZ-ppudh-R | TCAGTGATGGTGGTGATGGT |
| qPCR-ppudh-F | GCAACCACACCATCGGCTTCTAC |
| qPCR-ppudh-R | CCATAGCGGTCGAAGTAGAAGGAGG |
| Primers related to anmioxA gene | |
| anmioxA-F | ATCAATCATCCGTCAAGATGGAATTCGCACCAGTCGCTGTGTCTCCT |
| anmioxA-R | CCAGATCTCTGCAGGGTACCGAGCTCCTACCACTTGATCACCTTGTTGG |
| qPCR-anmioxA-F | GAAGCTCAACACCCTCATCGACG |
| qPCR-anmioxA-R | ATGAGACCGGTCAGCTGCATC |
| Primers related to scJEN1 gene | |
| YZ-scJEN1-F | ATGTCCTCCAGCATTACCGAT |
| YZ-scJEN1-R | GACCTTGGCGTAATCTTCCTT |
| qPCR-scJEN1-F | GACCACGAGAAGCTGTACCATAACCC |
| qPCR-scJEN1-R | GGGCTTATCTTCTTCGTCCTCCTCG |
| Primers related to aninoA gene | |
| aninoA-F | CACATCTAAACAATGGAATTCATGGCTCCCCACGCAAGC |
| aninoA-R | AGTGGATCCCTGCAGGGTACCCTAGAACAGCTTGTGCTCCAG |
| qPCR-aninoA-F | GGCCACCAACTACCACTTCAAGG |
| qPCR-aninoA-R | CCACAGAGCCGTAGTAGTTGGAGG |
| Primers related to llnox gene | |
| YZ-llnox-F | TCATCGGCACCAACCACG |
| YZ-llnox-R | ACGGTCATGTAGTTGTAGGGG |
| qPCR-llnox-F | ATCTGGTCATCAACTGCATCGG |
| qPCR-llnox-R | CCAGGGCGATGTAGGTGAAATC |
| Primers related to zwf gene | |
| zwf-S-F | TACACCTCAGCAATGGAGCTCAGAAGGAGCAGAACAGAATCT |
| zwf-S-R | GCTCCTGGAAGATCTGAGCTCATCCAGAGACTTTCCGTACT |
| zwf-A-F | CGCATCGAGCTGAAGGGTACCATCCAGAGACTTTCCGTACT |
| zwf-A-R | AAGTGGATCGCATGCGGTACCAGAAGGAGCAGAACAGAATCT |
| YZ-PglaA-F | ACCTGCGTTATAGCTTCCCG |
| YZ-gfp-F | AGGAGCGCACCATCTTCTTC |
| YZ-gfp-R | CTCGATGCGGTTCACCAGG |
| YZ-TtrpC-R | CTCCGGAGCTGACATCGAC |
| qPCR-zwf-F | GAACGAGAGGTGGGACGGTG |
| qPCR-zwf-R | CAGGCAGCTTGGAGTTCATCTTG |
| Primers related to pfkA gene | |
| pfkA-S-F | ATCAATCATCCGTCAAGATGGAATTCACTATGATGACACCCCGATCT |
| pfkA-S-R | GGTGCGCTCCTGGAGGTACCGAGCTCCAAAGTTGTCACGCAGGAAGT |
| pfkA-A-F | TGAACCGCATCGAGCTGAAGCTGCAGCAAAGTTGTCACGCAGGAAGT |
| pfkA-A-R | TGATTTCAGTAACGTTAAGTCTGCAGACTATGATGACACCCCGATCT |
| YZ-PpkiA-F | GCGAGGAGAAAATTCAGCACC |
| YZ-gfp-F | AGGAGCGCACCATCTTCTTC |
| YZ-gfp-R | CTCGATGCGGTTCACCAGG |
| YZ-TtrpC-R | CTCCGGAGCTGACATCGAC |
| qPCR-pfkA-F | ACCATTTCCAACAACGTGCCG |
| qPCR-pfkA-R | TGTCACGCAGGAAGTCAATGTCG |
| Primers related to actA gene | |
| qPCR-actA-F | TCCTCACCCTCAGATACCC |
| qPCR-actA-R | CACCGTCACCAGAGTCCA |
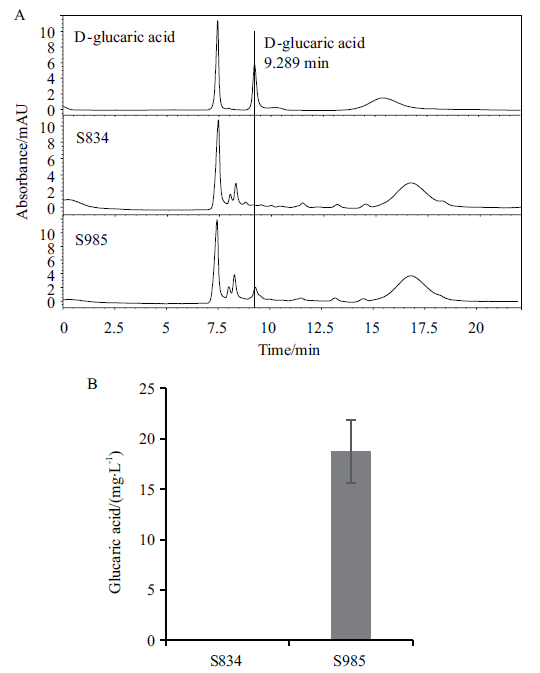
图2 表达ppudh基因对葡萄糖二酸产量的影响 A:100 mg/L葡萄糖二酸标准品和不同菌株发酵液的HPLC图;B:改造菌的葡萄糖二酸产量
Fig. 2 Effect of ppudh gene expression on glucaric acid production A:HPLC chromatogram of 100 mg/L glucaric acid standard and different strains fermentation. B:Glucaric acid yield of the modified strain
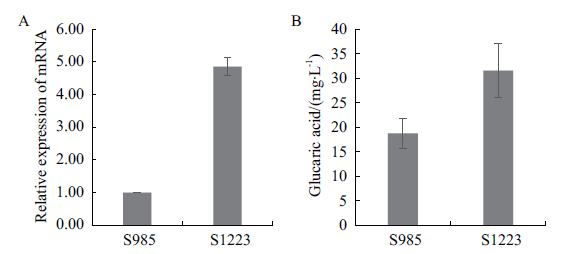
图3 表达anmioxA基因对葡萄糖二酸产量的影响 A:改造菌中anmioxA基因的相对表达量;B:改造菌的葡萄糖二酸的产量
Fig. 3 Effect of anmioxA gene expression on glucaric acid production A:The relative expression of anmioxA gene in the modified strain. B:Glucaric acid yield of the modified strain
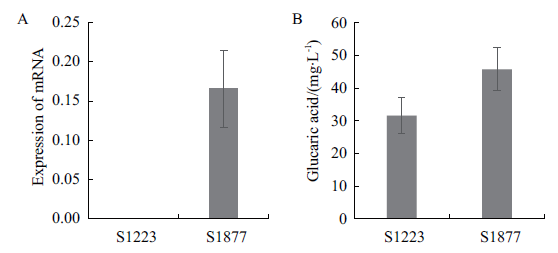
图4 表达scJEN1基因对葡萄糖二酸产量的影响 A:改造菌中scJEN1基因的表达量;B:改造菌的葡萄糖二酸的产量
Fig. 4 Effect of scJEN1 gene expression on glucaric acid production A:Expression of scJEN1 gene in the modified strain. B:Glucaric acid yield of the modified strain
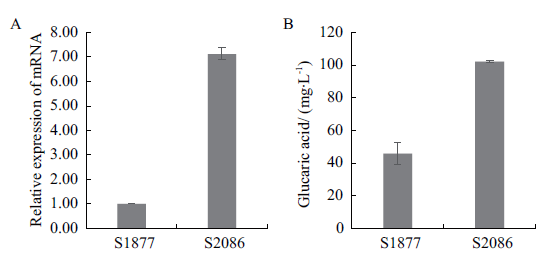
图5 表达aninoA基因对葡萄糖二酸产量的影响 A:改造菌中aninoA基因的相对表达量;B:改造菌的葡萄糖二酸的产量
Fig. 5 Effect of aninoA gene expression on glucaric acid production A:Relative expression of aninoA gene in the modified strain. B:Glucaric acid yield of the modified strain
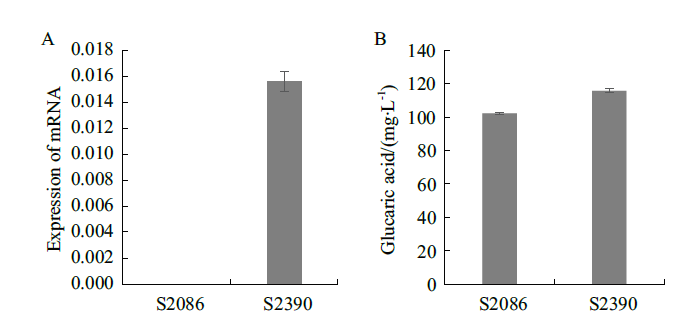
图6 表达llnox基因对葡萄糖二酸产量的影响 A:改造菌中llnox基因的表达量;B:改造菌的葡萄糖二酸的产量
Fig. 6 Effects of llnox gene expression on glucaric acid production A:Expression of llnox gene in the modified strain;B:Glucaric acid yield of the modified strain
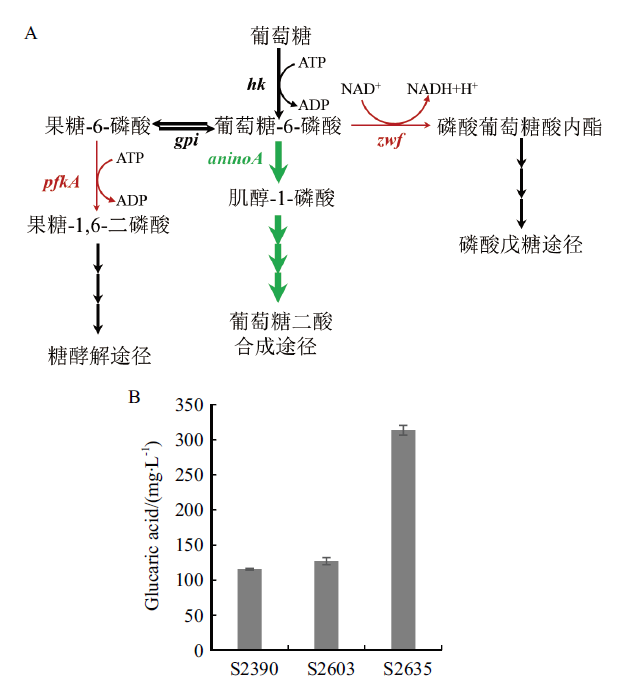
图7 干扰pfkA和zwf基因对葡萄糖二酸产量的影响 A:葡萄糖-6-磷酸的支路代谢(红色箭头表示弱化表达,绿色加粗箭头表示强化表达);B:改造菌的葡萄糖二酸的产量
Fig. 7 Effect of interfering pfkA and zwf gene on glucaric acid production A:Branch metabolism of glucose-6-phosphate(Red arrows indicate weakened expression and green bold arrows indicate enhanced expression). B:Glucaric acid yield of the modified strain
| [1] |
Walaszek Z, Szemraj J, Hanausek M, et al. D-Glucaric acid content of various fruits and vegetables and cholesterol-lowering effects of dietary d-glucarate in the rat[J]. Nutr Res, 1996, 16(4):673-681.
doi: 10.1016/0271-5317(96)00045-0 URL |
| [2] | Werpy T, Petersen G. Top Value Added Chemicals from Biomass:Volume I -- Results of Screening for Potential Candidates from Sugars and Synthesis Gas[R]. Office of Scientific and Technical Information(OSTI), 2004. |
| [3] |
Marsh CA. Metabolism of d-glucuronolactone in mammalian systems. 2. Conversion of d-glucuronolactone into d-glucaric acid by tissue preparations[J]. Biochem J, 1963, 87(1):82-90.
pmid: 16748999 |
| [4] | Zółtaszek R, Hanausek M, Kiliańska ZM, et al. The biological role of D-glucaric acid and its derivatives:potential use in medicine[J]. Postepy Hig Med Dosw(Online), 2008, 62:451-462. |
| [5] |
Walaszek Z. Potential use of D-glucaric acid derivatives in cancer prevention[J]. Cancer Lett, 1990, 54(1/2):1-8.
doi: 10.1016/0304-3835(90)90083-A URL |
| [6] | 余作龙, 苗江月, 曹飞, 等. 葡萄糖二酸的生物炼制及应用研究进展[J]. 化工进展, 2011, 30(11):2502-2508, 2535. |
| Yu ZL, Miao JY, Cao F, et al. Research progress in preparation and application of bio-refined D-glucaric acid[J]. Chem Ind Eng Prog, 2011, 30(11):2502-2508, 2535. | |
| [7] | 仇钰莹, 方芳, 堵国成, 等. 葡萄糖二酸研究进展[J]. 生物工程学报, 2015, 31(4):481-490. |
| Qiu YY, Fang F, Du GC, et al. Progress in glucaric acid[J]. Chin J Biotechnol, 2015, 31(4):481-490. | |
| [8] | Kiely DE, Hash KR, Sr. Method of oxidation using nitric acid:US9162959[P]. 2015-10-20. |
| [9] |
Ibert M, Fuertès P, Merbouh N, et al. Improved preparative electrochemical oxidation of d-glucose to d-glucaric acid[J]. Electrochimica Acta, 2010, 55(10):3589-3594.
doi: 10.1016/j.electacta.2009.11.041 URL |
| [10] | 朱俏俏, 庄军平, 余开荣. 生物质基葡萄糖二酸的研究与展望[J]. 工业催化, 2019, 27(9):13-18. |
| Zhu QQ, Zhuang JP, Yu KR. Current status and future prospect of biomass-based glucaric acid[J]. Ind Catal, 2019, 27(9):13-18. | |
| [11] | Pamuk V, Yılmaz M, Alıcılar A. The preparation of D-glucaric acid by oxidation of molasses in packed beds[J]. J Chem Technol Biotechnol, 2001, 76(2):186-190. |
| [12] |
Moon TS, Yoon SH, Lanza AM, et al. Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli[J]. Appl Environ Microbiol, 2009, 75(3):589-595.
doi: 10.1128/AEM.00973-08 URL |
| [13] |
Moon TS, Dueber JE, Shiue E, et al. Use of modular, synthetic scaffolds for improved production of glucaric acid in engineered E. coli[J]. Metab Eng, 2010, 12(3):298-305.
doi: 10.1016/j.ymben.2010.01.003 URL |
| [14] |
Gupta A, Hicks MA, Manchester SP, et al. Porting the synthetic D-glucaric acid pathway from Escherichia coli to Saccharomyces cerevisiae[J]. Biotechnol J, 2016, 11(9):1201-1208.
doi: 10.1002/biot.201500563 URL |
| [15] | 巩旭, 刘叶, 王毳, 等. 代谢工程改造酿酒酵母合成葡萄糖二酸[J]. 生物工程学报, 2017, 33(2):228-236. |
| Gong X, Liu Y, Wang C, et al. Metabolic engineering of Saccharomyces cerevisiae for production of glucaric acid[J]. Chin J Biotechnol, 2017, 33(2):228-236. | |
| [16] | 刘叶, 巩旭, 康振, 等. 代谢工程改造毕赤酵母生产葡萄糖二酸[J]. 食品与生物技术学报, 2018, 37(9):955-961. |
| Liu Y, Gong X, Kang Z, et al. Metabolic engineering of Pichia pastoris for production of glucaric acid[J]. J Food Sci Biotechnol, 2018, 37(9):955-961. | |
| [17] |
Shiue E, Prather KLJ. Improving D-glucaric acid production from myo-inositol in E. coli by increasing MIOX stability and myo-inositol transport[J]. Metab Eng, 2014, 22:22-31.
doi: 10.1016/j.ymben.2013.12.002 pmid: 24333274 |
| [18] |
Gupta A, Reizman IM, Reisch CR, et al. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit[J]. Nat Biotechnol, 2017, 35(3):273-279.
doi: 10.1038/nbt.3796 pmid: 28191902 |
| [19] |
Qu YN, Yan HJ, Guo Q, et al. Biosynthesis of D-glucaric acid from sucrose with routed carbon distribution in metabolically engineered Escherichia coli[J]. Metab Eng, 2018, 47:393-400.
doi: S1096-7176(17)30484-6 pmid: 29715517 |
| [20] |
Chen N, Wang JY, Zhao YY, et al. Metabolic engineering of Saccharomyces cerevisiae for efficient production of glucaric acid at high titer[J]. Microb Cell Fact, 2018, 17(1):67.
doi: 10.1186/s12934-018-0914-y pmid: 29729665 |
| [21] |
Su HH, Peng F, Ou XY, et al. Combinatorial synthetic pathway fine-tuning and cofactor regeneration for metabolic engineering of Escherichia coli significantly improve production of D-glucaric acid[J]. N Biotechnol, 2020, 59:51-58.
doi: 10.1016/j.nbt.2020.03.004 URL |
| [22] |
Zhang X, Xu C, Liu YL, et al. Enhancement of glucaric acid production in Saccharomyces cerevisiae by expressing Vitreoscilla hemoglobin[J]. Biotechnol Lett, 2020, 42(11):2169-2178.
doi: 10.1007/s10529-020-02966-2 pmid: 32691185 |
| [23] |
Schuster E, Dunn-Coleman N, Frisvad JC, et al. On the safety of Aspergillus niger-a review[J]. Appl Microbiol Biotechnol, 2002, 59(4/5):426-435.
doi: 10.1007/s00253-002-1032-6 URL |
| [24] |
Xu YX, Shan L, Zhou YT, et al. Development of a Cre-loxP-based genetic system in Aspergillus niger ATCC1015 and its application to construction of efficient organic acid-producing cell factories[J]. Appl Microbiol Biotechnol, 2019, 103(19):8105-8114.
doi: 10.1007/s00253-019-10054-3 URL |
| [25] |
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4):402-408.
doi: 10.1006/meth.2001.1262 pmid: 11846609 |
| [26] | 秦俊文, 谢琪璇, 蔡冬青, 等. shRNA慢病毒质粒的构建技巧[J]. 中国生物工程杂志, 2011, 31(3):124-127. |
| Qin JW, Xie QX, Cai DQ, et al. Construction techniques of shRNA lentivirus plasmid[J]. China Biotechnol, 2011, 31(3):124-127. | |
| [27] |
Chang SS, Zhang ZY, Liu Y. RNA interference pathways in fungi:mechanisms and functions[J]. Annu Rev Microbiol, 2012, 66:305-323.
doi: 10.1146/annurev-micro-092611-150138 URL |
| [28] |
Cairns TC, Nai C, Meyer V. How a fungus shapes biotechnology:100 years of Aspergillus niger research[J]. Fungal Biol Biotechnol, 2018, 5:13.
doi: 10.1186/s40694-018-0054-5 URL |
| [29] |
Andersen MR, Lehmann L, Nielsen J. Systemic analysis of the response of Aspergillus niger to ambient pH[J]. Genome Biol, 2009, 10(5):R47.
doi: 10.1186/gb-2009-10-5-r47 URL |
| [30] | 石慧, 陈卓逐, 阚建全. 大肠杆菌在食品加工贮藏中胁迫响应机制的研究进展[J]. 食品科学, 2016, 37(9):250-257. |
|
Shi H, Chen ZZ, Kan JQ. Progress in research on stress response in Escherichia coli during food processing and storage[J]. Food Sci, 2016, 37(9):250-257.
doi: 10.1111/j.1365-2621.1972.tb05828.x URL |
|
| [31] |
Yoon SH, Moon TS, Iranpour P, et al. Cloning and characterization of uronate dehydrogenases from two pseudomonads and Agrobacterium tumefaciens strain C58[J]. J Bacteriol, 2009, 191(5):1565-1573.
doi: 10.1128/JB.00586-08 URL |
| [32] | Ribas D, Sá-Pessoa J, Soares-Silva I, et al. Yeast as a tool to express sugar acid transporters with biotechnological interest[J]. FEMS Yeast Res, 2017, 17(2):2017 Mar 1;17(2). |
| [33] |
Shi XC, Zou YN, Chen Y, et al. A water-forming NADH oxidase regulates metabolism in anaerobic fermentation[J]. Biotechnol Biofuels, 2016, 9:103.
doi: 10.1186/s13068-016-0517-y URL |
| [1] | 薛鲜丽, 王静然, 毕杭杭, 王德培. 过表达Spt7对黑曲霉生长及抗逆性影响[J]. 生物技术通报, 2022, 38(5): 112-122. |
| [2] | 刘晓玫, 王东鑫, 张春, 魏双施. AAV介导的RNAi对SARS-CoV-2 S基因表达的抑制作用[J]. 生物技术通报, 2022, 38(3): 188-193. |
| [3] | 李红叶, 陈立佼, 刘明丽, 郭天杰, 王道平, 潘映红, 赵明. 黑曲霉单宁酶基因Tan2克隆与表达[J]. 生物技术通报, 2021, 37(3): 44-52. |
| [4] | 潘银来, 邱春辉, 王艺磊, 张子平. RNA药物的发展及其在水产上的应用[J]. 生物技术通报, 2021, 37(2): 203-215. |
| [5] | 孟晓建, 于建东, 郑小梅, 郑平, 李志敏, 孙际宾, 叶勤. 小分子代谢物对黑曲霉己糖激酶和丙酮酸激酶的酶活调控[J]. 生物技术通报, 2021, 37(12): 180-190. |
| [6] | 邓普荣, 刘勇波. RNAi与转Bt基因技术协同抗虫研究进展[J]. 生物技术通报, 2021, 37(10): 216-224. |
| [7] | 徐雪亮, 王奋山, 刘子荣, 范琳娟, 季香云, 蒋杰贤, 姚英娟. RNA干扰技术在昆虫学领域研究进展[J]. 生物技术通报, 2021, 37(1): 255-261. |
| [8] | 杨文文, 倪嘉瑶, 胡蕊洁, 王华忠. 一个RNAi载体上反向重复片段的测序策略[J]. 生物技术通报, 2020, 36(5): 205-210. |
| [9] | 宋华丽, 孙效迎, 孔祥会, 李莉, 裴超. RNA干扰技术在水产动物抗病毒和抗寄生虫研究中的应用研究进展[J]. 生物技术通报, 2020, 36(2): 193-205. |
| [10] | 许祥, 董维鹏, 张少华, 冯晨毅, 刘田福, 燕炯. Fsp27基因沉默载体的构建及其对细胞脂解的影响研究[J]. 生物技术通报, 2020, 36(1): 88-94. |
| [11] | 韩翠翠, 刘立琨, 王玉春, 杨莹, 刘吉成, 周忠光. TOX3基因RNAi慢病毒载体的构建及对乳腺癌ZR-75-1细胞增殖的影响[J]. 生物技术通报, 2019, 35(7): 141-147. |
| [12] | 王佳悦, 刘香男, 彭康莉, 赵博. RNA干扰USE1基因慢病毒载体的构建及鉴定[J]. 生物技术通报, 2019, 35(3): 117-122. |
| [13] | 陈浩宇, 徐瑞涛, 程志翔, 高强, 张健. H2O2对黑曲霉氧化胁迫机理的研究[J]. 生物技术通报, 2018, 34(4): 201-207. |
| [14] | 陈静, 张道伟, 钱正敏. 白背飞虱几丁质合成酶1基因的结构及特性研究[J]. 生物技术通报, 2018, 34(1): 195-201. |
| [15] | 魏姜勉, 鲁雷震, 焦国宝, 刘家扬, 陆隽鹤. 黑曲霉发酵菌渣对臧红T的吸附研究[J]. 生物技术通报, 2017, 33(10): 191-198. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||