








生物技术通报 ›› 2023, Vol. 39 ›› Issue (11): 191-204.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0723
收稿日期:2023-07-30
出版日期:2023-11-26
发布日期:2023-12-20
通讯作者:
郑道琼,男,博士,教授,研究方向:微生物遗传和基因组学;E-mail: zhengdaoqiong@zju.edu.cn作者简介:祝瑛萱,女,博士研究生,研究方向:环境因子驱动下的酵母基因组变异;E-mail: zhuyingxuan@zju.edu.cn
基金资助:
ZHU Ying-xuan( ), LI Ke-jing, HE Min, ZHENG Dao-qiong(
), LI Ke-jing, HE Min, ZHENG Dao-qiong( )
)
Received:2023-07-30
Published:2023-11-26
Online:2023-12-20
摘要:
基因组变异是遗传疾病发生和物种演化的分子基础,这个过程受到细胞内外源理化因子的共同作用。模式生物酿酒酵母(Saccharomyces cerevisiae)基因组小且易于开展分子遗传操作,在探究基因组变异进化调控机制的相关研究中应用广泛。本文总结了酵母模型中典型的DNA变异检测遗传体系,包括利用报告基因检测DNA突变率和红白扇形菌落筛选染色体重组子等;讨论了高通量测序技术在检测自发性和胁迫因子诱导基因组变异中的应用;综述了运用酵母模型揭示温度波动、氧化压力、抗肿瘤药物、金属离子和辐射等胁迫因子对基因组稳定性的影响及遗传机制的研究进展。酵母在多种胁迫条件下均会发生适应性进化现象,特定的染色体结构变异是适应性背后的重要遗传机制之一。在酵母中结合遗传筛选体系和高通量分析手段阐释细胞胁迫因子与基因组变异的关联机制,可为全面理解生物基因组不稳定机理和物种进化规律提供新的视角。
祝瑛萱, 李克景, 何敏, 郑道琼. 酵母模型揭示胁迫因子驱动基因组变异的研究进展[J]. 生物技术通报, 2023, 39(11): 191-204.
ZHU Ying-xuan, LI Ke-jing, HE Min, ZHENG Dao-qiong. Research Progress in the Exploring Genomic Variations Driven by Stress Factors Using the Yeast Model[J]. Biotechnology Bulletin, 2023, 39(11): 191-204.
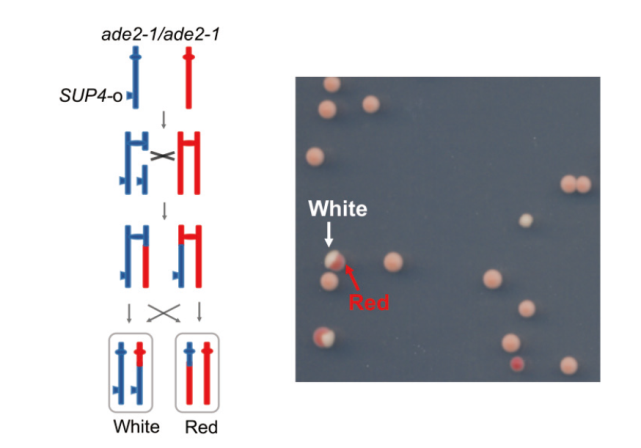
图1 酵母红白扇形菌落系统筛选染色体交换重组子 该二倍体酵母的ade2-1等位基因是赭石突变体,一份拷贝的SUP4-o基因可以部分抑制赭石突变,亲株菌落呈粉色。染色体交换重组事件可使一个子细胞具有两份拷贝的SUP4-o,在平板上形成白色菌落;另一个子细胞没有SUP4-o基因,形成的菌落显红色
Fig. 1 Yeast white/red sectoring colony system to screen chromosomal crossover recombinants The homologous allele ade2-1 in the diploid yeast strain is ocher mutant. One copy of the SUP4-o gene can partially suppress this ochre mutation of ade2-1, making the colonies appear pink. Chromosomal crossover events can lead to one daughter cell having two copies of the SUP4-o gene, causing the colonies to appear white; another daughter cell without the SUP4-o gene would result in red colonies
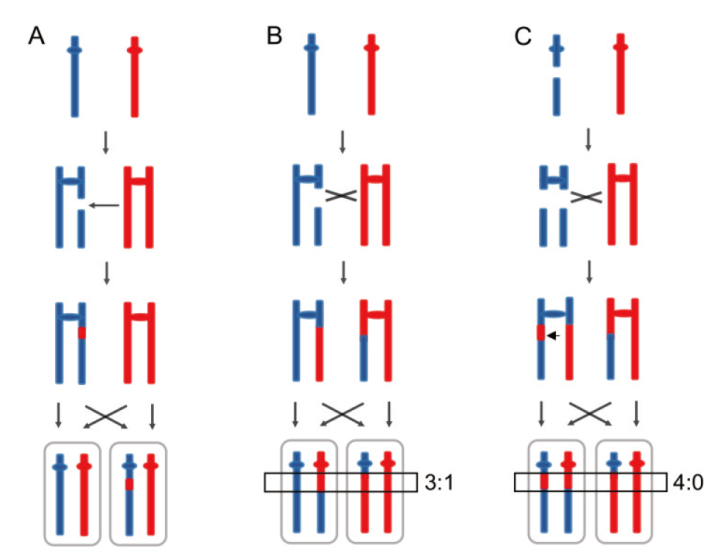
图2 DNA同源重组诱导杂合二倍体基因组杂合性丢失 A:同源重组修复姐妹染色体上双链断裂形成内部LOH,也称基因转换;B:同源重组修复姐妹染色体上双链断裂形成末端LOH,也称染色体交换。在断点附近形成3∶1的基因转换区域(黑框);C:G1期染色体断裂会使两条姐妹染色体上均存在DNA断裂。这种情况经修复后在断点处形成4∶0的基因转换区域
Fig. 2 LOH events induced by DNA homologous recombination in heterozygous diploid A: The interstitial LOH or gene conversion is resulted from repairing the DSB(double-strand DNA breaks)on a sister chromatid via homologous recombination. B: A 3∶1 pattern of the crossover associated with gene conversion tract(black rectangle)indicates repairing the DSB on one sister chromatid via homologous recombination. C: Chromosome breakage during the G1 phase results in DNA breaks on both sister chromosomes. A 4∶0 gene conversion district is formed at the breakpoint after this situation is repaired
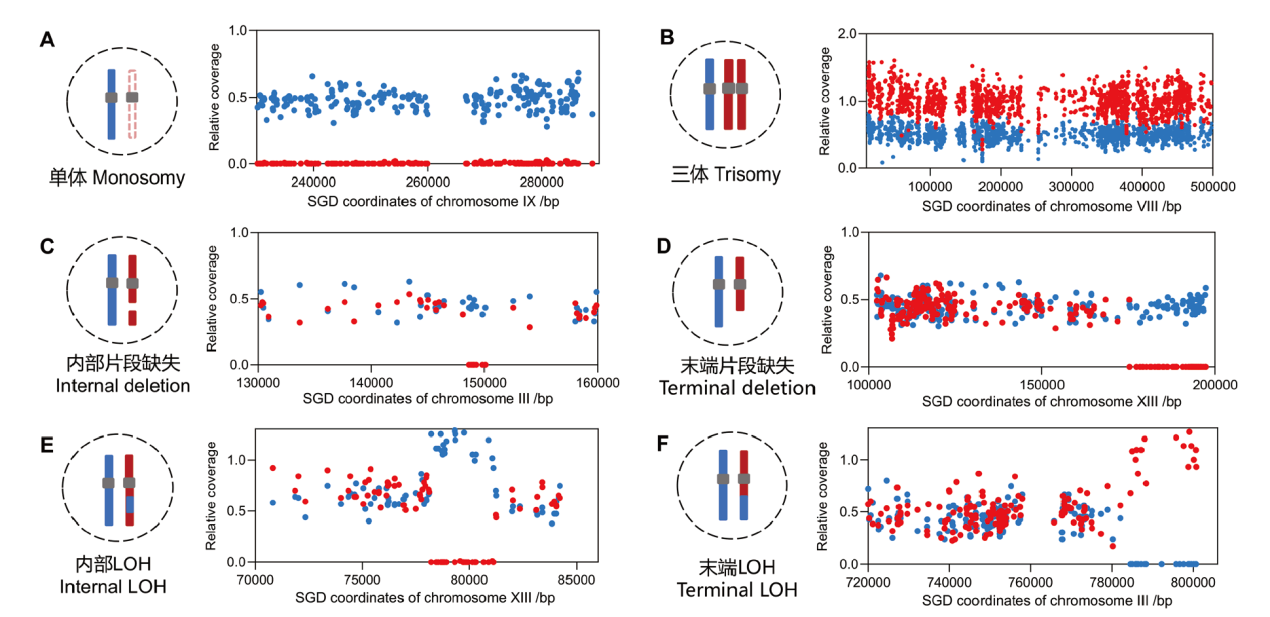
图3 利用SNP位点的相对测序深度分析基因组变异事件 A:染色体三体畸变事件;B:染色体单体畸变事件;C:染色体内部片段缺失;D:染色体末端缺失;E:基因转换引起内部杂合性丢失;F:染色体交换引起末端杂合性丢失。蓝色和红色的线条或点代表同源染色体或同源染色体上的SNP位点。SNP位点的相对测序深度0、0.5和1分别代表0、1和2份DNA拷贝
Fig. 3 Mapping genomic alterations by analyzing the relative sequencing coverages of SNP sites A: Chromosomal trisomy events. B: Chromosomal monosomy events. C: An internal deletion event in chromosome. D: A terminal deletion event in chromosome. E: Internal loss of heterozygosity(LOH)caused by gene conversion events. F: Crossover induced terminal LOH. The blue and red lines or points represent homologous chromosomes or SNP sites. The relative coverage value 0, 0.5, and 1 indicate zero, one, and two copies of DNA, respectively
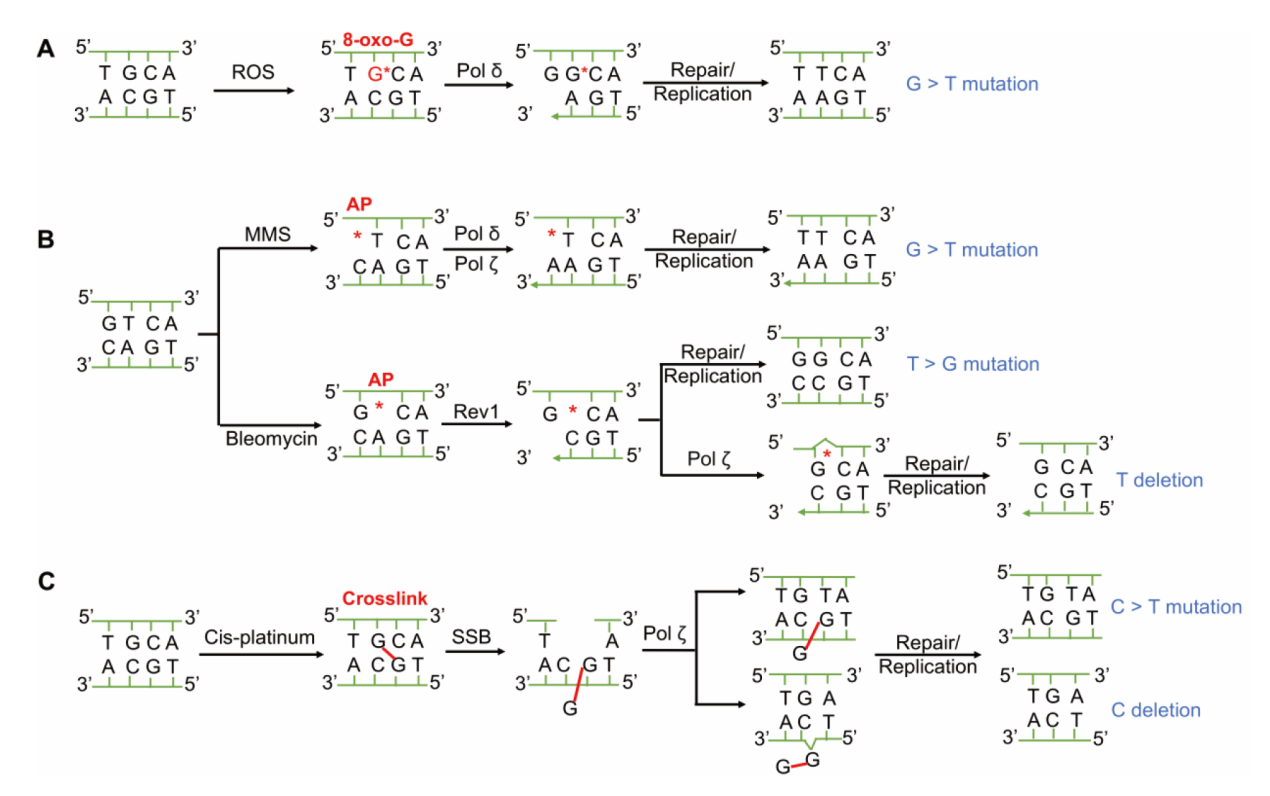
图4 不同化合物处理酵母细胞诱导的特征性变异及遗传机制 A:溴酸钾诱导细胞产生ROS将碱基G氧化成8-oxo-G,其偏好与A配对并引起G > T的特征性突变;B:MMS和博来霉素处理细胞诱导产生无碱基AP位点。DNA聚合酶δ和Rev1具有识别AP位点的能力并分别偏好掺入A和C,造成G > T和T > G两类特征性变异。Rev1插入的C在DNA聚合酶ζ作用与AP位点邻近的G配对,这种模板滑步现象会引起碱基T的缺失;C:顺铂处理诱导C > T或C缺失的特征性突变
Fig. 4 Characteristic mutations and genetic mechanisms of yeast cells induced by different compounds A: Potassium bromate induces cells to produce ROS, which oxidizes base G to 8-oxo-G that prefers to pair with A, causing G > T. B: MMS and bleomycin treatment induces the generation of AP sites without base. DNA polymerase δ and Rev1 can recognize AP sites and prefer to insert A and C, respectively, causing G > T and T > G. The C inserted by Rev1 pairs with the G adjacent to the AP site when DNA polymerase ζ acts, this template slippage will cause one base T deletion. C: Cisplatin treatment induces C > T substitution or C deletion
| 表型效应 Phenotypic effect | 变异类型 Variation type | 遗传机理 Genetic mechanism | 参考文献 References |
|---|---|---|---|
| H2O2耐受性提高 | Ⅳ号染色体扩增 | 编码硫氧还蛋白过氧化物酶的TSP2基因拷贝数上升 | [ |
| VII号染色体上572-734 kb区域扩增 | 编码过氧化氢酶T的 CTT1基因拷贝数上升 | [ | |
| 热胁迫耐受性提高 | III号染色体扩增 | 该染色体上至少14个与耐热相关基因表达量上调 | [ |
| 博来霉素耐受性提高 | SKY1突变, XIII号染色体右臂缺失 | SKY1基因编码蛋白激酶,其缺失可抑制膜转运蛋白活性,减少药物进入细胞 | [ |
| 乙醇耐受性提高 | III号染色体扩增 | 未知 | [ |
| 对亚硫酸盐环境耐受性提高 | VIII和XVI号染色体易位,XV和XVI号染色体易位 | 染色体易位引起SSU1基因表达量提高,SSU1基因编码可泵出亚硫酸盐的膜蛋白 | [ |
| 氮限制环境耐受性提高 | 含有GAP1基因区域形成eccDNA | GAP1基因编码氨基酸透性酶 | [ |
| 尿囊素利用能力提高 | DAL4基因所在区域扩增 | DAL4基因编码尿囊素通透酶 | [ |
| 尿素利用能力提高 | DUR3基因所在区域扩增 | DUR3基因编码尿素通透酶 | [ |
| 冻融耐受性增强 | AQY2基因所在区域扩增 | AQY2基因介导水分子跨膜运输通道 | [ |
| 氟康唑耐受性增强 | ERG11基因所在区域扩增 | ERG11基因编码羊毛甾醇14α-去甲基化酶,可与氟康唑结合发挥耐药性作用 | [ |
| 雷帕霉素耐受性提高 | XII号染色体扩增 | 维持核糖体DNA拷贝数 | [ |
| NaCl耐受性提高 | Gcn20基因所在区域缺失 | 未知 | [ |
| 5-氟胞嘧啶耐受性提高 | FCY2基因所在区域扩增缺失 | FCY2基因编码胞嘧啶通透酶 | [ |
| 葡萄糖限制环境耐受性提高 | IV号染色体扩增 | 己糖转运蛋白编码基因HXT6和HXT7扩增 | [ |
表1 酵母基因组变异驱动适应性进化的研究
Table 1 Examples of evolutionary adaptation induced by genomic alternations in yeast
| 表型效应 Phenotypic effect | 变异类型 Variation type | 遗传机理 Genetic mechanism | 参考文献 References |
|---|---|---|---|
| H2O2耐受性提高 | Ⅳ号染色体扩增 | 编码硫氧还蛋白过氧化物酶的TSP2基因拷贝数上升 | [ |
| VII号染色体上572-734 kb区域扩增 | 编码过氧化氢酶T的 CTT1基因拷贝数上升 | [ | |
| 热胁迫耐受性提高 | III号染色体扩增 | 该染色体上至少14个与耐热相关基因表达量上调 | [ |
| 博来霉素耐受性提高 | SKY1突变, XIII号染色体右臂缺失 | SKY1基因编码蛋白激酶,其缺失可抑制膜转运蛋白活性,减少药物进入细胞 | [ |
| 乙醇耐受性提高 | III号染色体扩增 | 未知 | [ |
| 对亚硫酸盐环境耐受性提高 | VIII和XVI号染色体易位,XV和XVI号染色体易位 | 染色体易位引起SSU1基因表达量提高,SSU1基因编码可泵出亚硫酸盐的膜蛋白 | [ |
| 氮限制环境耐受性提高 | 含有GAP1基因区域形成eccDNA | GAP1基因编码氨基酸透性酶 | [ |
| 尿囊素利用能力提高 | DAL4基因所在区域扩增 | DAL4基因编码尿囊素通透酶 | [ |
| 尿素利用能力提高 | DUR3基因所在区域扩增 | DUR3基因编码尿素通透酶 | [ |
| 冻融耐受性增强 | AQY2基因所在区域扩增 | AQY2基因介导水分子跨膜运输通道 | [ |
| 氟康唑耐受性增强 | ERG11基因所在区域扩增 | ERG11基因编码羊毛甾醇14α-去甲基化酶,可与氟康唑结合发挥耐药性作用 | [ |
| 雷帕霉素耐受性提高 | XII号染色体扩增 | 维持核糖体DNA拷贝数 | [ |
| NaCl耐受性提高 | Gcn20基因所在区域缺失 | 未知 | [ |
| 5-氟胞嘧啶耐受性提高 | FCY2基因所在区域扩增缺失 | FCY2基因编码胞嘧啶通透酶 | [ |
| 葡萄糖限制环境耐受性提高 | IV号染色体扩增 | 己糖转运蛋白编码基因HXT6和HXT7扩增 | [ |
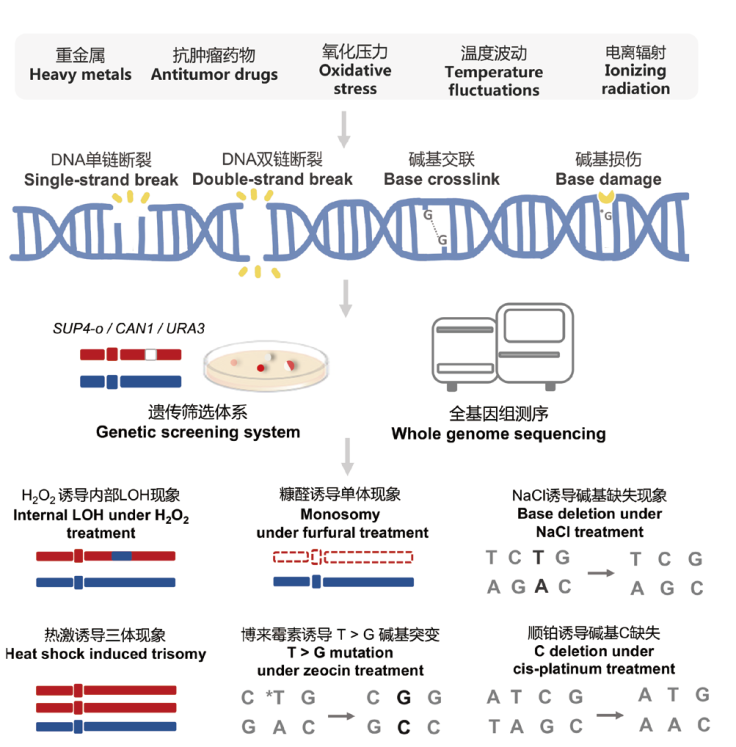
图5 不同环境胁迫因子诱导基因组产生差异化的特征性变异模式 多种胞外胁迫因子均会引起DNA损伤,包括DNA断裂、碱基损伤和交联等,进而诱导不同类型的变异形式。遗传筛选体系和全基因组测序是运用酵母模型检测环境因子诱导基因组变异的重要技术方法
Fig. 5 Different environmental stressors result in differentiated and characteristic variations in genomes Various extracellular stressors can cause DNA damage, including DNA breaks, base damage, and cross-linking, thereby inducing different types of variations. Genetic screening systems and whole-genome sequencing are crucial technical methods for detecting environmental factors induced genomic alterations in yeast
| [1] |
Jensen RB, Rothenberg E. Preserving genome integrity in human cells via DNA double-strand break repair[J]. Mol Biol Cell, 2020, 31(9): 859-865.
doi: 10.1091/mbc.E18-10-0668 pmid: 32286930 |
| [2] |
Behringer MG, Hall DW. The repeatability of genome-wide mutation rate and spectrum estimates[J]. Curr Genet, 2016, 62(3): 507-512.
doi: 10.1007/s00294-016-0573-7 pmid: 26919990 |
| [3] |
Waterman DP, Haber JE, Smolka MB. Checkpoint responses to DNA double-strand breaks[J]. Annu Rev Biochem, 2020, 89: 103-133.
doi: 10.1146/annurev-biochem-011520-104722 pmid: 32176524 |
| [4] | Huang RX, Zhou PK. DNA damage repair: historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy[J]. Signal Transduct Target Ther, 2021, 6(1): 254. |
| [5] |
Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection[J]. Nat Rev Immunol, 2017, 17(3): 151-164.
doi: 10.1038/nri.2016.147 pmid: 28138137 |
| [6] |
Venkataram S, Dunn B, Li YP, et al. Development of a comprehensive genotype-to-fitness map of adaptation-driving mutations in yeast[J]. Cell, 2016, 166(6): 1585-1596.e22.
doi: S0092-8674(16)31010-8 pmid: 27594428 |
| [7] |
Kciuk M, Marciniak B, Mojzych M, et al. Focus on UV-induced DNA damage and repair-disease relevance and protective strategies[J]. Int J Mol Sci, 2020, 21(19): 7264.
doi: 10.3390/ijms21197264 URL |
| [8] |
Martin H, Shales M, Fernandez-Piñar P, et al. Differential genetic interactions of yeast stress response MAPK pathways[J]. Mol Syst Biol, 2015, 11(4): 800.
doi: 10.15252/msb.20145606 pmid: 25888283 |
| [9] |
Coelho M C, Pinto R M, Murray A W. Heterozygous mutations cause genetic instability in a yeast model of cancer evolution[J]. Nature, 2019, 566(7743): 275-278.
doi: 10.1038/s41586-019-0887-y |
| [10] |
Beaupere C, Dinatto L, Wasko BM, et al. Genetic screen identifies adaptive aneuploidy as a key mediator of ER stress resistance in yeast[J]. Proc Natl Acad Sci USA, 2018, 115(38): 9586-9591.
doi: 10.1073/pnas.1804264115 pmid: 30185560 |
| [11] |
Puddu F, Herzog M, Selivanova A, et al. Genome architecture and stability in the Saccharomyces cerevisiae knockout collection[J]. Nature, 2019, 573(7774): 416-420.
doi: 10.1038/s41586-019-1549-9 |
| [12] |
Ko N, Nishihama R, Pringle JR. Control of 5-FOA and 5-FU resistance by Saccharomyces cerevisiae YJL055W[J]. Yeast, 2008, 25(2): 155-160.
doi: 10.1002/yea.v25:2 URL |
| [13] |
Ahmad M, Bussey H. Yeast arginine permease: nucleotide sequence of the CAN1 gene[J]. Curr Genet, 1986, 10(8): 587-592.
pmid: 3327612 |
| [14] |
Ekwall K, Ruusala T. Budding yeast CAN1 gene as a selection marker in fission yeast[J]. Nucleic Acids Res, 1991, 19(5): 1150.
pmid: 2020549 |
| [15] |
Lang GI, Murray AW. Estimating the per-base-pair mutation rate in the yeast Saccharomyces cerevisiae[J]. Genetics, 2008, 178(1): 67-82.
doi: 10.1534/genetics.107.071506 URL |
| [16] |
Zheng DQ, Wang YT, Zhu YX, et al. Uncovering bleomycin-induced genomic alterations and underlying mechanisms in the yeast Saccharomyces cerevisiae[J]. Appl Environ Microbiol, 2022, 88(2): e0170321.
doi: 10.1128/AEM.01703-21 URL |
| [17] | Kiktev DA, Sheng ZW, Lobachev KS, et al. GC content elevates mutation and recombination rates in the yeast Saccharomyces cerevisiae[J]. Proc Natl Acad Sci USA, 2018, 115(30): E7109-E7118. |
| [18] |
Lippert MJ, Kim N, Cho JE, et al. Role for topoisomerase 1 in transcription-associated mutagenesis in yeast[J]. Proc Natl Acad Sci USA, 2011, 108(2): 698-703.
doi: 10.1073/pnas.1012363108 pmid: 21177427 |
| [19] | Putnam CD, Kolodner RD. Determination of gross chromosomal rearrangement rates[J]. Cold Spring Harb Protoc, 2010, 2010(9): pdb.prot5492. |
| [20] |
Shibata M, Keyamura K, Shioiri T, et al. Diploid-associated adaptation to chronic low-dose UV irradiation requires homologous recombination in Saccharomyces cerevisiae[J]. Genetics, 2022, 222(1): iyac115.
doi: 10.1093/genetics/iyac115 URL |
| [21] |
St Charles J, Petes TD. High-resolution mapping of spontaneous mitotic recombination hotspots on the 1.1 Mb arm of yeast chromosome IV[J]. PLoS Genet, 2013, 9(4): e1003434.
doi: 10.1371/journal.pgen.1003434 URL |
| [22] |
Yin Y, Petes TD. Genome-wide high-resolution mapping of UV-induced mitotic recombination events in Saccharomyces cerevisiae[J]. PLoS Genet, 2013, 9(10): e1003894.
doi: 10.1371/journal.pgen.1003894 URL |
| [23] |
Yin Y, Dominska M, Yim E, et al. High-resolution mapping of heteroduplex DNA formed during UV-induced and spontaneous mitotic recombination events in yeast[J]. eLife, 2017, 6: e28069.
doi: 10.7554/eLife.28069 URL |
| [24] |
Zhang K, Zheng DQ, Sui Y, et al. Genome-wide analysis of genomic alterations induced by oxidative DNA damage in yeast[J]. Nucleic Acids Res, 2019, 47(7): 3521-3535.
doi: 10.1093/nar/gkz027 pmid: 30668788 |
| [25] |
Sheng H, Qi L, Sui Y, et al. Mapping chromosomal instability induced by small-molecular therapeutics in a yeast model[J]. Appl Microbiol Biotechnol, 2019, 103(12): 4869-4880.
doi: 10.1007/s00253-019-09845-5 pmid: 31053912 |
| [26] | Qi L, Zhang K, Wang YT, et al. Global analysis of furfural-induced genomic instability using a yeast model[J]. Appl Environ Microbiol, 2019, 85(18): e01237-e01219. |
| [27] |
Qi L, Zhu YX, Wang YK, et al. Nonlethal furfural exposure causes genomic alterations and adaptability evolution in Saccharomyces cerevisiae[J]. Microbiol Spectr, 2023, 11(4): e0121623.
doi: 10.1128/spectrum.01216-23 URL |
| [28] |
Shen L, Wang YT, Tang XX, et al. Heat shock drives genomic instability and phenotypic variations in yeast[J]. AMB Express, 2020, 10(1): 146.
doi: 10.1186/s13568-020-01091-7 pmid: 32804300 |
| [29] |
St Charles J, Hazkani-Covo E, Yin Y, et al. High-resolution genome-wide analysis of irradiated(UV and γ-rays)diploid yeast cells reveals a high frequency of genomic loss of heterozygosity(LOH)events[J]. Genetics, 2012, 190(4): 1267-1284.
doi: 10.1534/genetics.111.137927 pmid: 22267500 |
| [30] |
Lynch M, Sung W, Morris K, et al. A genome-wide view of the spectrum of spontaneous mutations in yeast[J]. Proc Natl Acad Sci U S A, 2008, 105(27): 9272-9277.
doi: 10.1073/pnas.0803466105 URL |
| [31] |
Peter J, De Chiara M, Friedrich A, et al. Genome evolution across 1, 011 Saccharomyces cerevisiae isolates[J]. Nature, 2018, 556(7701): 339-344.
doi: 10.1038/s41586-018-0030-5 |
| [32] | Song W, Dominska M, Greenwell PW, et al. Genome-wide high-resolution mapping of chromosome fragile sites in Saccharomyces cerevisiae[J]. Proc Natl Acad Sci USA, 2014, 111(21): E2210-E2218. |
| [33] | Zhu YO, Siegal ML, Hall DW, et al. Precise estimates of mutation rate and spectrum in yeast[J]. Proc Natl Acad Sci USA, 2014, 111(22): E2310-E2318. |
| [34] |
Sui Y, Qi L, Wu JK, et al. Genome-wide mapping of spontaneous genetic alterations in diploid yeast cells[J]. Proc Natl Acad Sci USA, 2020, 117(45): 28191-28200.
doi: 10.1073/pnas.2018633117 pmid: 33106417 |
| [35] |
Rhoads A, Au KF. PacBio sequencing and its applications[J]. Genomics Proteomics Bioinformatics, 2015, 13(5): 278-289.
doi: 10.1016/j.gpb.2015.08.002 URL |
| [36] |
Hu TS, Chitnis N, Monos D, et al. Next-generation sequencing technologies: an overview[J]. Hum Immunol, 2021, 82(11): 801-811.
doi: 10.1016/j.humimm.2021.02.012 pmid: 33745759 |
| [37] |
O'Donnell S, Yue JX, Saada OA, et al. Telomere-to-telomere assemblies of 142 strains characterize the genome structural landscape in Saccharomyces cerevisiae[J]. Nat Genet, 2023, 55(8): 1390-1399.
doi: 10.1038/s41588-023-01459-y |
| [38] |
Qi L, Sui Y, Tang XX, et al. Shuffling the yeast genome using CRISPR/Cas9-generated DSBs that target the transposable Ty1 elements[J]. PLoS Genet, 2023, 19(1): e1010590.
doi: 10.1371/journal.pgen.1010590 URL |
| [39] |
Ogur M, Ogur S, St John R. Temperature dependence of the spontaneous mutation rate to respiration deficiency in Saccharomyces[J]. Genetics, 1960, 45(2): 189-194.
doi: 10.1093/genetics/45.2.189 pmid: 17247917 |
| [40] | Zhang K, Wu XC, Zheng DQ, et al. Effects of temperature on the meiotic recombination landscape of the yeast Saccharomyces cerevisiae[J]. mBio, 2017, 8(6): e02099-e02017. |
| [41] | Huang CJ, Lu MY, Chang YW, et al. Experimental evolution of yeast for high-temperature tolerance[J]. Mol Biol Evol, 2018, 35(8): 1823-1839. |
| [42] |
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing[J]. Nature, 2000, 408(6809): 239-247.
doi: 10.1038/35041687 |
| [43] |
Hayashi M, Umezu K. Homologous recombination is required for recovery from oxidative DNA damage[J]. Genes Genet Syst, 2017, 92(2): 73-80.
doi: 10.1266/ggs.16-00066 pmid: 28381656 |
| [44] |
Poetsch AR. The genomics of oxidative DNA damage, repair, and resulting mutagenesis[J]. Comput Struct Biotechnol J, 2020, 18: 207-219.
doi: 10.1016/j.csbj.2019.12.013 URL |
| [45] |
Ikner A, Shiozaki K. Yeast signaling pathways in the oxidative stress response[J]. Mutat Res, 2005, 569(1-2): 13-27.
doi: 10.1016/j.mrfmmm.2004.09.006 URL |
| [46] |
Degtyareva NP, Heyburn L, Sterling J, et al. Oxidative stress-induced mutagenesis in single-strand DNA occurs primarily at cytosines and is DNA polymerase zeta-dependent only for adenines and guanines[J]. Nucleic Acids Res, 2013, 41(19): 8995-9005.
doi: 10.1093/nar/gkt671 pmid: 23925127 |
| [47] |
Degtyareva NP, Placentra VC, Gabel SA, et al. Changes in metabolic landscapes shape divergent but distinct mutational signatures and cytotoxic consequences of redox stress[J]. Nucleic Acids Res, 2023, 51(10): 5056-5072.
doi: 10.1093/nar/gkad305 URL |
| [48] |
Choi JE, Heo SH, Kim MJ, et al. Lack of superoxide dismutase in a rad51 mutant exacerbates genomic instability and oxidative stress-mediated cytotoxicity in Saccharomyces cerevisiae[J]. Free Radic Biol Med, 2018, 129: 97-106.
doi: 10.1016/j.freeradbiomed.2018.09.015 URL |
| [49] | Bjornsti MA, Knab AM, Benedetti P. Yeast Saccharomyces cerevisiae as a model system to study the cytotoxic activity of the antitumor drug camptothecin[J]. Cancer Chemother Pharmacol, 1994, 34 Suppl: S1-S5. |
| [50] |
Puddu F, Salguero I, Herzog M, et al. Chromatin determinants impart camptothecin sensitivity[J]. EMBO Rep, 2017, 18(6): 1000-1012.
doi: 10.15252/embr.201643560 pmid: 28389464 |
| [51] |
Pommier Y, Sun YL, Huang SY N, et al. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability[J]. Nat Rev Mol Cell Biol, 2016, 17(11): 703-721.
doi: 10.1038/nrm.2016.111 |
| [52] |
Sloan R, Huang SY N, Pommier Y, et al. Effects of camptothecin or TOP1 overexpression on genetic stability in Saccharomyces cerevisiae[J]. DNA Repair, 2017, 59: 69-75.
doi: 10.1016/j.dnarep.2017.09.004 URL |
| [53] | Thomas AD, Johnson GE. DNA repair and its influence on points of departure for alkylating agent genotoxicity[M]// Thresholds of Genotoxic Carcinogens. Amsterdam: Elsevier, 2016: 67-82. |
| [54] |
Lundin C, North M, Erixon K, et al. Methyl methanesulfonate(MMS)produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks[J]. Nucleic Acids Res, 2005, 33(12): 3799-3811.
doi: 10.1093/nar/gki681 URL |
| [55] |
Haracska L, Unk I, Johnson RE, et al. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites[J]. Genes Dev, 2001, 15(8): 945-954.
doi: 10.1101/gad.882301 URL |
| [56] |
Dolling JA, Boreham DR, Brown DL, et al. Cisplatin-modification of DNA repair and ionizing radiation lethality in yeast, Saccharomyces cerevisiae[J]. Mutat Res, 1999, 433(2): 127-136.
doi: 10.1016/S0921-8777(98)00069-X URL |
| [57] |
Segovia R, Shen YQ, Lujan SA, et al. Hypermutation signature reveals a slippage and realignment model of translesion synthesis by Rev3 polymerase in cisplatin-treated yeast[J]. Proc Natl Acad Sci USA, 2017, 114(10): 2663-2668.
doi: 10.1073/pnas.1618555114 pmid: 28223526 |
| [58] |
Ward JF. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: a review[J]. Int J Radiat Biol, 1990, 57(6): 1141-1150.
doi: 10.1080/09553009014551251 pmid: 1971840 |
| [59] |
Brennan RJ, Schiestl RH. Persistent genomic instability in the yeast Saccharomyces cerevisiae induced by ionizing radiation and DNA-damaging agents[J]. Radiat Res, 2001, 155(6): 768-777.
pmid: 11352758 |
| [60] |
Argueso JL, Westmoreland J, Mieczkowski PA, et al. Double-strand breaks associated with repetitive DNA can reshape the genome[J]. Proc Natl Acad Sci USA, 2008, 105(33): 11845-11850.
doi: 10.1073/pnas.0804529105 pmid: 18701715 |
| [61] |
Franklin WA, Doetsch PW, Haseltine WA. Structural determination of the ultraviolet light-induced thymine-cytosine pyrimidine-pyrimidone(6-4)photoproduct[J]. Nucleic Acids Res, 1985, 13(14): 5317-5325.
pmid: 4022781 |
| [62] |
Bhavya G, Hiremath KY, Jogaiah S, et al. Heavy metal-induced oxidative stress and alteration in secretory proteins in yeast isolates[J]. Arch Microbiol, 2022, 204(3): 172.
doi: 10.1007/s00203-022-02756-6 pmid: 35165751 |
| [63] |
Diaconu M, Pavel LV, Hlihor RM, et al. Characterization of heavy metal toxicity in some plants and microorganisms-a preliminary approach for environmental bioremediation[J]. N Biotechnol, 2020, 56: 130-139.
doi: 10.1016/j.nbt.2020.01.003 URL |
| [64] |
Yu SS, Qin W, Zhuang GQ, et al. Monitoring oxidative stress and DNA damage induced by heavy metals in yeast expressing a redox-sensitive green fluorescent protein[J]. Curr Microbiol, 2009, 58(5): 504-510.
doi: 10.1007/s00284-008-9354-y pmid: 19184609 |
| [65] |
Wu K, Li HC, Wang YH, et al. Silver nanoparticles elevate mutagenesis of eukaryotic genomes[J]. G3, 2023, 13(3): jkad008.
doi: 10.1093/g3journal/jkad008 URL |
| [66] |
Liu HX, Zhang JZ. Yeast spontaneous mutation rate and spectrum vary with environment[J]. Curr Biol, 2019, 29(10): 1584-1591.e3.
doi: S0960-9822(19)30382-3 pmid: 31056389 |
| [67] |
Voordeckers K, Colding C, Grasso L, et al. Ethanol exposure increases mutation rate through error-prone polymerases[J]. Nat Commun, 2020, 11(1): 3664.
doi: 10.1038/s41467-020-17447-3 pmid: 32694532 |
| [68] |
Yona AH, Frumkin I, Pilpel Y. A relay race on the evolutionary adaptation spectrum[J]. Cell, 2015, 163(3): 549-559.
doi: 10.1016/j.cell.2015.10.005 pmid: 26496602 |
| [69] |
Zhang K, Fang YH, Gao KH, et al. Effects of genome duplication on phenotypes and industrial applications of Saccharomyces cerevisiae strains[J]. Appl Microbiol Biotechnol, 2017, 101(13): 5405-5414.
doi: 10.1007/s00253-017-8284-7 pmid: 28429058 |
| [70] |
Linder RA, Greco JP, Seidl F, et al. The stress-inducible peroxidase TSA2 underlies a conditionally beneficial chromosomal duplication in Saccharomyces cerevisiae[J]. G3, 2017, 7(9): 3177-3184.
doi: 10.1534/g3.117.300069 URL |
| [71] | Li J, Stenberg S, Yue JX, et al. Genome instability footprint under rapamycin and hydroxyurea treatments[J]. bioRxiv, 2023. DOI: 10.1101/2023.02.28.530484. |
| [72] |
Noer JB, Hørsdal OK, Xiang X, et al. Extrachromosomal circular DNA in cancer: history, current knowledge, and methods[J]. Trends Genet, 2022, 38(7): 766-781.
doi: 10.1016/j.tig.2022.02.007 pmid: 35277298 |
| [73] |
Gresham D, Usaite R, Germann SM, et al. Adaptation to diverse nitrogen-limited environments by deletion or extrachromosomal element formation of the GAP1 locus[J]. Proc Natl Acad Sci USA, 2010, 107(43): 18551-18556.
doi: 10.1073/pnas.1014023107 pmid: 20937885 |
| [74] |
Kang JP, Li JY, Guo Z, et al. Enhancement and mapping of tolerance to salt stress and 5-fluorocytosine in synthetic yeast strains via SCRaMbLE[J]. Synth Syst Biotechnol, 2022, 7(3): 869-877.
doi: 10.1016/j.synbio.2022.04.003 pmid: 35601823 |
| [75] |
Yona AH, Manor YS, Herbst RH, et al. Chromosomal duplication is a transient evolutionary solution to stress[J]. Proc Natl Acad Sci USA, 2012, 109(51): 21010-21015.
doi: 10.1073/pnas.1211150109 pmid: 23197825 |
| [76] |
Morard M, Macías LG, Adam AC, et al. Aneuploidy and ethanol tolerance in Saccharomyces cerevisiae[J]. Front Genet, 2019, 10: 82.
doi: 10.3389/fgene.2019.00082 URL |
| [77] |
García-Ríos E, Nuévalos M, Barrio E, et al. A new chromosomal rearrangement improves the adaptation of wine yeasts to sulfite[J]. Environ Microbiol, 2019, 21(5): 1771-1781.
doi: 10.1111/1462-2920.14586 pmid: 30859719 |
| [78] |
Hong J, Gresham D. Molecular specificity, convergence and constraint shape adaptive evolution in nutrient-poor environments[J]. PLoS Genet, 2014, 10(1): e1004041.
doi: 10.1371/journal.pgen.1004041 URL |
| [79] |
Hose J, Yong CM, Sardi M, et al. Dosage compensation can buffer copy-number variation in wild yeast[J]. eLife, 2015, 4: e05462.
doi: 10.7554/eLife.05462 URL |
| [80] |
Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans[J]. Science, 2006, 313(5785): 367-370.
pmid: 16857942 |
| [81] |
Dunham MJ, Badrane H, Ferea T, et al. Characteristic genome rearrangements in experimental evolution of Saccharomyces cerevisiae[J]. Proc Natl Acad Sci USA, 2002, 99(25): 16144-16149.
doi: 10.1073/pnas.242624799 URL |
| [1] | 徐发迪, 徐康, 孙东明, 李萌蕾, 赵建志, 鲍晓明. 基于杨木(Populus sp.)的二代燃料乙醇技术研究进展[J]. 生物技术通报, 2023, 39(9): 27-39. |
| [2] | 成婷, 苑帅, 张晓元, 林良才, 李欣, 张翠英. 酿酒酵母异丁醇合成途径调控的研究进展[J]. 生物技术通报, 2023, 39(7): 80-90. |
| [3] | 王晨宇, 周楚源, 何堤, 樊梓豪, 王梦梦, 杨柳燕. 多聚磷酸盐在微生物抗环境胁迫中的作用及机制[J]. 生物技术通报, 2023, 39(11): 168-181. |
| [4] | 孙言秋, 谢采芸, 汤岳琴. 耐高温酿酒酵母的构建与高温耐受机制解析[J]. 生物技术通报, 2023, 39(11): 226-237. |
| [5] | 王文韬, 冯颀, 刘晨光, 白凤武, 赵心清. 氧化还原敏感型基因元件增强酵母木质纤维素水解液抑制物胁迫耐受性[J]. 生物技术通报, 2023, 39(11): 360-372. |
| [6] | 崔新刚, 孙雅欣, 崔晓静, 邓雁文, 孙恩浩, 王俊芳, 崔红晶. 酿酒酵母TAP42基因在细胞壁应激反应中的作用[J]. 生物技术通报, 2021, 37(10): 57-62. |
| [7] | 顾翰琦, 邵玲智, 刘冉, 刘晓光, 李玲, 刘倩, 李洁, 张雅丽. 酿酒酵母酚类抑制物耐受性脂质组学研究[J]. 生物技术通报, 2021, 37(1): 15-23. |
| [8] | 吴宇, 王金华, 赵筱. GLN1基因过表达对提高酿酒酵母糠醛耐受性的研究[J]. 生物技术通报, 2020, 36(8): 69-78. |
| [9] | 顾翰琦, 刘冉, 邵玲智, 徐岩岩, 王东岩, 张冬梅, 李洁. 酿酒酵母对酚类抑制物耐受性研究[J]. 生物技术通报, 2020, 36(6): 136-142. |
| [10] | 陈永灿, 张建志, 司同. 酿酒酵母中基于CRISPR/dCás9的基因转录调控工具的开发与应用[J]. 生物技术通报, 2020, 36(4): 1-12. |
| [11] | 李佳秀, 蔡倩茹, 吴杰群. 萜类化合物在酿酒酵母中的合成生物学研究进展[J]. 生物技术通报, 2020, 36(12): 199-207. |
| [12] | 曹文妍, 王心凝, 沈煜, 李在禄, 鲍晓明. 酿酒酵母关联多药耐受性转录因子Yrr1p的研究进展[J]. 生物技术通报, 2020, 36(11): 148-154. |
| [13] | 陈和锋, 朱晁谊, 李爽. 产瓦伦西亚烯酿酒酵母的表达载体适配及发酵碳氮源优化[J]. 生物技术通报, 2020, 36(1): 209-219. |
| [14] | 黄贞杰, 陈由强, 薛婷. 过表达INO1基因提高酿酒酵母乙醇耐受性[J]. 生物技术通报, 2019, 35(3): 87-92. |
| [15] | 陈雪莲, 江高飞, 钟增涛. 基因水平转移在根瘤菌进化中的研究进展[J]. 生物技术通报, 2019, 35(10): 18-24. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||