








生物技术通报 ›› 2024, Vol. 40 ›› Issue (1): 45-56.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0596
收稿日期:2023-06-25
出版日期:2024-01-26
发布日期:2024-02-06
通讯作者:
韩楠玉,女,博士,副教授,研究方向:蛋白质工程;E-mail: nyhan@ynnu.edu.cn作者简介:向霞,女,硕士,研究方向:微生物分子生物学;E-mail: xiangxia202206@163.com
基金资助:
XIANG Xia1( ), ZHU En-heng2, HAN Nan-yu1,2(
), ZHU En-heng2, HAN Nan-yu1,2( )
)
Received:2023-06-25
Published:2024-01-26
Online:2024-02-06
摘要:
饲料生产贮藏时常被真菌毒素污染,主要包括黄曲霉毒素、玉米赤霉烯酮、呕吐毒素、伏马毒素B1、赭曲霉毒素A和T-2毒素,真菌毒素会对畜禽造成严重的身体伤害甚至死亡,而真菌毒素的共存将导致更大程度的经济损失。真菌毒素的降解主要包括化学降解法、物理降解法和生物酶解法,而生物酶解法相比于另外两种方法更为环保、高效,因此备受关注。本文对毒性强、污染广的黄曲霉毒素、玉米赤霉烯酮和呕吐毒素的危害机制、降解途径以及相关的真菌毒素降解酶进行详细分析及探讨。利用分子对接等手段揭示了毒素小分子在降解反应中与真菌毒素降解酶的相互作用,并筛选出了降解过程中的关键氨基酸。虽然酶解法在去除真菌毒素方面具有优势,但是由于成本高等原因目前应用仍然受限,急需进一步研究和开发。因此,优化酶解法的工艺和条件,以实现高效、经济地去除真菌毒素将成为未来研究重点。本研究为指导真菌毒素降解酶的设计和优化提供了重要参考。
向霞, 朱恩恒, 韩楠玉. 三种主要真菌毒素及其毒素降解酶的研究进展[J]. 生物技术通报, 2024, 40(1): 45-56.
XIANG Xia, ZHU En-heng, HAN Nan-yu. Research Progress in Three Major Mycotoxins and Their Toxin-degrading Enzymes[J]. Biotechnology Bulletin, 2024, 40(1): 45-56.
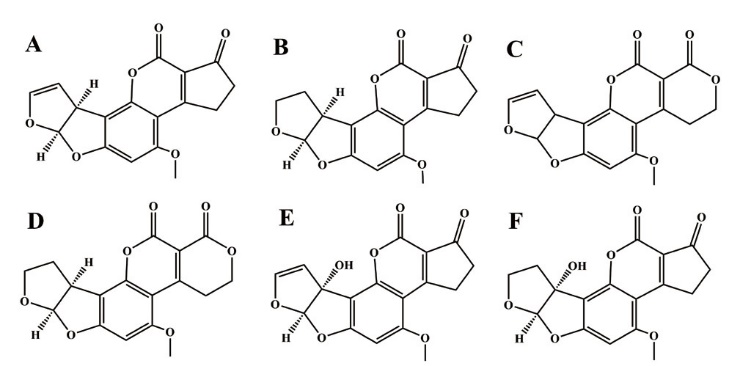
图1 AFs毒素衍生物 A:黄曲霉毒素B1;B:黄曲霉毒素B2;C:黄曲霉毒素M1;D:黄曲霉毒素M2;E:黄曲霉毒素G1;F:黄曲霉毒素G2
Fig. 1 AFs toxin derivatives A: aflatoxin B1; B: aflatoxin B2; C: aflatoxin M1; D: aflatoxin M2; E: aflatoxin G1; F: aflatoxin G2
| Mode | Affinity/(kcal·mol-1) | Mode | Affinity/(kcal·mol-1) | |
|---|---|---|---|---|
| 1 | -8.7 | 6 | -6.7 | |
| 2 | -8.5 | 7 | -6.6 | |
| 3 | -7.5 | 8 | -6.6 | |
| 4 | -7.1 | 9 | -6.3 | |
| 5 | -7.1 | 10 | -6.2 |
表1 黄曲霉毒素氧化酶与小分子对接最优模型结果
Table 1 Results of optimal model of aflatoxin oxidase docking with small molecules
| Mode | Affinity/(kcal·mol-1) | Mode | Affinity/(kcal·mol-1) | |
|---|---|---|---|---|
| 1 | -8.7 | 6 | -6.7 | |
| 2 | -8.5 | 7 | -6.6 | |
| 3 | -7.5 | 8 | -6.6 | |
| 4 | -7.1 | 9 | -6.3 | |
| 5 | -7.1 | 10 | -6.2 |
| Mode | Affinity/(kcal·mol-1) | Mode | Affinity/(kcal·mol-1) | |
|---|---|---|---|---|
| 1 | -8.5 | 6 | -7.3 | |
| 2 | -7.9 | 7 | -7.0 | |
| 3 | -7.4 | 8 | -6.7 | |
| 4 | -7.4 | 9 | -6.6 | |
| 5 | -7.3 | 10 | -6.5 |
表2 玉米赤酶烯酮降解酶与ZEN对接最优模型结果
Table 2 Optimal model results for maize erythrene ketone degradation enzyme docking with ZEN
| Mode | Affinity/(kcal·mol-1) | Mode | Affinity/(kcal·mol-1) | |
|---|---|---|---|---|
| 1 | -8.5 | 6 | -7.3 | |
| 2 | -7.9 | 7 | -7.0 | |
| 3 | -7.4 | 8 | -6.7 | |
| 4 | -7.4 | 9 | -6.6 | |
| 5 | -7.3 | 10 | -6.5 |

图8 内酯水解酶zhd101与ZEN形成的相互作用,参与底物结合的氨基酸显示为棒状
Fig. 8 Interaction formed by lactone hydrolase zhd101 and ZEN, and the amino acids involved in substrate binding are shown as rods
| Mode | Affinity/(kcal·mol-1) | Mode | Affinity/(kcal·mol-1) | |
|---|---|---|---|---|
| 1 | -7.6 | 6 | -6.3 | |
| 2 | -7.5 | 7 | -6.2 | |
| 3 | -7.1 | 8 | -6.2 | |
| 4 | -7.0 | 9 | -6.1 | |
| 5 | -6.9 | 10 | -5.9 |
表3 特异乙醛化酶与DON对接最优模型结果
Table 3 Results of the optimal model for the docking of specific glyoxylase with DON
| Mode | Affinity/(kcal·mol-1) | Mode | Affinity/(kcal·mol-1) | |
|---|---|---|---|---|
| 1 | -7.6 | 6 | -6.3 | |
| 2 | -7.5 | 7 | -6.2 | |
| 3 | -7.1 | 8 | -6.2 | |
| 4 | -7.0 | 9 | -6.1 | |
| 5 | -6.9 | 10 | -5.9 |
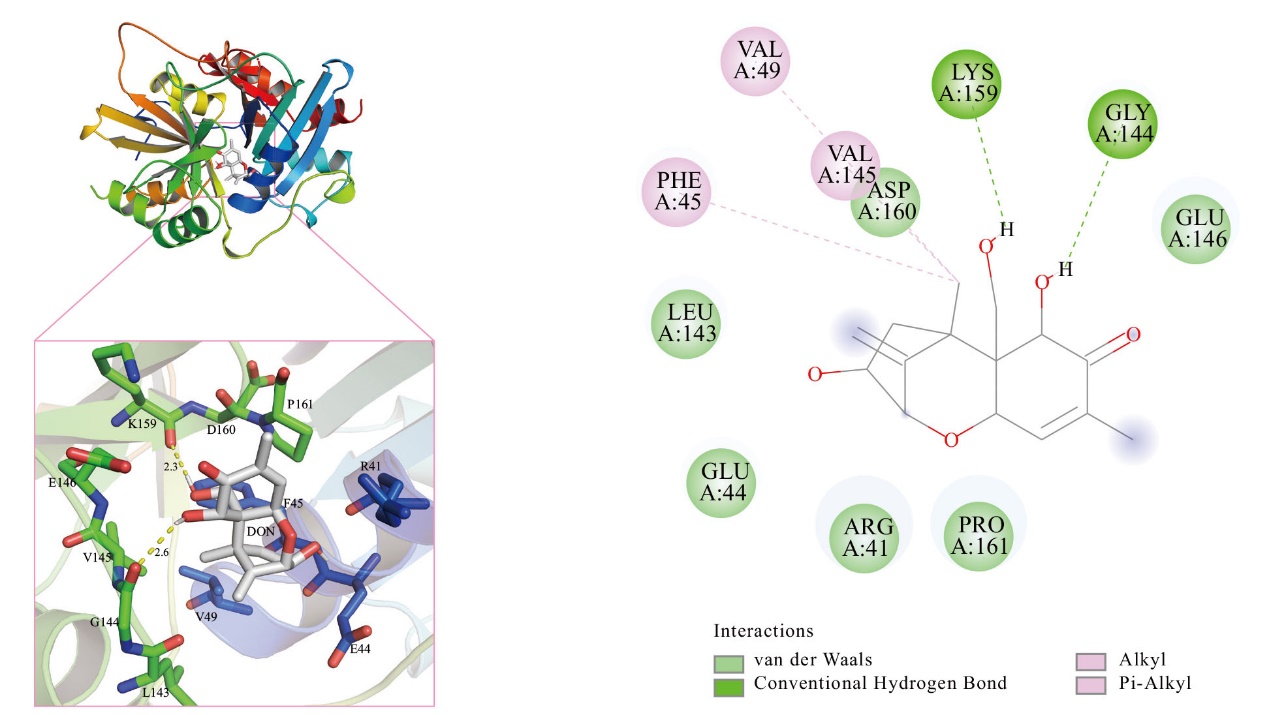
图12 特异乙醛化酶SPG与DON形成的相互作用,参与底物结合的氨基酸显示为棒状
Fig. 12 Interaction of specific glyoxylase SPG with DON formation, amino acids involved in substrate binding shown as rods
| [1] |
Schoeman A, Flett BC, Janse van Rensburg B. Evaluating three commonly used growth media for assessing fumonisin analogues FB1,FB2 and FB3 production by nineFusarium verticillioidesisolates[J]. Food Addit Contam A, 2017, 34(2): 291-298.
doi: 10.1080/19440049.2016.1266397 pmid: 27899061 |
| [2] |
Haque MA, Wang YH, Shen ZQ, et al. Mycotoxin contamination and control strategy in human, domestic animal and poultry: a review[J]. Microb Pathog, 2020, 142: 104095.
doi: 10.1016/j.micpath.2020.104095 URL |
| [3] |
Paterson RR, Lima N. Toxicology of mycotoxins[J]. EXS, 2010, 100: 31-63.
pmid: 20358681 |
| [4] |
Magnussen A, Parsi MA. Aflatoxins, hepatocellular carcinoma and public health[J]. World J Gastroenterol, 2013, 19(10): 1508-1512.
doi: 10.3748/wjg.v19.i10.1508 URL |
| [5] |
Grenier B, Applegate TJ. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals[J]. Toxins, 2013, 5(2): 396-430.
doi: 10.3390/toxins5020396 pmid: 23430606 |
| [6] |
Guthrie WD, Lillehoj EB, McMillian WW, et al. Effect of hybrids with different levels of susceptibility to second-generation European corn borers on aflatoxin contamination in corn[J]. J Agric Food Chem, 1981, 29(6): 1170-1172.
doi: 10.1021/jf00108a018 URL |
| [7] |
Akbari P, Braber S, Varasteh S, et al. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins[J]. Arch Toxicol, 2017, 91(3): 1007-1029.
doi: 10.1007/s00204-016-1794-8 pmid: 27417439 |
| [8] |
Swamy HVLN, Smith TK, MacDonald EJ, et al. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on swine performance, brain regional neurochemistry, and serum chemistry and the efficacy of a polymeric glucomannan mycotoxin adsorbent1[J]. J Anim Sci, 2002, 80(12): 3257-3267.
pmid: 12542167 |
| [9] |
张欣昕, 张福金, 张尧, 等. 中国青贮玉米中霉菌毒素的污染情况分析与动物健康风险评估[J]. 中国畜牧兽医, 2021, 48(12): 4451-4459.
doi: 10.16431/j.cnki.1671-7236.2021.12.013 |
| Zhang XX, Zhang FJ, Zhang Y, et al. Analysis of mycotoxin contamination and animal health risk assessment in silage corn of China[J]. China Anim Husb Vet Med, 2021, 48(12): 4451-4459. | |
| [10] | GB 13078-2017饲料卫生标准[J]. 饲料与畜牧, 2018(1): 16-24. |
| GB 13078-2017 Hygienic standard for feed[J]. Anim Agric, 2018(1): 16-24. | |
| [11] |
Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals[J]. Toxicology, 2001, 167(2): 101-134.
doi: 10.1016/s0300-483x(01)00471-1 pmid: 11567776 |
| [12] |
Vila-Donat P, Marín S, Sanchis V, et al. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination[J]. Food Chem Toxicol, 2018, 114: 246-259.
doi: S0278-6915(18)30118-2 pmid: 29476792 |
| [13] | Szabó A, Szabó-Fodor J, Fébel H, et al. Individual and combined effects of fumonisin B1, deoxynivalenol and Zearalenone on the hepatic and renal membrane lipid integrity of rats[J]. Toxins: Basel, 2017, 10(1): 4. |
| [14] |
王会娟, 刘阳, 邢福国. 高产漆酶平菇的筛选及其在降解黄曲霉毒素B1中的应用[J]. 核农学报, 2012, 26(7): 1025-1030.
doi: 10.11869/hnxb.2012.07.1025 |
| Wang HJ, Liu Y, Xing FG. Screening high-production laccase of pleurotus ostreatus and degradation of afb1 by pleurotus ostreatus laccase[J]. J Nucl Agric Sci, 2012, 26(7): 1025-1030. | |
| [15] |
Zavala-Franco A, Arámbula-Villa G, Ramírez-Noguera P, et al. Aflatoxin detoxification in tortillas using an infrared radiation thermo-alkaline process: Cytotoxic and genotoxic evaluation[J]. Food Contr, 2020, 112: 107084.
doi: 10.1016/j.foodcont.2019.107084 URL |
| [16] |
Ezekiel CN, Sulyok M, Babalola DA, et al. Incidence and consumer awareness of toxigenic Aspergillus section Flavi and aflatoxin B1 in peanut cake from Nigeria[J]. Food Contr, 2013, 30(2): 596-601.
doi: 10.1016/j.foodcont.2012.07.048 URL |
| [17] | 黄玮玮, 洪军, 常娟, 等. 饲料中常见霉菌毒素的毒性作用及其脱毒方法的研究进展[J]. 饲料研究, 2021, 44(21): 145-149. |
| Huang WW, Hong J, Chang J, et al. Advance of toxic effect and detoxification methods of common mycotoxins in feed[J]. Feed Res, 2021, 44(21): 145-149. | |
| [18] |
Matumba L, Sulyok M, Njoroge SMC, et al. Uncommon occurrence ratios of aflatoxin B1, B2, G1, and G2 in maize and groundnuts from Malawi[J]. Mycotoxin Res, 2015, 31(1): 57-62.
doi: 10.1007/s12550-014-0209-z URL |
| [19] |
Dey DK, Kang SC. Aflatoxin B1 induces reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, inhibits Allium cepa root cell division, and triggers inflammatory response in zebrafish larvae[J]. Sci Total Environ, 2020, 737: 139704.
doi: 10.1016/j.scitotenv.2020.139704 URL |
| [20] |
Watanakij N, Visessanguan W, Petchkongkaew A. Aflatoxin B1-degrading activity from Bacillus subtilis BCC 42005 isolated from fermented cereal products[J]. Food Addit Contam A, 2020, 37(9): 1579-1589.
doi: 10.1080/19440049.2020.1778182 pmid: 32723015 |
| [21] |
Gallo A, Fancello F, Ghilardelli F, et al. Effects of several commercial or pure lactic acid bacteria inoculants on fermentation and mycotoxin levels in high-moisture corn silage[J]. Anim Feed Sci Technol, 2022, 286: 115256.
doi: 10.1016/j.anifeedsci.2022.115256 URL |
| [22] | 王卫国. 与饲料有关的健康危害分析[J]. 饲料工业, 2021, 42(17): 1-7. |
| Wang WG. Health hazards analysis associated with feed[J]. Feed Ind, 2021, 42(17): 1-7. | |
| [23] |
Shier WT, Shier AC, Xie W, et al. Structure-activity relationships for human estrogenic activity in Zearalenone mycotoxins[J]. Toxicon, 2001, 39(9): 1435-1438.
pmid: 11384734 |
| [24] | 于心蕊. 真菌毒素玉米赤霉烯酮降解酶基因资源挖掘及其催化效率分子改良研究[D]. 北京: 中国农业科学院, 2020. |
| Yu XR. Gene resource mining and improvement in catalytic efficiency of mycotoxin Zearalenone hydrolase[D]. Beijing: Chinese Academy of Agricultural Sciences, 2020. | |
| [25] |
Savard C, Gawhary S, Boyer A, et al. Assessment of Zearalenone-induced cell survival and of global gene regulation in mouse TM4 Sertoli cells[J]. Toxins, 2022, 14(2): 98.
doi: 10.3390/toxins14020098 URL |
| [26] |
Zhao LJ, Xiao YY, Li CM, et al. Zearalenone perturbs the circadian clock and inhibits testosterone synthesis in mouse Leydig cells[J]. J Toxicol Environ Health A, 2021, 84(3): 112-124.
doi: 10.1080/15287394.2020.1841699 URL |
| [27] |
Song TT, Yang WR, Huang LB, et al. Zearalenone exposure affects the Wnt/β-catenin signaling pathway and related genes of porcine endometrial epithelial cells in vitro[J]. Anim Biosci, 2021, 34(6): 993-1005.
doi: 10.5713/ajas.20.0292 URL |
| [28] |
Cheng L, Jiang T, Zhang JD. Photoelectrocatalytic degradation of deoxynivalenol on CuO-Cu2O/WO3 ternary film: mechanism and reaction pathways[J]. Sci Total Environ, 2021, 776: 145840.
doi: 10.1016/j.scitotenv.2021.145840 URL |
| [29] |
Kang RF, Li RN, Dai PY, et al. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production[J]. Environ Pollut, 2019, 251: 689-698.
doi: S0269-7491(19)30073-9 pmid: 31108302 |
| [30] |
Cao L, Jiang YJ, Zhu L, et al. Deoxynivalenol induces caspase-8-mediated apoptosis through the mitochondrial pathway in hippocampal nerve cells of piglet[J]. Toxins, 2021, 13(2): 73.
doi: 10.3390/toxins13020073 URL |
| [31] | 尹清强, 常娟, 王平, 等. 饲料中多种霉菌毒素的危害与生物防控[J]. 饲料工业, 2021, 42(21): 9-14. |
| Yin QQ, Chang J, Wang P, et al. Hazard and biological control of multi-mycotoxins in feed[J]. Feed Ind, 2021, 42(21): 9-14. | |
| [32] |
Sanner MF. Python: a programming language for software integration and development[J]. J Mol Graph Model, 1999, 17(1): 57-61.
pmid: 10660911 |
| [33] |
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading[J]. J Comput Chem, 2010, 31(2): 455-461.
doi: 10.1002/jcc.21334 pmid: 19499576 |
| [34] |
Delano WL. The PyMOL molecular graphics system[J]. Proteins Structure Function and Bioinformatics, 2002, 30:442-454.
doi: 10.1002/(ISSN)1097-0134 URL |
| [35] |
Volkamer A, Griewel A, Grombacher T, et al. Analyzing the topology of active sites: on the prediction of pockets and subpockets[J]. J Chem Inf Model, 2010, 50(11): 2041-2052.
doi: 10.1021/ci100241y pmid: 20945875 |
| [36] |
Sarrami S, Jafari P, Tajabadi Ebrahimi M, et al. Effects of various Toxeat concentrations on growth performance, immune response, cecal microflora, and gut morphology in broilers fed with aflatoxin contaminated diets[J]. Turk J Vet Anim Sci, 2019, 43(3): 299-305.
doi: 10.3906/vet-1811-50 URL |
| [37] |
Limaye A, Yu RC, Chou CC, et al. Protective and detoxifying effects conferred by dietary selenium and curcumin against AFB1-mediated toxicity in livestock: a review[J]. Toxins, 2018, 10(1): 25.
doi: 10.3390/toxins10010025 URL |
| [38] |
Solis-Cruz B, Hernandez-Patlan D, Petrone VM, et al. Evaluation of cellulosic polymers and curcumin to reduce aflatoxin B1 toxic effects on performance, biochemical, and immunological parameters of broiler chickens[J]. Toxins, 2019, 11(2): 121.
doi: 10.3390/toxins11020121 URL |
| [39] |
Liu YQ, Song CG, Ding G, et al. High-performance functional Fe-MOF for removing aflatoxin B1 and other organic pollutants[J]. Adv Materials Inter, 2022, 9(9): 2102480.
doi: 10.1002/admi.v9.9 URL |
| [40] |
Broekaert N, Devreese M, De Baere S, et al. Modified Fusarium mycotoxins unmasked: from occurrence in cereals to animal and human excretion[J]. Food Chem Toxicol, 2015, 80: 17-31.
doi: S0278-6915(15)00061-7 pmid: 25725190 |
| [41] |
Alexander NJ, McCormick SP, Waalwijk C, et al. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium[J]. Fungal Genet Biol, 2011, 48(5): 485-495.
doi: 10.1016/j.fgb.2011.01.003 pmid: 21216300 |
| [42] | Grove JF. The trichothecenes and their biosynthesis[J]. Fortschr Chem Org Naturst, 2007, 88: 63-130. |
| [43] | 唐语谦, 刘晨迪, 潘药银, 等. 呕吐毒素降解微生物的研究进展[J]. 食品与生物技术学报, 2021, 40(6): 1-7. |
| Tang YQ, Liu CD, Pan YY, et al. Research progress on microbial degradation of deoxynivalenol[J]. J Food Sci Biotechnol, 2021, 40(6): 1-7. | |
| [44] | 何伟杰, 刘易科, 朱展望, 等. 镰刀菌毒素脱氧雪腐镰刀菌烯醇脱毒菌及脱毒酶研究进展[J]. 植物病理学报, 2019, 49(5): 577-589. |
| He WJ, Liu YK, Zhu ZW, et al. Recent progress on microbial and enzymatic detoxification of Fusarium mycotoxin deoxynivalenol[J]. Acta Phytopathol Sin, 2019, 49(5): 577-589. | |
| [45] |
张静, 张琼琼, 计成, 等. 微生物及生物酶对脱氧雪腐镰刀菌烯醇生物转化研究进展[J]. 动物营养学报, 2020, 32(10): 4807-4820.
doi: 10.3969/j.issn.1006-267x.2020.10.032 |
| Zhang J, Zhang QQ, Ji C, et al. Research advance on biotransformation of deoxynivalenol by microbes and biological enzymes[J]. Chin J Anim Nutr, 2020, 32(10): 4807-4820. | |
| [46] |
Xiao K, Liu CC, Qin Q, et al. EPA and DHA attenuate deoxynivalenol-induced intestinal porcine epithelial cell injury and protect barrier function integrity by inhibiting necroptosis signaling pathway[J]. FASEB J, 2020, 34(2): 2483-2496.
doi: 10.1096/fj.201902298R pmid: 31909535 |
| [47] |
Li P, Su RX, Yin RY, et al. Detoxification of mycotoxins through biotransformation[J]. Toxins, 2020, 12(2): 121.
doi: 10.3390/toxins12020121 URL |
| [48] | 赵雪芹, 朱风华, 陈甫, 等. 6种益生菌对玉米赤霉烯酮和呕吐毒素降解能力的研究[J]. 黑龙江畜牧兽医, 2020(14): 108-111. |
| Zhao XQ, Zhu FH, Chen F, et al. Study on the degradation ability of six probiotics to Zearalenone and vomiting toxin[J]. Heilongjiang Anim Sci Vet Med, 2020(14): 108-111. | |
| [49] |
Chlebicz A, Śliżewska K. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and Zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast[J]. Probiotics Antimicrob Proteins, 2020, 12(1): 289-301.
doi: 10.1007/s12602-018-9512-x |
| [50] |
杨冬, 唐璎. 枯草芽孢杆菌WTX1胞外酶降解AFB1酶学特性及降解位点分析[J]. 生物技术通报, 2023, 39(4): 93-102.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-1384 |
| Yang D, Tang Y. Enzymatic characterization and degradation sites of AFB1 degradation by the extracellular enzyme of Bacillus subtilis strain WTX1[J]. Biotechnol Bull, 2023, 39(4): 93-102. | |
| [51] | 陈毅保, 杨趁仙, 刘昆仑, 等. 生物技术对黄曲霉毒素B1的脱除及机制研究进展[J]. 中国油脂, 2023, 48(7): 109-114. |
| Chen YB, Yang CX, Liu KL, et al. Advances in biological detoxification of aflatoxin B1 and its mechanism[J]. China Oils Fats, 2023, 48(7): 109-114. | |
| [52] | 刘晨, 谢岩黎, 曹荣耀, 等. 玉米赤霉烯酮降解菌的筛选、鉴定及降解酶初步提取[J]. 现代食品科技, 2022, 38(8): 73-79. |
| Liu C, Xie YL, Cao RY, et al. Screening and identification of Zearalenone-degrading bacteria and preliminary extraction of Zearalenone-degrading enzyme[J]. Mod Food Sci Technol, 2022, 38(8): 73-79. | |
| [53] |
Takahashi-Ando N, Kimura M, Kakeya H, et al. A novel lactonohydrolase responsible for the detoxification of Zearalenone: enzyme purification and gene cloning[J]. Biochem J, 2002, 365(Pt 1): 1-6.
pmid: 11978180 |
| [54] |
Higa-Nishiyama A, Takahashi-Ando N, Shimizu T, et al. A model transgenic cereal plant with detoxification activity for the estrogenic mycotoxin Zearalenone[J]. Transgenic Res, 2005, 14(5): 713-717.
pmid: 16245162 |
| [55] |
Igawa T, Takahashi-Ando N, Ochiai N, et al. Reduced contamination by the Fusarium mycotoxin Zearalenone in maize kernels through genetic modification with a detoxification gene[J]. Appl Environ Microbiol, 2007, 73(5): 1622-1629.
doi: 10.1128/AEM.01077-06 URL |
| [56] | 吴宛芹, 曲睿, 艾重阳, 等. 乳酸菌去除脱氧雪腐镰刀菌烯醇的研究进展[J]. 饲料工业, 2019, 40(16): 51-59. |
| Wu WQ, Qu R, Ai CY, et al. Progress in removal of deoxynivalenol by Lactobacillus[J]. Feed Ind, 2019, 40(16): 51-59. | |
| [57] |
Hu YM, Li H, Min J, et al. Crystal structure and biochemical analysis of the specialized deoxynivalenol-detoxifying glyoxalase SPG from Gossypium hirsutum[J]. Int J Biol Macromol, 2022, 200: 388-396.
doi: 10.1016/j.ijbiomac.2022.01.055 URL |
| [58] | 王娟, 王改琴, 李钒, 等. 2020年饲料原料霉菌毒素污染状况调查[J]. 食品安全质量检测学报, 2021, 12(15): 6275-6282. |
| Wang J, Wang GQ, Li F, et al. Investigation on mycotoxin contamination in feed raw materials in 2020[J]. J Food Saf Qual, 2021, 12(15): 6275-6282. | |
| [59] |
Karlovsky P, Suman M, Berthiller F, et al. Impact of food processing and detoxification treatments on mycotoxin contamination[J]. Mycotoxin Res, 2016, 32(4): 179-205.
pmid: 27554261 |
| [60] |
Yao YZ, Long M. The biological detoxification of deoxynivalenol: a review[J]. Food Chem Toxicol, 2020, 145: 111649.
doi: S0278-6915(20)30539-1 pmid: 32745571 |
| [61] | 朱晓慧, 陆启荣, 胡嗣祎, 等. 单端孢霉烯族毒素生物脱毒技术研究进展[J]. 动物医学进展, 2021, 42(5): 108-113. |
| Zhu XH, Lu QR, Hu SY, et al. Progress on bio-detoxification technology of trichothecenes[J]. Prog Vet Med, 2021, 42(5): 108-113. |
| [1] | 李柳, 穆迎春, 刘璐, 张洪玉, 徐锦华, 杨臻, 乔璐, 宋金龙. 氟喹诺酮类抗生素及耐药基因污染控制的研究进展[J]. 生物技术通报, 2022, 38(9): 84-95. |
| [2] | 卫晓博, 侯颖, 程豪杰, 秦翠丽, 牛明福, 徐建强. 一种苯酚降解菌Pseudoxanthomonas sp. BF-6的分离鉴定及其降解特性及途径研究[J]. 生物技术通报, 2021, 37(10): 72-80. |
| [3] | 赵颖, 王楠, 陆安祥, 冯晓元, 郭晓军, 栾云霞. 核酸适配体侧流层析分析技术在真菌毒素检测中的应用[J]. 生物技术通报, 2020, 36(8): 217-227. |
| [4] | 王龑, 刘阳, 刘伊宁. 产毒真菌基因组研究进展[J]. 生物技术通报, 2015, 31(2): 26-34. |
| [5] | 夏云婷;张宗棋;王妮莎;辛宝平;刘晓晴;. 酶法催化降解2,4,6-三硝基甲苯的研究进展[J]. , 2012, 0(04): 45-50. |
| [6] | 邱平;李节. 真核生物中mRNA的降解[J]. , 1994, 0(04): 10-12. |
| [7] | 王璋瑜;. 对阿弗雷毒素的担忧在升级[J]. , 1990, 0(06): 17-17. |
| [8] | . 体外培养和遗传育种[J]. , 1989, 0(11): 75-80. |
| [9] | 王璋瑜;. 真菌毒素有希望成为除草剂[J]. , 1987, 0(04): 11-13. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||