








生物技术通报 ›› 2024, Vol. 40 ›› Issue (10): 160-171.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1227
收稿日期:2024-01-02
出版日期:2024-10-26
发布日期:2024-11-20
通讯作者:
陈晶瑜,女,博士,教授,研究方向:分子生物学及合成生物学;E-mail: chenjy@cau.edu.cn作者简介:琚康辉,男,硕士研究生,研究方向:分子生物学及合成生物学;E-mail: jkh72552365@163.com
基金资助:
JU Kang-hui1( ), TIAN Xiao-ya1, WANG Li2, CHEN Jing-yu1(
), TIAN Xiao-ya1, WANG Li2, CHEN Jing-yu1( )
)
Received:2024-01-02
Published:2024-10-26
Online:2024-11-20
摘要:
合成生物学是近年来蓬勃发展的一门涉及分子生物学、生物工程、微生物学、系统生物学等多个学科新型交叉学科,旨在利用生物学原理和工程方法创造全新的生物学系统和生物产品。合成生物学的发展也受到了高效“细胞工厂”理念的推动,这使得生物工程技术朝着工业化应用的方向迈出了重要一步。然而,受制于生产效率低、遗传不稳定、调控过程难等问题,如何获得转化效率高、鲁棒性强的“细胞工厂”仍然是合成生物学领域面临的重要任务。近年来,细胞工程和基因工程领域发展迅速,新型细胞元件、细胞底盘以及基因回路构造方式等技术逐渐成熟,其通过精确的基因编辑和调控,可实现对细胞的特定功能进行编程,例如增强细胞的代谢能力、改变细胞的分化路径以及设计新的细胞功能模块等,具有广泛的应用前景。本文从新型细胞元件、底盘细胞以及基因回路构造方式等角度,综述了近年来在细胞工程和基因工程领域发展迅速的细胞编程技术,这些技术的进步已经或者将要用于合成生物学的发展中,将会赋予工程菌更加强大的工作能力。
琚康辉, 田晓雅, 王立, 陈晶瑜. 细胞编程技术:助力高效细胞工厂的设计[J]. 生物技术通报, 2024, 40(10): 160-171.
JU Kang-hui, TIAN Xiao-ya, WANG Li, CHEN Jing-yu. Cell Programming Technology: Paving the Way for Efficient Cell Factories[J]. Biotechnology Bulletin, 2024, 40(10): 160-171.
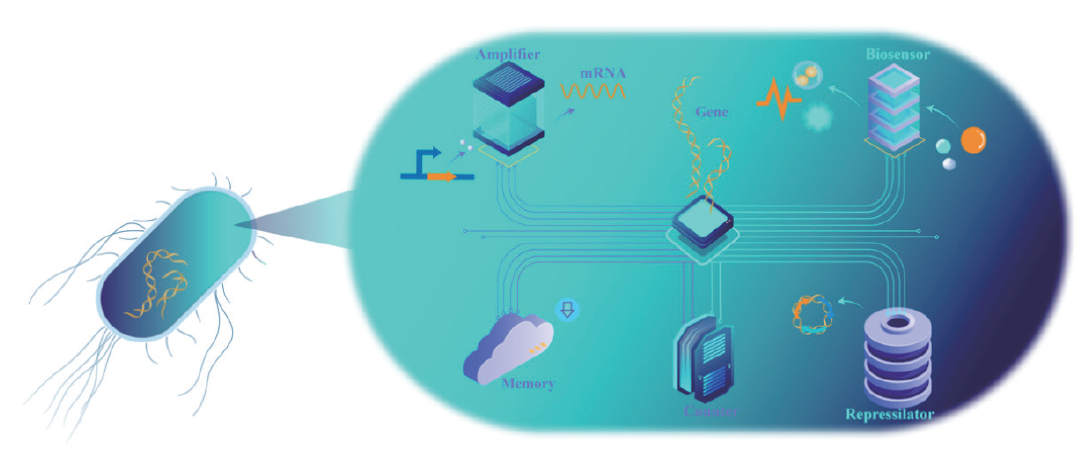
图1 细胞编程拓扑概念 在基因(gene)的调控下,各元件执行相应功能,放大器(amplifier):能够在级联基因网络中放大具有增益控制的转录信号,并且可以通过预测和动态调制转录信号实现细胞内外的功能控制;生物传感器(biosensor):对细胞工厂内外环境成分进行可视化的动态监测,进行实时调控;存储器(memory):细胞工厂中的记忆元件,延长细胞的工作时间;基因振荡器(repressilator):探究细胞内基因调控动力学的工具;计数器(counter):通过计算细胞内部各单元的级联反应,实现更加复杂的细胞编程和生物技术应用
Fig. 1 Topology concepts on cell programming Under genetic regulation, each element assumes a distinct role. Amplifier: It amplifies the transcriptional signals with gain control within cascade gene networks, and functional control inside and outside the cell can be achieved by predicting and dynamically modulating transcriptional signals. Biosensor: Real-time regulation through visual and dynamic monitoring of environmental components both inside and outside the cell factory. Memory: Memory elements within the cell factory extend its working time by preserving essential information. Repressilator: Probing intracellular regulatory dynamics. Counter: Catalyzing complex cell programming, the counter concept facilitates more intricate cell programming and broader biotechnological applications by calculating the cascade reactions of units within the cell
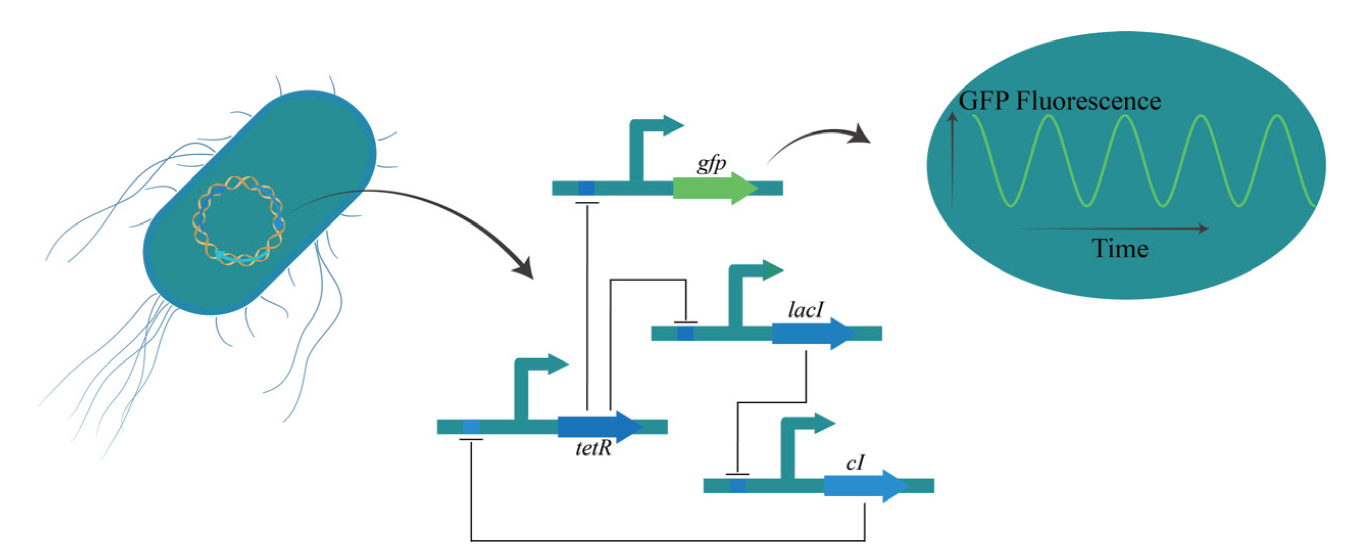
图2 初代“repressilator”工作原理 “repressilator”由3个操纵子相互作用,彼此抑制。其中,tetR基因负责调节绿色荧光蛋白(green flurescent protein, GFP)报告节点。使用延时荧光显微镜观察GFP报告情况,电路会表现出周期性的振荡
Fig. 2 Work mechanism of initial “repressilator” The “repressilator” comprises three operons interacting and inhibiting with each other. Notably, the tetR gene assumes a pivotal role in governing the regulatory node of GFP. Through the application of a time-lapse fluorescence microscope to monitor the GFP signal, the circuit manifests periodic oscillations
| 细胞编程 Cell reprogramming | 描述 Description | 来源 Reference | |
|---|---|---|---|
| 遗传元件 Genetic elements | 振荡器 | ||
| 抑制振荡器-RFL | 初代“repressilator”,3个转录抑制子建立的振荡网络,开发早、研究范围广、独立控制尚未实现 | [ | |
| 双反馈振荡器-DFO | 由两个诱导操纵子控制,可以通过控制诱导剂的浓度实现振荡器电路的独立控制,使得振荡器能够用于更复杂的电路设计 | [ | |
| 存储器 | |||
| 功能稳态型存储器 | 利用λ噬菌体的Cro蛋白诱导的CI/Cro转录开关作为“记忆元件”使得工程菌能够维持功能稳定至少6个月之久 | [ | |
| 状态机 | 使用重组酶特有的DNA切除和反转功能在大肠杆菌中构建状态机(State machines),读取基因表达的状态,记录所有输入的时序,并对基因表达进行多输入、多输出的控制 | [ | |
| 生物磁带记录器 | 使用基于CRISPR的适应系统,构建了一种“生物磁带记录器”,将其用来描述细胞动态和环境变化,从而相对准确地描述时间间隔生物信号和调节程序的能力 | [ | |
| 生物传感器 | |||
| 产物响应型生物传感器 | 基于产物响应的生物传感器,产物生成量越高,细胞传感荧光水平越强,以此建立了超高通量的筛选方式 | [ | |
| 温敏型生物传感器 | 利用温敏型生物传感器控制启动子表达,解偶联细胞生长与合成的关系,极大地提高了类胡萝卜的产生 | [ | |
| 细胞底盘 Cell chassis | 枯草芽孢杆菌 Bacillus subtilis | 模式化的细胞工厂,生产工业用酶的优良底盘 | [ |
| 酪丁酸梭菌 Clostridium tyrobutyricum | 在生产丁酸及丁醇的生产上有极高的生产效率,短链脂肪酸潜在的理性“细胞工厂” | [ | |
| 米曲霉 Aspergillus oryzae | 在天然产物的生物合成领域有重要的研究价值 | [ | |
| 嗜盐碱单胞菌 Natronomonas kamekura | 用于高分子材料、化学品和燃料的生物制造 | [ | |
| 解脂耶氏酵母 Yarrowia lipolytica | 适合用于油脂及其衍生物的生物合成 | [ | |
| 蓝藻 | 与上转换纳米粒子一起封装,能够创建工程化的微氧细胞工厂 | [ | |
| Minicell | 无染色体细胞,药物递送的优良载体 | [ | |
| Simcell | 无染色体细胞,安全可编程的通用平台 | [ | |
| 复杂基因逻辑回路设计Complex gene logic circuit design | AND、NOT联用型遗传电路设计 | 利用AND和NOT联用的基因电路,巧妙实现了细胞生长过渡到生物合成时间点的精确把控,减少外源电路引入对宿主菌株的代谢负担 | [ |
| CASwitch | 将CRISPR-Cas内环核酸酶与Tet-On3G诱导基因系统相结合而设计的高性能基因电路,用于哺乳动物系统中高性能诱导表达 | [ | |
表1 本文涉及到的部分细胞编程化改造基础与技术
Table 1 Partial modification basis and technology of the cell programming involved in this study
| 细胞编程 Cell reprogramming | 描述 Description | 来源 Reference | |
|---|---|---|---|
| 遗传元件 Genetic elements | 振荡器 | ||
| 抑制振荡器-RFL | 初代“repressilator”,3个转录抑制子建立的振荡网络,开发早、研究范围广、独立控制尚未实现 | [ | |
| 双反馈振荡器-DFO | 由两个诱导操纵子控制,可以通过控制诱导剂的浓度实现振荡器电路的独立控制,使得振荡器能够用于更复杂的电路设计 | [ | |
| 存储器 | |||
| 功能稳态型存储器 | 利用λ噬菌体的Cro蛋白诱导的CI/Cro转录开关作为“记忆元件”使得工程菌能够维持功能稳定至少6个月之久 | [ | |
| 状态机 | 使用重组酶特有的DNA切除和反转功能在大肠杆菌中构建状态机(State machines),读取基因表达的状态,记录所有输入的时序,并对基因表达进行多输入、多输出的控制 | [ | |
| 生物磁带记录器 | 使用基于CRISPR的适应系统,构建了一种“生物磁带记录器”,将其用来描述细胞动态和环境变化,从而相对准确地描述时间间隔生物信号和调节程序的能力 | [ | |
| 生物传感器 | |||
| 产物响应型生物传感器 | 基于产物响应的生物传感器,产物生成量越高,细胞传感荧光水平越强,以此建立了超高通量的筛选方式 | [ | |
| 温敏型生物传感器 | 利用温敏型生物传感器控制启动子表达,解偶联细胞生长与合成的关系,极大地提高了类胡萝卜的产生 | [ | |
| 细胞底盘 Cell chassis | 枯草芽孢杆菌 Bacillus subtilis | 模式化的细胞工厂,生产工业用酶的优良底盘 | [ |
| 酪丁酸梭菌 Clostridium tyrobutyricum | 在生产丁酸及丁醇的生产上有极高的生产效率,短链脂肪酸潜在的理性“细胞工厂” | [ | |
| 米曲霉 Aspergillus oryzae | 在天然产物的生物合成领域有重要的研究价值 | [ | |
| 嗜盐碱单胞菌 Natronomonas kamekura | 用于高分子材料、化学品和燃料的生物制造 | [ | |
| 解脂耶氏酵母 Yarrowia lipolytica | 适合用于油脂及其衍生物的生物合成 | [ | |
| 蓝藻 | 与上转换纳米粒子一起封装,能够创建工程化的微氧细胞工厂 | [ | |
| Minicell | 无染色体细胞,药物递送的优良载体 | [ | |
| Simcell | 无染色体细胞,安全可编程的通用平台 | [ | |
| 复杂基因逻辑回路设计Complex gene logic circuit design | AND、NOT联用型遗传电路设计 | 利用AND和NOT联用的基因电路,巧妙实现了细胞生长过渡到生物合成时间点的精确把控,减少外源电路引入对宿主菌株的代谢负担 | [ |
| CASwitch | 将CRISPR-Cas内环核酸酶与Tet-On3G诱导基因系统相结合而设计的高性能基因电路,用于哺乳动物系统中高性能诱导表达 | [ | |

图3 基于不同元件响应的生物传感器原理 细胞感受到外部核酸、抗体、酶分子等生物分子信号后,经过基于启动子(a)、核糖开关(b)和转录调节因子(c)等元件所设计的生物传感器后,转化为荧光信号、电信号、化学信号等输出胞外被捕捉分析
Fig. 3 Principle of biosensor based on the responses of different components Upon detecting external biomolecular signals encompassing gene, antibody, and enzyme molecules, cells navigate through biosensors meticulously designed with elements such as promoters(a), riboswitch(b), and transcription regulators(c). These biosensors efficiently convert the sensed signals into diverse outputs, including fluorescence signals, electrical signals, and chemical signals. The translated signals are then captured and analyzed outside the cell, providing valuable insights into the intricate cellular responses to external stimuli
| 回路类型 Loop type | 电路原理图 Electrical circuit diagram | 原理 Principle | 来源 Reference |
|---|---|---|---|
| OR | 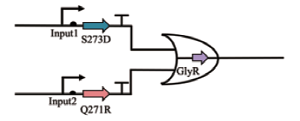 | Input1和Input2分别控制S273和Q271R的形成,二者均可作为甘氨酸合成的前体 | [ |
| NOT | 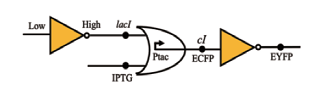 | Lac I和IPTG共同作用于Ptac启动子,作为OR门电路的两个输入。当有高电平输出信号时,会刺激cI和ECFP的表达,进而通过非门抑制EYFP的表达 | [ |
| AND | 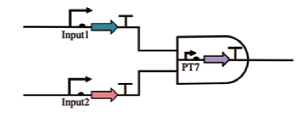 | Input1控制T7 RNA聚合酶基因的转录,Input2控制琥珀酸抑制剂t RNA sup D。当两种成分转录后,合成T7 RNA聚合酶,实现后续的基因表达 | [ |
| NAND | 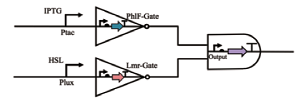 | Phlf和Lmr是两个非门,当IPTG和HSL同时诱导时,黄色荧光蛋白的表达会停止,其余情况正常 | [ |
| NOR | 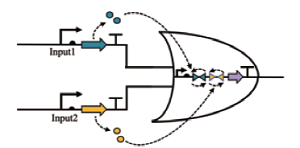 | Input1和Input2合成信号分子,翻转两个终止子的状态,当两个输入信号都表达时,关闭输出启动子 | [ |
表2 基本逻辑电路构造方式汇总
Table 2 Summary of basic logic circuit construction
| 回路类型 Loop type | 电路原理图 Electrical circuit diagram | 原理 Principle | 来源 Reference |
|---|---|---|---|
| OR |  | Input1和Input2分别控制S273和Q271R的形成,二者均可作为甘氨酸合成的前体 | [ |
| NOT |  | Lac I和IPTG共同作用于Ptac启动子,作为OR门电路的两个输入。当有高电平输出信号时,会刺激cI和ECFP的表达,进而通过非门抑制EYFP的表达 | [ |
| AND |  | Input1控制T7 RNA聚合酶基因的转录,Input2控制琥珀酸抑制剂t RNA sup D。当两种成分转录后,合成T7 RNA聚合酶,实现后续的基因表达 | [ |
| NAND |  | Phlf和Lmr是两个非门,当IPTG和HSL同时诱导时,黄色荧光蛋白的表达会停止,其余情况正常 | [ |
| NOR |  | Input1和Input2合成信号分子,翻转两个终止子的状态,当两个输入信号都表达时,关闭输出启动子 | [ |
| [1] | Majidian P, Tabatabaei M, Zeinolabedini M, et al. Metabolic engineering of microorganisms for biofuel production[J]. Renew Sustain Energy Rev, 2018, 82: 3863-3885. |
| [2] | Gu PF, Liu LW, Ma QQ, et al. Metabolic engineering of Escherichia coli for the production of isobutanol: a review[J]. World J Microbiol Biotechnol, 2021, 37(10): 168. |
| [3] |
Fidock DA. Eliminating malaria[J]. Science, 2013, 340(6140): 1531-1533.
doi: 10.1126/science.1240539 pmid: 23812705 |
| [4] |
Kannan K, Gibson DG. Yeast genome, by design[J]. Science, 2017, 355(6329): 1024-1025.
doi: 10.1126/science.aam9739 pmid: 28280169 |
| [5] | Joshi YJ, Jawale YK, Athale CA. Modeling the tunability of the dual-feedback genetic oscillator[J]. Phys Rev E, 2020, 101(1-1): 012417. |
| [6] |
Zúñiga A, Guiziou S, Mayonove P, et al. Rational programming of history-dependent logic in cellular populations[J]. Nat Commun, 2020, 11(1): 4758.
doi: 10.1038/s41467-020-18455-z pmid: 32958811 |
| [7] |
Tyler J, Shiu A, Walton J. Revisiting a synthetic intracellular regulatory network that exhibits oscillations[J]. J Math Biol, 2019, 78(7): 2341-2368.
doi: 10.1007/s00285-019-01346-3 pmid: 30929046 |
| [8] | Strelkowa N, Barahona M. Transient dynamics around unstable periodic orbits in the generalized repressilator model[J]. Chaos, 2011, 21(2): 023104. |
| [9] |
Zhang FY, Sun YH, Zhang YH, et al. Independent control of amplitude and period in a synthetic oscillator circuit with modified repressilator[J]. Commun Biol, 2022, 5(1): 23.
doi: 10.1038/s42003-021-02987-1 pmid: 35017621 |
| [10] |
Ozdemir T, Fedorec AJH, Danino T, et al. Synthetic biology and engineered live biotherapeutics: toward increasing system complexity[J]. Cell Syst, 2018, 7(1): 5-16.
doi: S2405-4712(18)30248-5 pmid: 30048620 |
| [11] |
Riglar DT, Giessen TW, Baym M, et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation[J]. Nat Biotechnol, 2017, 35: 653-658.
doi: 10.1038/nbt.3879 pmid: 28553941 |
| [12] | Roquet N, Soleimany AP, Ferris AC, et al. Synthetic recombinase-based state machines in living cells[J]. Science, 2016, 353(6297): aad8559. |
| [13] |
Sheth RU, Yim SS, Wu FL, et al. Multiplex recording of cellular events over time on CRISPR biological tape[J]. Science, 2017, 358(6369): 1457-1461.
doi: 10.1126/science.aao0958 pmid: 29170279 |
| [14] |
Adolfsen KJ, Callihan I, Monahan CE, et al. Improvement of a synthetic live bacterial therapeutic for phenylketonuria with biosensor-enabled enzyme engineering[J]. Nat Commun, 2021, 12(1): 6215.
doi: 10.1038/s41467-021-26524-0 pmid: 34711827 |
| [15] | Zhou PP, Li M, Shen B, et al. Directed coevolution of β-carotene ketolase and hydroxylase and its application in temperature-regulated biosynthesis of astaxanthin[J]. J Agric Food Chem, 2019, 67(4): 1072-1080. |
| [16] | 马平英, 罗雯, 詹怡昕, 等. 枯草芽孢杆菌表达系统研究进展[J]. 江西科学, 2020, 38(6): 867-871. |
| Ma PY, Luo W, Zhan YX, et al. Research progress of Bacillus subtilis expression system[J]. Jiangxi Sci, 2020, 38(6): 867-871. | |
| [17] |
Liu CZ, Qin Y, Li XJ, et al. Preparation and characterization of starch nanoparticles via self-assembly at moderate temperature[J]. Int J Biol Macromol, 2016, 84: 354-360.
doi: 10.1016/j.ijbiomac.2015.12.040 pmid: 26708434 |
| [18] | Dong JW, Li XJ, Yang C, et al. The antioxidant activity and total phenolic and total flavonoid contents of Pyracantha fortuneana fruit can be improved by solid-state fermentation with Rhizopus oryzae and Penicillium commune[J]. Journal of Chinese Pharmaceutical Sciences, 2022, 31(6):452-460. |
| [19] | 晋彪, 张静, 洪坤强, 等. 嗜盐单胞菌利用乙酸盐合成PHB的研究[J]. 化学工业与工程, 2022, 39(5): 119-126. |
| Jin B, Zhang J, Hong KQ, et al. Studies on PHB production in Ha-lomonas using acetate as substrate[J]. Chem Ind Eng, 2022, 39(5): 119-126. | |
| [20] | 赵禹, 刘士琦, 李建, 等. 解脂耶氏酵母作为微生物细胞工厂的应用研究进展[J]. 食品科学, 2021, 42(19): 388-400. |
| Zhao Y, Liu SQ, Li J, et al. Advances in the application of Yarrowia lipolytica as a microbial cell factory[J]. Food Sci, 2021, 42(19): 388-400. | |
| [21] |
Wang WL, Zheng HZ, Jiang J, et al. Engineering micro oxygen factories to slow tumour progression via hyperoxic microenvironments[J]. Nat Commun, 2022, 13(1): 4495.
doi: 10.1038/s41467-022-32066-w pmid: 35918337 |
| [22] | Chen JX, Steel H, Wu YH, et al. Development of aspirin-inducible biosensors in Escherichia coli and SimCells[J]. Appl Environ Microbiol, 2019, 85(6): e02959-e02918. |
| [23] |
Fan C, Davison PA, Habgood R, et al. Chromosome-free bacterial cells are safe and programmable platforms for synthetic biology[J]. Proc Natl Acad Sci USA, 2020, 117(12): 6752-6761.
doi: 10.1073/pnas.1918859117 pmid: 32144140 |
| [24] |
Salis HM, Mirsky EA, Voigt CA. Automated design of synthetic ribosome binding sites to control protein expression[J]. Nat Biotechnol, 2009, 27(10): 946-950.
doi: 10.1038/nbt.1568 pmid: 19801975 |
| [25] |
De Carluccio G, Fusco V, di Bernardo D. Engineering a synthetic gene circuit for high-performance inducible expression in mammalian systems[J]. Nat Commun, 2024, 15(1): 3311.
doi: 10.1038/s41467-024-47592-y pmid: 38632224 |
| [26] | Schuster SC, Swanson RV, Alex LA, et al. Assembly and function of a quaternary signal transduction complex monitored by surface plasmon resonance[J]. Nature, 1993, 365(6444): 343-347. |
| [27] |
Thouand G, Horry H, Durand MJ, et al. Development of a biosensor for on-line detection of tributyltin with a recombinant bioluminescent Escherichia coli strain[J]. Appl Microbiol Biotechnol, 2003, 62(2-3): 218-225.
pmid: 12883867 |
| [28] | Misawa N, Osaki T, Takeuchi S. Membrane protein-based biosensors[J]. J R Soc Interface, 2018, 15(141): 20170952. |
| [29] |
Maskow T, Kemp R, Buchholz F, et al. What heat is telling us about microbial conversions in nature and technology: from chip- to megacalorimetry[J]. Microb Biotechnol, 2010, 3(3): 269-284.
doi: 10.1111/j.1751-7915.2009.00121.x pmid: 21255327 |
| [30] |
Zhu C, Gerald RE II, Huang J. Micromachined optical fiber sensors for biomedical applications[J]. Methods Mol Biol, 2022, 2393: 367-414.
doi: 10.1007/978-1-0716-1803-5_20 pmid: 34837190 |
| [31] |
Koveal D, Rosen PC, Meyer DJ, et al. A high-throughput multiparameter screen for accelerated development and optimization of soluble genetically encoded fluorescent biosensors[J]. Nat Commun, 2022, 13(1): 2919.
doi: 10.1038/s41467-022-30685-x pmid: 35614105 |
| [32] |
Bourque K, Pétrin D, Sleno R, et al. Distinct conformational dynamics of three G protein-coupled receptors measured using FlAsH-BRET biosensors[J]. Front Endocrinol, 2017, 8: 61.
doi: 10.3389/fendo.2017.00061 pmid: 28439254 |
| [33] |
Alloush HM, Anderson E, Martin AD, et al. A bioluminescent microbial biosensor for in vitro pretreatment assessment of cytarabine efficacy in leukemia[J]. Clin Chem, 2010, 56(12): 1862-1870.
doi: 10.1373/clinchem.2010.145581 pmid: 20921267 |
| [34] |
Dhyani R, Shankar K, Bhatt A, et al. Homogentisic acid-based whole-cell biosensor for detection of alkaptonuria disease[J]. Anal Chem, 2021, 93(10): 4521-4527.
doi: 10.1021/acs.analchem.0c04914 pmid: 33655752 |
| [35] |
Dabirian Y, Li XW, Chen Y, et al. Expanding the dynamic range of a transcription factor-based biosensor in Saccharomyces cerevisiae[J]. ACS Synth Biol, 2019, 8(9): 1968-1975.
doi: 10.1021/acssynbio.9b00144 pmid: 31373795 |
| [36] |
Serganov A, Nudler E. A decade of riboswitches[J]. Cell, 2013, 152(1-2): 17-24.
doi: 10.1016/j.cell.2012.12.024 pmid: 23332744 |
| [37] |
Ma Q, Zhang QW, Xu QY, et al. Systems metabolic engineering strategies for the production of amino acids[J]. Synth Syst Biotechnol, 2017, 2(2): 87-96.
doi: 10.1016/j.synbio.2017.07.003 pmid: 29062965 |
| [38] |
Friedland AE, Lu TK, Wang X, et al. Synthetic gene networks that count[J]. Science, 2009, 324(5931): 1199-1202.
doi: 10.1126/science.1172005 pmid: 19478183 |
| [39] |
Rubens JR, Selvaggio G, Lu TK. Synthetic mixed-signal computation in living cells[J]. Nat Commun, 2016, 7: 11658.
doi: 10.1038/ncomms11658 pmid: 27255669 |
| [40] |
Kong WT, Blanchard AE, Liao C, et al. Engineering robust and tunable spatial structures with synthetic gene circuits[J]. Nucleic Acids Res, 2017, 45(2): 1005-1014.
doi: 10.1093/nar/gkw1045 pmid: 27899571 |
| [41] | Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532. |
| [42] |
MacDiarmid JA, Mugridge NB, Weiss JC, et al. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics[J]. Cancer Cell, 2007, 11(5): 431-445.
pmid: 17482133 |
| [43] |
Ali MK, Liu Q, Liang K, et al. Bacteria-derived minicells for cancer therapy[J]. Cancer Lett, 2020, 491: 11-21.
doi: S0304-3835(20)30379-7 pmid: 32721550 |
| [44] |
Rampley CPN, Davison PA, Qian P, et al. Development of SimCells as a novel chassis for functional biosensors[J]. Sci Rep, 2017, 7(1): 7261.
doi: 10.1038/s41598-017-07391-6 pmid: 28775370 |
| [45] |
Brophy JAN, Voigt CA. Principles of genetic circuit design[J]. Nat Methods, 2014, 11(5): 508-520.
doi: 10.1038/nmeth.2926 pmid: 24781324 |
| [46] | Han L, Shan Q. Pair of residue substitutions at the outer mouth of the channel pore act as inputs for a Boolean logic “OR” gate based on the Glycine receptor[J]. ACS Chem Neurosci, 2020, 11(20): 3409-3417. |
| [47] | Yokobayashi Y, Weiss R, Arnold FH. Directed evolution of a genetic circuit[J]. Proc Natl Acad Sci U S A, 2002, 99(26): 16587-16591. |
| [48] |
Anderson JC, Voigt CA, Arkin AP. Environmental signal integration by a modular AND gate[J]. Mol Syst Biol, 2007, 3: 133.
pmid: 17700541 |
| [49] |
Stanton BC, Nielsen AAK, Tamsir A, et al. Genomic mining of prokaryotic repressors for orthogonal logic gates[J]. Nat Chem Biol, 2014, 10(2): 99-105.
doi: 10.1038/nchembio.1411 pmid: 24316737 |
| [50] |
Bonnet J, Yin P, Ortiz ME, et al. Amplifying genetic logic gates[J]. Science, 2013, 340(6132): 599-603.
doi: 10.1126/science.1232758 pmid: 23539178 |
| [51] |
Siuti P, Yazbek J, Lu TK. Synthetic circuits integrating logic and memory in living cells[J]. Nat Biotechnol, 2013, 31(5): 448-452.
doi: 10.1038/nbt.2510 pmid: 23396014 |
| [52] | Li XM, Jiang W, Qi QS, et al. A gene circuit combining the endogenous I-E type CRISPR-cas system and a light sensor to produce poly-β-hydroxybutyric acid efficiently[J]. Biosensors, 2022, 12(8): 642. |
| [53] |
Mutalik VK, Guimaraes JC, Cambray G, et al. Precise and reliable gene expression via standard transcription and translation initiation elements[J]. Nat Methods, 2013, 10(4): 354-360.
doi: 10.1038/nmeth.2404 pmid: 23474465 |
| [54] |
Cameron DE, Bashor CJ, Collins JJ. A brief history of synthetic biology[J]. Nat Rev Microbiol, 2014, 12(5): 381-390.
doi: 10.1038/nrmicro3239 pmid: 24686414 |
| [55] | MacKenzie A. From validating to verifying: public appeals in synthetic biology[J]. Sci Cult, 2013, 22(4): 476-496. |
| [1] | 成婷, 苑帅, 张晓元, 林良才, 李欣, 张翠英. 酿酒酵母异丁醇合成途径调控的研究进展[J]. 生物技术通报, 2023, 39(7): 80-90. |
| [2] | 王晓梅, 杨小薇, 李辉尚, 何微, 辛竹琳. 全球合成生物学发展现状及对我国的启示[J]. 生物技术通报, 2023, 39(2): 292-302. |
| [3] | 陈晓琳, 刘洋儿, 许文涛, 郭明璋, 刘慧琳. 合成生物学细胞传感技术在食品安全快速检测中的应用[J]. 生物技术通报, 2023, 39(1): 137-149. |
| [4] | 周琳, 梁轩铭, 赵磊. 天然类胡萝卜素的生物合成研究进展[J]. 生物技术通报, 2022, 38(7): 119-127. |
| [5] | 邱益彬, 马艳琴, 沙媛媛, 朱逸凡, 苏二正, 雷鹏, 李莎, 徐虹. 解淀粉芽孢杆菌分子遗传操作及其应用研究进展[J]. 生物技术通报, 2022, 38(2): 205-217. |
| [6] | 郭晓真, 张学福. 植物合成生物学领域发展态势的文献计量分析[J]. 生物技术通报, 2022, 38(2): 289-296. |
| [7] | 赵玉雪, 王芸, 余璐瑶, 刘京晶, 斯金平, 张新凤, 张磊. 植物中C-糖基转移酶的结构与应用[J]. 生物技术通报, 2022, 38(10): 18-28. |
| [8] | 叶敏, 高教琪, 周雍进. 非常规酵母细胞工厂合成天然产物[J]. 生物技术通报, 2021, 37(8): 12-24. |
| [9] | 叶健文, 陈江楠, 张旭, 吴赴清, 陈国强. 动态调控:一种高效的细胞工厂工程化代谢改造策略[J]. 生物技术通报, 2020, 36(6): 1-12. |
| [10] | 常瀚文, 郑鑫铃, 骆健美, 王敏, 申雁冰. 抗逆元件及其在高效微生物细胞工厂构建中的应用进展[J]. 生物技术通报, 2020, 36(6): 13-34. |
| [11] | 张慧, 田方方, 吴毅. 合成型酵母基因组重排技术[J]. 生物技术通报, 2020, 36(4): 13-18. |
| [12] | 曹燕亭, 刘延峰, 李江华, 刘龙, 堵国成. 基于细胞亚群调控提升生物合成效率的研究进展[J]. 生物技术通报, 2020, 36(4): 19-25. |
| [13] | 李佳秀, 蔡倩茹, 吴杰群. 萜类化合物在酿酒酵母中的合成生物学研究进展[J]. 生物技术通报, 2020, 36(12): 199-207. |
| [14] | 刘新平, 谭玉萌, 张雪, 冯雁, 杨广宇. 神经节苷脂氟化寡糖在大肠杆菌中的生物合成[J]. 生物技术通报, 2019, 35(8): 162-169. |
| [15] | 刘洋儿, 郭明璋, 杜若曦, 贺晓云, 黄昆仑, 许文涛. 乳酸菌在合成生物学中的研究现状及展望[J]. 生物技术通报, 2019, 35(8): 193-204. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||