








生物技术通报 ›› 2025, Vol. 41 ›› Issue (7): 37-48.doi: 10.13560/j.cnki.biotech.bull.1985.2024-1061
林佳怡1( ), 陈强2, 张磊1, 刘宏鑫1, 郑晓明3,4,5(
), 陈强2, 张磊1, 刘宏鑫1, 郑晓明3,4,5( ), 逄洪波1(
), 逄洪波1( )
)
收稿日期:2024-10-30
出版日期:2025-07-26
发布日期:2025-07-22
通讯作者:
:逄洪波,女,博士,教授,研究方向 :植物逆境分子生物学;E-mail: panghb@synu.edu.cn作者简介:林佳怡,女,硕士研究生,研究方向 :生物化学与分子生物学;E-mail: 15998495879@163.com基金资助:
LIN Jia-yi1( ), CHEN Qiang2, ZHANG Lei1, LIU Hong-xin1, ZHENG Xiao-ming3,4,5(
), CHEN Qiang2, ZHANG Lei1, LIU Hong-xin1, ZHENG Xiao-ming3,4,5( ), PANG Hong-bo1(
), PANG Hong-bo1( )
)
Received:2024-10-30
Published:2025-07-26
Online:2025-07-22
摘要:
褪黑素(melatonin, MT)是植物中普遍存在的吲哚胺类生物活性分子,近年来其参与植物非生物胁迫响应的分子机制已成为研究的前沿热点。本文系统综述了褪黑素在植物低温胁迫响应中的最新研究进展,深入解析其通过多维度调控网络增强植物耐寒性的分子机理。在生理生化层面,褪黑素通过稳定细胞膜脂质双分子层结构、保护光合系统Ⅱ反应中心复合体、清除过量活性氧(ROS)等核心途径,有效缓解低温引发的膜脂过氧化和光抑制效应。在分子调控网络层面,褪黑素通过复杂的信号网络调控植物的低温响应,如:(1)激活ICE1-CBF-COR转录级联通路,上调冷响应基因的表达;(2)通过受体介导的信号途径传递胁迫信号;(3)调控钙离子、一氧化氮、H2O2等第二信使的动态平衡;(4)与植物激素(如ABA、JA、IAA)协同或拮抗,形成信号网络;(5)激活MAPK、CDPK等蛋白激酶级联反应,放大冷胁迫信号,这些机制共同作用增强植物的耐寒性。褪黑素在植物低温耐受性调控中的应用潜力巨大,外源施用褪黑素已被证明对多种作物的低温耐受性具有积极作用,通过基因编辑技术提升植物内源褪黑素的合成能力也是提高作物耐寒性的重要策略。未来研究应以多学科交叉为基础,深入探讨褪黑素在植物低温胁迫中的作用机制及其潜在应用价值,为培育更加耐寒的作物品种提供理论支撑和技术指导。
林佳怡, 陈强, 张磊, 刘宏鑫, 郑晓明, 逄洪波. 褪黑素在植物低温胁迫中的研究进展[J]. 生物技术通报, 2025, 41(7): 37-48.
LIN Jia-yi, CHEN Qiang, ZHANG Lei, LIU Hong-xin, ZHENG Xiao-ming, PANG Hong-bo. Research Progress in Melatonin in Plant Low-temperature Stress[J]. Biotechnology Bulletin, 2025, 41(7): 37-48.
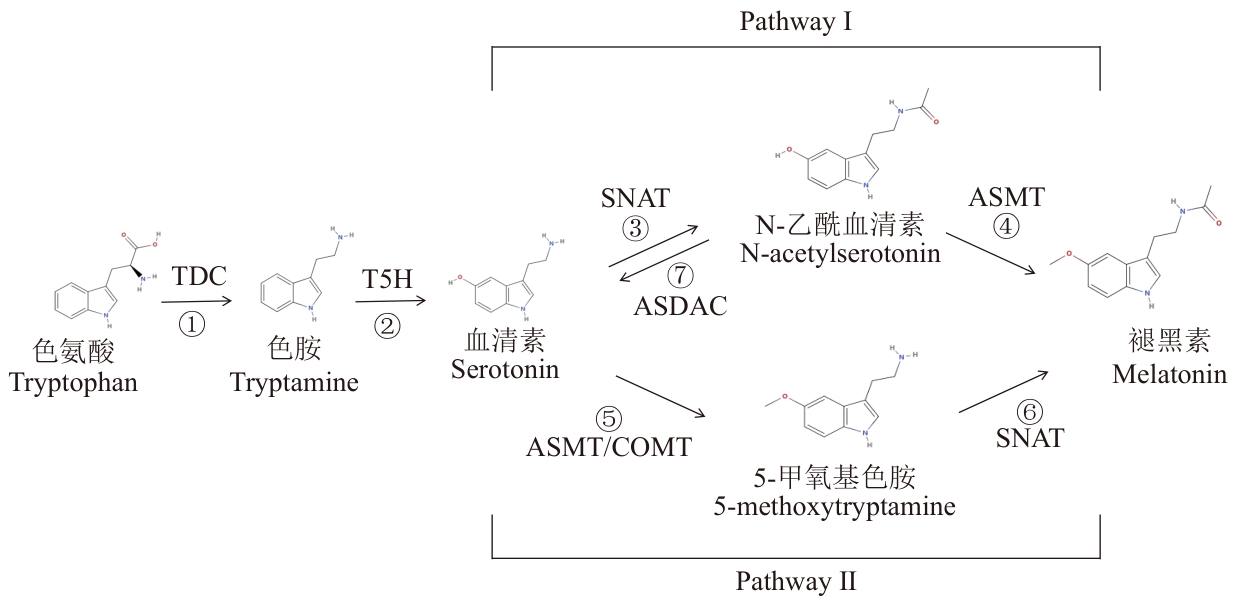
图2 植物中褪黑素的合成途径①:色氨酸在色氨酸脱羧酶(TDC)的催化下转化为色胺;②:色胺的C-5位置被色胺5-羟化酶(T5H)羟化形成血清素;③:血清素经血清素N-乙酰转移酶(SNAT)催化形成N-乙酰血清素;④:N-乙酰血清素被乙酰5-羟色胺甲基转移酶(ASMT)转化为褪黑素;⑤:胁迫条件下,羟化形成的血清素在乙酰5-羟色胺甲基转移酶(ASMT)或咖啡酸3-O-甲基转移酶(COMT)作用下生成5-甲氧基色胺;⑥:5-甲氧基色胺经血清素N-乙酰转移酶(SNAT)催化生成褪黑素;⑦:N-乙酰血清素在N-乙酰血清素脱乙酰酶(ASDAC)的作用下重新形成血清素
Fig. 2 Pathway of melatonin synthesis in plants①: Tryptophan is converted to tryptamine through the catalytic action of tryptophan decarboxylase (TDC). ②: Tryptamine undergoes hydroxylation at the C-5 position by tryptamine 5-hydroxylase (T5H), resulting in the formation of serotonin. ③: Serotonin is catalyzed by serotonin N-acetyltransferase (SNAT) to form N-acetylserotonin. ④: N-acetylserotonin is converted to melatonin by acetylserotonin O-methyltransferase (ASMT). ⑤: Serotonin, after hydroxylation, can also be converted into 5-methoxytryptamine via acetylserotonin O-methyltransferase (ASMT) or caffeic acid O-methyltransferase (COMT) under stress conditions. ⑥: 5-methoxytryptamine is further catalyzed by serotonin N-acetyltransferase (SNAT) to produce melatonin. ⑦: N-acetylserotonin can be deacetylated back into serotonin by N-acetylserotonin deacetylase (ASDAC)
| [1] | Heidarvand L, Maali Amiri R. What happens in plant molecular responses to cold stress? [J]. Acta Physiol Plant, 2010, 32(3): 419-431. |
| [2] | Yadav SK. Cold stress tolerance mechanisms in plants. A review [J]. Agron Sustain Dev, 2010, 30(3): 515-527. |
| [3] | Ding F, Liu B, Zhang SX. Exogenous melatonin ameliorates cold-induced damage in tomato plants [J]. Sci Hortic, 2017, 219: 264-271. |
| [4] | Sanghera GS, Wani SH, Hussain W, et al. Engineering cold stress tolerance in crop plants [J]. Curr Genomics, 2011, 12(1): 30-43. |
| [5] | Soualiou S, Duan FY, Li X, et al. Crop production under cold stress: an understanding of plant responses, acclimation processes, and management strategies [J]. Plant Physiol Biochem, 2022, 190: 47-61. |
| [6] | Janmohammadi M, Zolla L, Rinalducci S. Low temperature tolerance in plants: changes at the protein level [J]. Phytochemistry, 2015, 117: 76-89. |
| [7] | Arnao MB, Hernández-Ruiz J. Melatonin against environmental plant stressors: a review [J]. Curr Protein Pept Sci, 2021, 22(5): 413-429. |
| [8] | Turk H, Erdal S, Genisel M, et al. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings [J]. Plant Growth Regul, 2014, 74(2): 139-152. |
| [9] | Li H, Dong YC, Chang JJ, et al. High-throughput microRNA and mRNA sequencing reveals that microRNAs may be involved in melatonin-mediated cold tolerance in Citrullus lanatus L [J]. Front Plant Sci, 2016, 7: 1231. |
| [10] | Li X, Wei JP, Scott ER, et al. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L [J]. Molecules, 2018, 23(1): 165. |
| [11] | Du HY, Liu DX, Liu GT, et al. Polyamines conjugated to the bio-membranes and membrane conformations are involved in the melatonin-mediated resistance of harvested plum fruit to cold stress [J]. Postharvest Biol Technol, 2023, 204: 112480. |
| [12] | Liu GS, Zhang YX, Yun Z, et al. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit [J]. Foods, 2020, 9(4): 454. |
| [13] | Zhang YP, Yang SJ, Chen YY. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery [J]. Biol Plant, 2017, 61(3): 571-578. |
| [14] | Lerner AB, Case JD, Takahashi Y, et al. Isolation of melatonin, the pineal gland factor that lightens MELANOCYTES1 [J]. J Am Chem Soc, 1958, 80(10): 2587. |
| [15] | Dubbels R, Reiter RJ, Klenke E, et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry [J]. J Pineal Res, 1995, 18(1): 28-31. |
| [16] | Hattori A, Migitaka H, Iigo M, et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates [J]. Biochem Mol Biol Int, 1995, 35(3): 627-634. |
| [17] | Hernández-Ruiz J, Arnao MB. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light [J]. J Agric Food Chem, 2008, 56(22): 10567-10573. |
| [18] | Kolár J, Machácková I. Melatonin in higher plants: occurrence and possible functions [J]. J Pineal Res, 2005, 39(4): 333-341. |
| [19] | Posmyk MM, Janas KM. Melatonin in plants [J]. Acta Physiol Plant, 2009, 31(1): 1-11. |
| [20] | Reiter RJ, Tan DX, Burkhardt S, et al. Melatonin in plants [J]. Nutr Rev, 2001, 59(9): 286-290. |
| [21] | Zhao Y, Tan DX, Lei Q, et al. Melatonin and its potential biological functions in the fruits of sweet cherry [J]. J Pineal Res, 2013, 55(1): 79-88. |
| [22] | Arnao MB, Hernández-Ruiz J. Melatonin: a new plant hormone and/or a plant master regulator? [J]. Trends Plant Sci, 2019, 24(1): 38-48. |
| [23] | Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination [J]. J Pineal Res, 2011, 51(1): 1-16. |
| [24] | García JJ, López-Pingarrón L, Almeida-Souza P, et al. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review [J]. J Pineal Res, 2014, 56(3): 225-237. |
| [25] | Ahmad I, Zhu GL, Zhou GS, et al. Melatonin role in plant growth and physiology under abiotic stress [J]. Int J Mol Sci, 2023, 24(10): 8759. |
| [26] | Wang KX, Xing QF, Ahammed GJ, et al. Functions and prospects of melatonin in plant growth, yield, and quality [J]. J Exp Bot, 2022, 73(17): 5928-5946. |
| [27] | Altaf MA, Shahid R, Ren MX, et al. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system [J]. Antioxidants, 2022, 11(2): 309. |
| [28] | Bian LP, Wang YS, Bai HW, et al. Melatonin-ROS signal module regulates plant lateral root development [J]. Plant Signal Behav, 2021, 16(5): 1901447. |
| [29] | Arnao MB, Hernández-Ruiz J. Melatonin: plant growth regulator and/or biostimulator during stress? [J]. Trends Plant Sci, 2014, 19(12): 789-797. |
| [30] | Sun H, Wang XQ, Zeng ZL, et al. Exogenous melatonin strongly affects dynamic photosynthesis and enhances water-water cycle in tobacco [J]. Front Plant Sci, 2022, 13: 917784. |
| [31] | Hassan MU, Mahmood A, Awan MI, et al. Melatonin-induced protection against plant abiotic stress: mechanisms and prospects [J]. Front Plant Sci, 2022, 13: 902694. |
| [32] | Tan DX, Manchester LC, Liu XY, et al. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes [J]. J Pineal Res, 2013, 54(2): 127-138. |
| [33] | Kang K, Kong K, Park S, et al. Molecular cloning of a plant N-acetylserotonin methyltransferase and its expression characteristics in rice [J]. J Pineal Res, 2011, 50(3): 304-309. |
| [34] | Back K, Tan DX, Reiter RJ. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts [J]. J Pineal Res, 2016, 61(4): 426-437. |
| [35] | Kang K, Lee K, Park S, et al. Molecular cloning of rice serotonin N-acetyltransferase, the penultimate gene in plant melatonin biosynthesis [J]. J Pineal Res, 2013, 55(1): 7-13. |
| [36] | Lee K, Lee HY, Back K. Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants [J]. J Pineal Res, 2018, 64(2). DOI:10.1111/jpi.12460 . |
| [37] | Bajwa VS, Shukla MR, Sherif SM, et al. Role of melatonin in alleviating cold stress in Arabidopsis thaliana [J]. J Pineal Res, 2014, 56(3): 238-245. |
| [38] | Zeng W, Mostafa S, Lu ZG, et al. Melatonin-mediated abiotic stress tolerance in plants [J]. Front Plant Sci, 2022, 13: 847175. |
| [39] | Irshad A, Rehman RNU, Kareem HA, et al. Addressing the challenge of cold stress resilience with the synergistic effect of Rhizobium inoculation and exogenous melatonin application in Medicago truncatula [J]. Ecotoxicol Environ Saf, 2021, 226: 112816. |
| [40] | Qari SH, Hassan MU, Chattha MU, et al. Melatonin induced cold tolerance in plants: physiological and molecular responses [J]. Front Plant Sci, 2022, 13: 843071. |
| [41] | Saita E, Albanesi D, de Mendoza D. Sensing membrane thickness: Lessons learned from cold stress [J]. Biochim Biophys Acta, 2016, 1861(8 Pt B): 837-846. |
| [42] | Hirata Y, Cai RQ, Volchuk A, et al. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis [J]. Curr Biol, 2023, 33(7): 1282-1294. |
| [43] | Quinn PJ. A lipid-phase separation model of low-temperature damage to biological membranes [J]. Cryobiology, 1985, 22(2): 128-146. |
| [44] | Khan A, Numan M, Khan AL, et al. Melatonin: awakening the defense mechanisms during plant oxidative stress [J]. Plants, 2020, 9(4): 407. |
| [45] | Karbownik M, Lewinski A, Reiter RJ. Anticarcinogenic actions of melatonin which involve antioxidative processes: comparison with other antioxidants [J]. Int J Biochem Cell Biol, 2001, 33(8): 735-753. |
| [46] | Bawa G, Feng LY, Shi JY, et al. Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway [J]. Funct Plant Biol, 2020, 47(9): 815-824. |
| [47] | Martinez V, Nieves-Cordones M, Lopez-Delacalle M, et al. Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin [J]. Molecules, 2018, 23(3): 535. |
| [48] | Takahashi D, Li B, Nakayama T, et al. Plant plasma membrane proteomics for improving cold tolerance [J]. Front Plant Sci, 2013, 4: 90. |
| [49] | Yu YC, Wang AM, Li X, et al. Melatonin-stimulated triacylglycerol breakdown and energy turnover under salinity stress contributes to the maintenance of plasma membrane H+-ATPase activity and K+/Na+ homeostasis in sweet potato [J]. Front Plant Sci, 2018, 9: 256. |
| [50] | Wang ZQ, Zhang L, Duan WH, et al. Melatonin maintained higher contents of unsaturated fatty acid and cell membrane structure integrity in banana peel and alleviated postharvest chilling injury [J]. Food Chem, 2022, 397: 133836. |
| [51] | Fan JB, Hu ZR, Xie Y, et al. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass [J]. Front Plant Sci, 2015, 6: 925. |
| [52] | Han QH, Huang B, Ding CB, et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings [J]. Front Plant Sci, 2017, 8: 785. |
| [53] | Posmyk MM, Bałabusta M, Wieczorek M, et al. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress [J]. J Pineal Res, 2009, 46(2): 214-223. |
| [54] | Zhang XW, Feng YQ, Jing TT, et al. Melatonin promotes the chilling tolerance of cucumber seedlings by regulating antioxidant system and relieving photoinhibition [J]. Front Plant Sci, 2021, 12: 789617. |
| [55] | Korkmaz A, Değer Ö, Szafrańska K, et al. Melatonin effects in enhancing chilling stress tolerance of pepper [J]. Sci Hortic, 2021, 289: 110434. |
| [56] | Arnao MB, Hernández-Ruiz J. Melatonin and its relationship to plant hormones [J]. Ann Bot, 2018, 121(2): 195-207. |
| [57] | Mittler R. Oxidative stress, antioxidants and stress tolerance [J]. Trends Plant Sci, 2002, 7(9): 405-410. |
| [58] | Chinnusamy V, Zhu JH, Zhu JK. Cold stress regulation of gene expression in plants [J]. Trends Plant Sci, 2007, 12(10): 444-451. |
| [59] | Reiter RJ, Tan DX, Zhou Z, et al. Phytomelatonin: assisting plants to survive and thrive [J]. Molecules, 2015, 20(4): 7396-7437. |
| [60] | Kong XM, Ge WY, Wei BD, et al. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity [J]. Postharvest Biol Technol, 2020, 170: 111315. |
| [61] | Yan L, Huang H, Raza A, et al. Exogenous melatonin confers cold tolerance in rapeseed (Brassica napus L.) seedlings by improving antioxidants and genes expression [J]. Plant Signal Behav, 2022, 17(1): 2129289. |
| [62] | Shi HT, Jiang C, Ye TT, et al. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin [J]. J Exp Bot, 2015, 66(3): 681-694. |
| [63] | Wang ML, Zhang SX, Ding F. Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants [J]. Antioxidants, 2020, 9(3): 218. |
| [64] | Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks [J]. J Exp Bot, 2012, 63(4): 1593-1608. |
| [65] | Nawaz MA, Huang Y, Bie ZL, et al. Melatonin: current status and future perspectives in plant science [J]. Front Plant Sci, 2016, 6: 1230. |
| [66] | Mariama K, Dong JX, Huan C, et al. Melatonin treatment alleviates chilling injury in mango fruit ‘Keitt’ by modulating proline metabolism under chilling stress [J]. J Integr Agric, 2023, 22(3): 935-944. |
| [67] | Ghosh UK, Islam MN, Siddiqui MN, et al. Understanding the roles of osmolytes for acclimatizing plants to changing environment: a review of potential mechanism [J]. Plant Signal Behav, 2021, 16(8): 1913306. |
| [68] | Aghdam MS, Luo ZS, Jannatizadeh A, et al. Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity [J]. Food Chem, 2019, 275: 549-556. |
| [69] | Cao SF, Song CB, Shao JR, et al. Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage [J]. J Agric Food Chem, 2016, 64(25): 5215-5222. |
| [70] | Breton G, Danyluk J, Charron JF, et al. Expression profiling and bioinformatic analyses of a novel stress-regulated multispanning transmembrane protein family from cereals and Arabidopsis [J]. Plant Physiol, 2003, 132(1): 64-74. |
| [71] | Du HY, Liu GT, Hua CM, et al. Exogenous melatonin alleviated chilling injury in harvested plum fruit via affecting the levels of polyamines conjugated to plasma membrane [J]. Postharvest Biol Technol, 2021, 179: 111585. |
| [72] | Szafrańska K, Szewczyk R, Janas KM. Involvement of melatonin applied to Vigna radiata L. seeds in plant response to chilling stress [J]. Cent Eur J Biol, 2014, 9(11): 1117-1126. |
| [73] | Jaglo KR, Kleff S, Amundsen KL, et al. Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species [J]. Plant Physiol, 2001, 127(3): 910-917. |
| [74] | Zhou XT, Zhao HL, Cao K, et al. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress [J]. Front Plant Sci, 2016, 7: 1823. |
| [75] | Wei J, Li DX, Zhang JR, et al. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana [J]. J Pineal Res, 2018, 65(2): e12500. |
| [76] | Li DX, Wei J, Peng ZP, et al. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis [J]. J Pineal Res, 2020, 68(3): e12640. |
| [77] | Barman D, Kumar MN, Dalal M, et al. Identification of rice melatonin receptor OsPMTR and its comparative in silico analysis with Arabidopsis AtCAND2 receptor [J]. S Afr N J Bot, 2023, 162: 813-829. |
| [78] | Kong MM, Sheng T, Liang J, et al. Melatonin and its homologs induce immune responses via receptors trP47363-trP13076 in Nicotiana benthamiana [J]. Front Plant Sci, 2021, 12: 691835. |
| [79] | Wang LF, Lu KK, Li TT, et al. Maize PHYTOMELATONIN RECEPTOR1 functions in plant tolerance to osmotic and drought stress [J]. J Exp Bot, 2022, 73(17): 5961-5973. |
| [80] | Zhan HS, Nie XJ, Zhang T, et al. Melatonin: a small molecule but important for salt stress tolerance in plants [J]. Int J Mol Sci, 2019, 20(3): 709. |
| [81] | Dodd AN, Kudla J, Sanders D. The language of calcium signaling [J]. Annu Rev Plant Biol, 2010, 61: 593-620. |
| [82] | Ranty B, Aldon D, Cotelle V, et al. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses [J]. Front Plant Sci, 2016, 7: 327. |
| [83] | Chang JJ, Guo YL, Li JY, et al. Positive interaction between H2O2 and Ca2+ mediates melatonin-induced CBF pathway and cold tolerance in watermelon (Citrullus lanatus L.) [J]. Antioxidants, 2021, 10(9): 1457. |
| [84] | Chin K, DeFalco TA, Moeder W, et al. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition [J]. Plant Physiol, 2013, 163(2): 611-624. |
| [85] | Lu M, Zhang YY, Tang SK, et al. AtCNGC2 is involved in jasmonic acid-induced calcium mobilization [J]. J Exp Bot, 2016, 67(3): 809-819. |
| [86] | Ma C, Pei ZQ, Zhu Q, et al. Melatonin-mediated low-temperature tolerance of cucumber seedlings requires Ca2+/CPKs signaling pathway [J]. Plant Physiol Biochem, 2024, 214: 108962. |
| [87] | Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants [J]. New Phytol, 2003, 159(1): 11-35. |
| [88] | Arnao MB, Hernández-Ruiz J. Functions of melatonin in plants: a review [J]. J Pineal Res, 2015, 59(2): 133-150. |
| [89] | Feng YQ, Fu X, Han LJ, et al. Nitric oxide functions as a downstream signal for melatonin-induced cold tolerance in cucumber seedlings [J]. Front Plant Sci, 2021, 12: 686545. |
| [90] | Eremina M, Rozhon W, Poppenberger B. Hormonal control of cold stress responses in plants [J]. Cell Mol Life Sci, 2016, 73(4): 797-810. |
| [91] | Zhang HJ, Zhang N, Yang RC, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA₄ interaction in cucumber (Cucumis sativus L.) [J]. J Pineal Res, 2014, 57(3): 269-279. |
| [92] | Chang JJ, Guo YL, Zhang ZX, et al. CBF-responsive pathway and phytohormones are involved in melatonin-improved photosynthesis and redox homeostasis under aerial cold stress in watermelon [J]. Acta Physiol Plant, 2020, 42(10): 159. |
| [93] | Li JD, Lv K, Wu JP, et al. Exogenous melatonin promotes cold tolerance in grape seedlings: physiological, transcriptomic, and functional evidence [J]. J Agric Food Chem, 2023, 71(50): 19970-19985. |
| [94] | Li H, Guo YL, Lan ZX, et al. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants [J]. Hortic Res, 2021, 8(1): 57. |
| [95] | Ren CY, Wang HM, Zhou ZH, et al. Genome-wide identification of the B3 gene family in soybean and the response to melatonin under cold stress [J]. Front Plant Sci, 2023, 13: 1091907. |
| [96] | Gong B, Yan YY, Wen D, et al. Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum [J]. Physiol Plant, 2017, 160(4): 396-409. |
| [97] | Lee HY, Back K. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana [J]. J Pineal Res, 2017, 62(2): e12379. |
| [98] | Ma C, Pei ZQ, Bai X, et al. Involvement of NO and Ca2+ in the enhancement of cold tolerance induced by melatonin in winter turnip rape (Brassica rapa L.) [J]. Plant Physiol Biochem, 2022, 190: 262-276. |
| [99] | Weeda S, Zhang N, Zhao XL, et al. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems [J]. PLoS One, 2014, 9(3): e93462. |
| [100] | Taj G, Agarwal P, Grant M, et al. MAPK machinery in plants [J]. Plant Signal Behav, 2010, 5(11): 1370-1378. |
| [101] | Sinha AK, Jaggi M, Raghuram B, et al. Mitogen-activated protein kinase signaling in plants under abiotic stress [J]. Plant Signal Behav, 2011, 6(2): 196-203. |
| [102] | Kimball SR, Abbas A, Jefferson LS. Melatonin represses oxidative stress-induced activation of the MAP kinase and mTOR signaling pathways in H4IIE hepatoma cells through inhibition of Ras [J]. J Pineal Res, 2008, 44(4): 379-386. |
| [103] | Yu Y, Lv Y, Shi YN, et al. The role of Phyto-melatonin and related metabolites in response to stress [J]. Molecules, 2018, 23(8): 1887. |
| [104] | Lee HY, Back K. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants [J]. J Pineal Res, 2016, 60(3): 327-335. |
| [105] | Hayat S, Ahmad, Alyemeni M. Salicylic Acid: Plant Growth and Development[M]. Dordrecht: Springer Science & Business Media, 2013. |
| [106] | Kang K, Lee K, Park S, et al. Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings [J]. J Pineal Res, 2010, 49(2): 176-182. |
| [107] | Khan M, Ali S, Manghwar H, et al. Melatonin function and crosstalk with other phytohormones under normal and stressful conditions [J]. Genes, 2022, 13(10): 1699. |
| [108] | Arnao MB, Hernández-Ruiz J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations [J]. Plant Biol, 2021, 23(): 7-19. |
| [109] | Raza A, Charagh S, García-Caparrós P, et al. Melatonin-mediated temperature stress tolerance in plants [J]. GM Crops Food, 2022, 13(1): 196-217. |
| [1] | 张学琼, 潘素君, 李魏, 戴良英. 植物磷酸盐转运蛋白在胁迫响应中的研究进展[J]. 生物技术通报, 2025, 41(7): 28-36. |
| [2] | 李新妮, 李俊怡, 马雪华, 何卫, 李佳丽, 于佳, 曹晓宁, 乔治军, 刘思辰. 谷子果胶甲酯酶抑制子PMEI基因家族鉴定及其对非生物胁迫的响应分析[J]. 生物技术通报, 2025, 41(7): 150-163. |
| [3] | 高婧, 陈益存, 高暝, 赵耘霄, 汪阳东. 植物单宁合成调控及其对环境的响应机制[J]. 生物技术通报, 2025, 41(7): 49-59. |
| [4] | 李思博, 钱虹萍, 徐昌文, 王笑, 林金星, 崔亚宁. 植物内源激发子调控的胞内转运参与植物生长发育和逆境胁迫响应的研究进展[J]. 生物技术通报, 2025, 41(7): 17-27. |
| [5] | 王从欢, 伍国强, 魏明. 植物CBL调控逆境胁迫响应的作用机制[J]. 生物技术通报, 2025, 41(7): 1-16. |
| [6] | 郭秀娟, 冯宇, 吴瑞香, 王利琴, 杨建春. Ca2+处理对胡麻种子萌发影响的转录组分析[J]. 生物技术通报, 2025, 41(7): 139-149. |
| [7] | 张钧杰, 刘爽, 胡明珠, 石雪瑞, 代金霞. 荒漠植物根际土壤固氮微生物的筛选及其抗逆促生特性[J]. 生物技术通报, 2025, 41(6): 317-326. |
| [8] | 何卫, 李俊怡, 李新妮, 马雪华, 邢媛, 曹晓宁, 乔治军, 刘思辰. 谷子泛素连接酶U-box E3基因家族的鉴定及响应非生物胁迫分析[J]. 生物技术通报, 2025, 41(5): 104-118. |
| [9] | 胡若群, 曾菁菁, 梁婉凤, 曹佳玉, 黄小苇, 梁晓英, 仇明月, 陈莹. 转录组和代谢组联合分析探究不同遮光条件下金线莲类胡萝卜素合成代谢机制[J]. 生物技术通报, 2025, 41(5): 231-243. |
| [10] | 姜丽思, 李文远, 张雨祺, 杨洋雯迪, 刘子瑞, 富薇. 纳米二氧化钛对植物的毒性效应研究进展[J]. 生物技术通报, 2025, 41(5): 32-41. |
| [11] | 刘园园, 陈析丰, 钱前, 高振宇. 水稻穗发育调控的分子机制研究进展[J]. 生物技术通报, 2025, 41(5): 1-13. |
| [12] | 樊玥妮, 仙保山, 师艺萍, 任梦圆, 徐佳慧, 魏绍巍, 许晓敬, 罗晓峰, 舒凯. SPINDLY和SECRET AGENT介导的蛋白糖基化调控植物发育与逆境响应[J]. 生物技术通报, 2025, 41(4): 1-8. |
| [13] | 夏馨媛, 薛道晟, 李鑫静, 龙俊杰, 陆开形, 丁沃娜, 李梦莎. 稻油轮作土壤多功能促生菌的鉴定及其对油菜生长和根际细菌群落的影响[J]. 生物技术通报, 2025, 41(4): 289-301. |
| [14] | 唐游, 赵俊伟, 孙兰茜, 李翔. 多组学技术在植物代谢通路解析中的联合应用[J]. 生物技术通报, 2025, 41(4): 76-87. |
| [15] | 田琴, 刘奎, 吴翔纬, 纪媛媛, 曹一博, 张凌云. 转录因子VcMYB17调控蓝莓抗旱性的功能研究[J]. 生物技术通报, 2025, 41(4): 198-210. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||