








生物技术通报 ›› 2025, Vol. 41 ›› Issue (11): 177-189.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0392
• 未来食品工程专题 • 上一篇
徐远志1,2( ), 胡珊2, 代思泽2, 游帅1, 郑明明1,2(
), 胡珊2, 代思泽2, 游帅1, 郑明明1,2( ), 单凯3
), 单凯3
收稿日期:2025-04-17
出版日期:2025-11-26
发布日期:2025-12-09
通讯作者:
郑明明,男,博士,教授,研究方向 :高活性酶制剂创制和功能性脂质开发;E-mail: zhengmingming@caas.cn作者简介:徐远志,男,硕士研究生,研究方向 :油脂加工;E-mail: 2292385667@qq.com
基金资助:
XU Yuan-zhi1,2( ), HU Shan2, DAI Si-ze2, YOU Shuai1, ZHENG Ming-ming1,2(
), HU Shan2, DAI Si-ze2, YOU Shuai1, ZHENG Ming-ming1,2( ), SHAN Kai3
), SHAN Kai3
Received:2025-04-17
Published:2025-11-26
Online:2025-12-09
摘要:
目的 针对国产脂肪酶催化效率低、热稳定性差等问题,筛选高活性脂肪酶菌株,解析催化特性用于酶法高效制备甘油二酯。 方法 采用中性红橄榄油平板初筛及对硝基苯酚比色法复筛的方法,从富含油脂的土壤中筛选高产脂肪酶菌株,结合形态学与16S rDNA序列分析进行菌种鉴定,通过PCR扩增获得高活性脂肪酶基因序列,探究其酶学性质,建立无溶剂体系评价其甘油二酯合成效率。 结果 从18份土壤样品中成功发掘到一株高产脂肪酶菌株E12C,胞外酶活为(80 826.4±1 838.9)U/L,经形态学与16S rDNA序列鉴定为洋葱伯克霍尔德菌(Burkholderia cepacia),命名为B. cepacian OCRI-Lip100,已保藏于中国典型培养物保藏中心。该脂肪酶命名为Lip-12c,最适反应温度为60 ℃、最适反应pH为9.0,在此条件下酶活为(190 761.2±5 181.5)U/L,比活力为(39 254.5±271.3)U/g蛋白,显著高于进口脂肪酶,在40-70 ℃和pH 5.0-10.0范围可维持较高活性,金属离子Na+和Mg2+对酶活性提升效果显著。在无溶剂体系中40 ℃酶法水解橄榄油4 h,甘油二酯含量达33.5%。 结论 土壤中发掘的高产脂肪酶菌株B. cepacian OCRI-Lip100,在无溶剂体系中展现高效的甘油二酯合成能力,不仅丰富了现有脂肪酶菌种资源库,还为功能脂质的高效生物制造提供了技术支撑。
徐远志, 胡珊, 代思泽, 游帅, 郑明明, 单凯. 高活性脂肪酶的发掘、评价及在甘油二脂合成中应用[J]. 生物技术通报, 2025, 41(11): 177-189.
XU Yuan-zhi, HU Shan, DAI Si-ze, YOU Shuai, ZHENG Ming-ming, SHAN Kai. Discovery and Evaluation of High-activity Lipase and Its Application in Diacylglycerol Synthesis[J]. Biotechnology Bulletin, 2025, 41(11): 177-189.
引物名称 Primer name | 引物序列 Sequence of primer(5′-3′) | 功能 Function |
|---|---|---|
| 27-F | AGAGTTTGATCCTGGCTCAG | 扩增16S rDNA序列用于菌种鉴定 |
| 1492-R | TACGGCTACCTTGTTACGACTT | |
| F | CGTGCTTCACTCCGCATT | 扩增脂肪酶前段序列bclipF |
| R | GACCGGGTCTTCCGCA | |
| 12C-F | TTCGTCAATGTATTCGGCA | 扩增脂肪酶后段序列bclipR |
| 11-R | GAGCGCATCGAGATACGC | |
| KZ-F | CAAATGGGTCGCGGATCC | 扩增完整脂肪酶序列bclip12c |
| KZ-R | TCGAGTGCGGCCGC |
表1 PCR中用到的主要引物
Table 1 Main primers used in PCR
引物名称 Primer name | 引物序列 Sequence of primer(5′-3′) | 功能 Function |
|---|---|---|
| 27-F | AGAGTTTGATCCTGGCTCAG | 扩增16S rDNA序列用于菌种鉴定 |
| 1492-R | TACGGCTACCTTGTTACGACTT | |
| F | CGTGCTTCACTCCGCATT | 扩增脂肪酶前段序列bclipF |
| R | GACCGGGTCTTCCGCA | |
| 12C-F | TTCGTCAATGTATTCGGCA | 扩增脂肪酶后段序列bclipR |
| 11-R | GAGCGCATCGAGATACGC | |
| KZ-F | CAAATGGGTCGCGGATCC | 扩增完整脂肪酶序列bclip12c |
| KZ-R | TCGAGTGCGGCCGC |
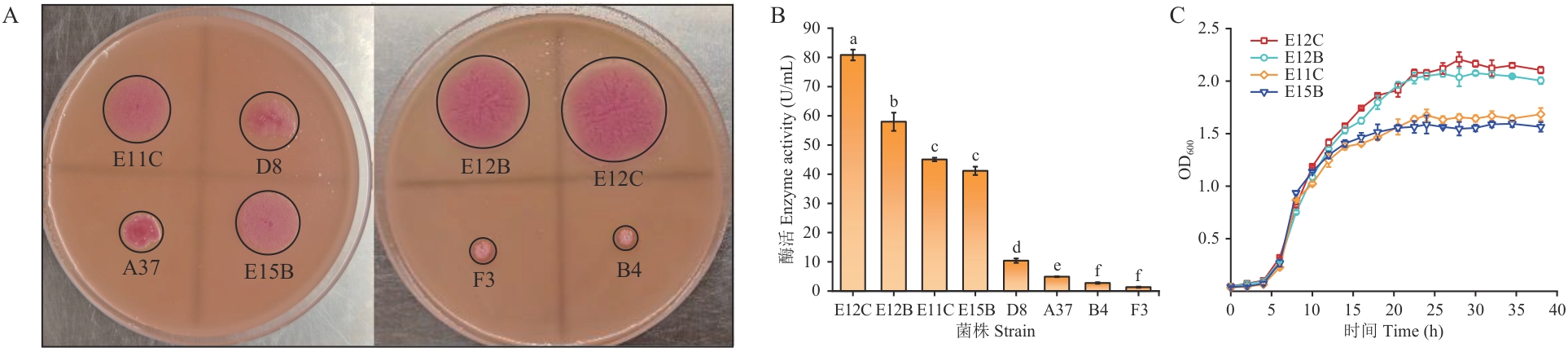
图1 产脂肪酶菌株的发掘和生长曲线A:产脂肪酶菌株在平板上的变色圈;B:各菌株的脂肪酶活力;C:菌株生长曲线。不同字母表示有显著差异(P≤0.05),下同
Fig. 1 Excavation and growth curve of lipase-producing strainsA: Discoloration circle of lipase-producing strains on the plate. B: Lipase activity of each strain. C: Strain growth curve. Different letters indicate significant differences (P≤0.05), the same below
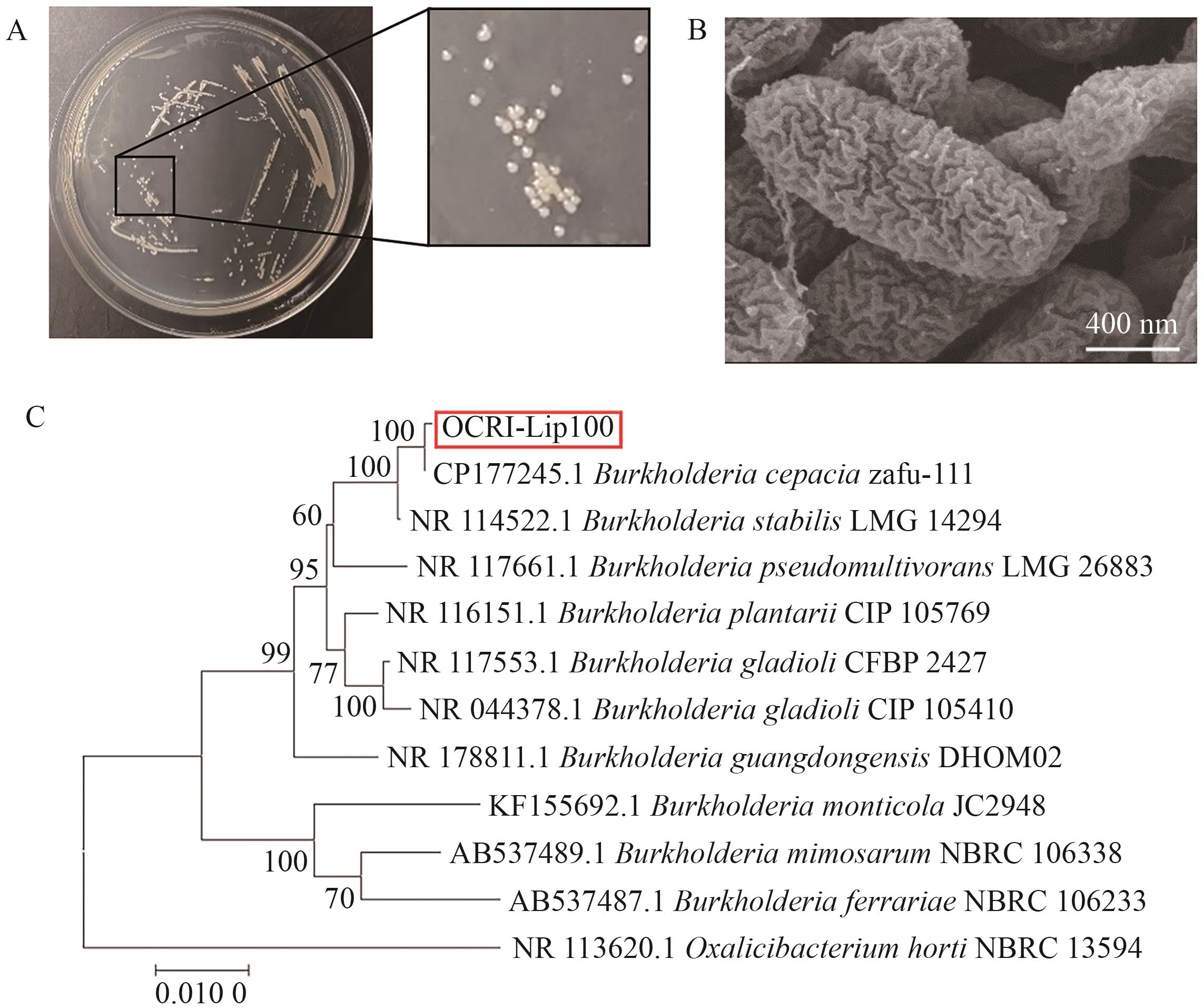
图2 菌株E12C的鉴定A:E12C菌株在LB平板上的菌落形态;B:在400 nm尺寸下的扫描电镜;C:E12C菌株的16S rDNA系统发育树
Fig. 2 Identification of strain E12CA: Colony morphology of E12C strain on LB plate. B: Scanning electron microscope at 400 nm. C: Phylogenetic tree of16S rDNA for strain E12C
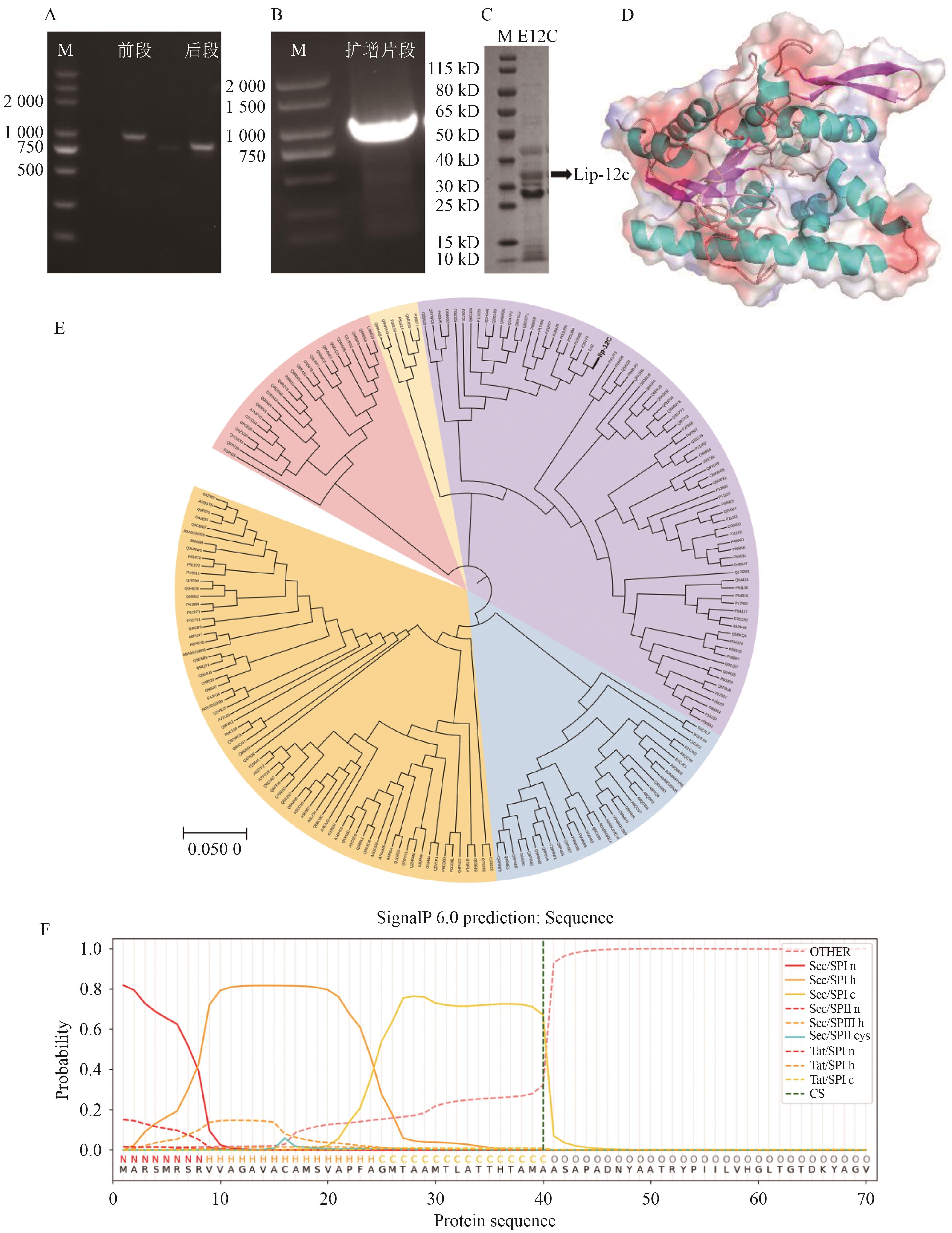
图3 脂肪酶Lip-12c的基因鉴定A:脂肪酶基因序列bclipF和bclipR;B:成熟脂肪酶基因序列bclip12c;C:脂肪酶Lip-12c的SDS-PAGE结果分析;D:预测的脂肪酶Lip-12c三维结构;E:脂肪酶Lip-12c的系统发育树图;F:信号肽预测结果
Fig. 3 Identification of lipase Lip-12c geneA: Lipase gene sequence bclipF and bclipR. B: Mature lipase gene sequence bclip12c. C: Analysis of SDS-PAGE results of lipase Lip-12c. D: Predicted three-dimensional structure of lipase Lip-12c. E: Phylogenetic tree of lipase Lip-12c. F: Signal peptide prediction results
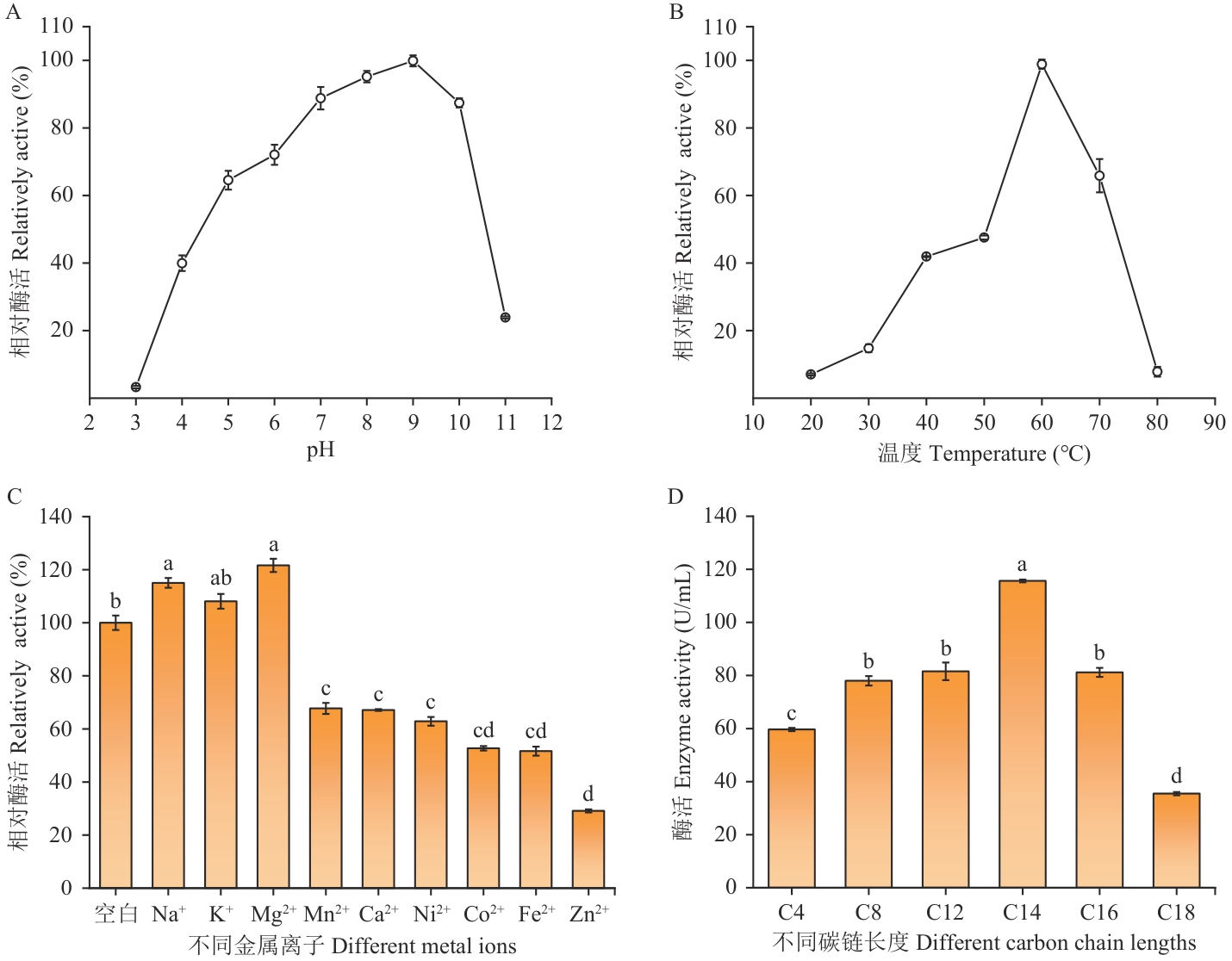
图4 酶学性质分析A:pH对酶活的影响;B:温度对酶活的影响;C:金属离子对酶活的影响;D:底物链长特异性
Fig. 4 Analysis of enzymatic propertiesA: Effect of pH on enzyme activity. B: Effect of temperature on enzyme activity. C: Effect of metal ions on enzyme activity. D: Substrate chain length specificity
有机溶剂 Organic solvent | 油水分配系数 Log P | 相对酶活 Relative activity (%) | 有机溶剂 Organic solvent | 油水分配系数 Log P | 相对酶活 Relative activity (%) |
|---|---|---|---|---|---|
| 无有机溶剂 | - | 100 ± 1.44ab | 乙酸 | -0.17 | 70.62 ± 1.08g |
| 正己烷 | 3.5 | 85.37 ± 1.77de | 丙酮 | -0.24 | 77.44 ± 2.71f |
| 环己烷 | 3.44 | 88.50 ± 1.03d | 乙醇 | -0.31 | 91.69 ± 1.48c |
| 二氯甲烷 | 1.25 | 77.01 ± 0.71f | 乙腈 | -0.33 | 90.66 ± 2.97cd |
| 乙醚 | 0.85 | 103.68 ± 1.29a | 甲醇 | -0.77 | 97.23 ± 0.76bc |
| 乙酸乙酯 | 0.68 | 81.44 ± 0.64e | 丙三醇 | -1.76 | 87.42 ± 0.74d |
| 异丙醇 | 0.05 | 79.94 ± 1.16ef |
表2 有机溶剂对脂肪酶Lip-12c活力的影响
Table 2 Effect of organic solvents on the activity of lipase lip-12c
有机溶剂 Organic solvent | 油水分配系数 Log P | 相对酶活 Relative activity (%) | 有机溶剂 Organic solvent | 油水分配系数 Log P | 相对酶活 Relative activity (%) |
|---|---|---|---|---|---|
| 无有机溶剂 | - | 100 ± 1.44ab | 乙酸 | -0.17 | 70.62 ± 1.08g |
| 正己烷 | 3.5 | 85.37 ± 1.77de | 丙酮 | -0.24 | 77.44 ± 2.71f |
| 环己烷 | 3.44 | 88.50 ± 1.03d | 乙醇 | -0.31 | 91.69 ± 1.48c |
| 二氯甲烷 | 1.25 | 77.01 ± 0.71f | 乙腈 | -0.33 | 90.66 ± 2.97cd |
| 乙醚 | 0.85 | 103.68 ± 1.29a | 甲醇 | -0.77 | 97.23 ± 0.76bc |
| 乙酸乙酯 | 0.68 | 81.44 ± 0.64e | 丙三醇 | -1.76 | 87.42 ± 0.74d |
| 异丙醇 | 0.05 | 79.94 ± 1.16ef |
脂肪酶 Lipase | 蛋白浓度 Protein concentration (mg/mL) | 比活力 Specific activity (U/g protein) | 酶来源 Enzyme source |
|---|---|---|---|
| Lip-12c | 1.57 ± 0.04b | 39 254.5 ± 271.3a | 本研究 |
| Candida rugosa lipase AY 400SD | 2.49 ± 0.12a | 6 171.1 ± 507.3b | 商品酶 |
| C. rugosa lipase CRL | 1.21 ± 0.18b | 3 376.6 ± 223.3c | |
| C. rugosa lipase AY SD | 0.27 ± 0.05c | 298.0 ± 31.3d | |
| C. rugosa lipase AYS | 0.26 ± 0.05c | 220.1 ± 15.5d | |
| Geobacillus thermocatenulatus KCTC | - | 22 700 | [ |
| Aeromonas caviae LipT51 | - | 34 200 | [ |
表3 不同脂肪酶的比活力对比
Table 3 Comparison of specific activity of different lipases
脂肪酶 Lipase | 蛋白浓度 Protein concentration (mg/mL) | 比活力 Specific activity (U/g protein) | 酶来源 Enzyme source |
|---|---|---|---|
| Lip-12c | 1.57 ± 0.04b | 39 254.5 ± 271.3a | 本研究 |
| Candida rugosa lipase AY 400SD | 2.49 ± 0.12a | 6 171.1 ± 507.3b | 商品酶 |
| C. rugosa lipase CRL | 1.21 ± 0.18b | 3 376.6 ± 223.3c | |
| C. rugosa lipase AY SD | 0.27 ± 0.05c | 298.0 ± 31.3d | |
| C. rugosa lipase AYS | 0.26 ± 0.05c | 220.1 ± 15.5d | |
| Geobacillus thermocatenulatus KCTC | - | 22 700 | [ |
| Aeromonas caviae LipT51 | - | 34 200 | [ |
| [15] | Chandra P, Enespa, Singh R, et al. Microbial lipases and their industrial applications: a comprehensive review [J]. Microb Cell Fact, 2020, 19(1): 169. |
| [16] | 张静, 贾佳, 董帅豪, 等. 国产毕赤酵母菌脂肪酶催化酯交换制备零反式、低饱和人造奶油基料油 [J]. 中国油脂, 2024, 49(8): 120-126. |
| Zhang J, Jia J, Dong SH, et al. Preparation of zero trans, low saturated margarine base oil by transesterification catalyzed by domestic Pichia pastoris lipase [J]. China Oils and Fats, 2024, 49(8): 120-126. | |
| [17] | 徐碧林, 朱庆. 微生物脂肪酶稳定性研究进展 [J]. 微生物学通报, 2020, 47(6): 1958-1972. |
| Xu BL, Zhu Q. Recent advances in stability studies of microbial lipase [J]. Microbiol China, 2020, 47(6): 1958-1972. | |
| [18] | Mahadik ND, Puntambekar US, Bastawde KB, et al. Production of acidic lipase by Aspergillus niger in solid state fermentation [J]. Process Biochem, 2002, 38(5): 715-721. |
| [19] | 布坎南 吉本斯. 伯杰细菌鉴定手册: 第八版 [M]. 8版. 北京: 科学出版社, 1984. |
| Buchanan G. Bergey’s manual of determinative bacteriology [M]. 8th ed. Beijing: Science Press, 1984. | |
| [20] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册 [M]. 北京: 科学出版社, 2001. |
| Dong XZ, Cai MY. Handbook of identification of common bacterial systems [M]. Beijing: Science Press, 2001. | |
| [21] | 贾彬. 洋葱伯克霍尔德菌的筛选、鉴定及其脂肪酶基因的高效表达 [D]. 武汉: 华中科技大学, 2010. |
| Jia B. Screening and identification of Burkholderia cepacia and its high-level expression of lipase gene [D]. Wuhan: Huazhong University of Science and Technology, 2010. | |
| [22] | Xu LJ, Zhang Y, Zivkovic V, et al. Deacidification of high-acid rice bran oil by the tandem continuous-flow enzymatic reactors [J]. Food Chem, 2022, 393: 133440. |
| [23] | Mezzetti A, Schrag JD, Cheong CS, et al. Mirror-image packing in enantiomer discrimination molecular basis for the enantioselectivity of B.cepacia lipase toward 2-methyl-3-phenyl-1-propanol [J]. Chem Biol, 2005, 12(4): 427-437. |
| [24] | Gurkok S, Ozdal M. Purification and characterization of a novel extracellular, alkaline, thermoactive, and detergent-compatible lipase from Aeromonas caviae LipT51 for application in detergent industry [J]. Protein Expr Purif, 2021, 180: 105819. |
| [25] | Jo E, Kim J, Lee A, et al. Identification and characterization of a novel thermostable GDSL-type lipase from Geobacillus thermocatenulatus [J]. J Microbiol Biotechnol, 2021, 31(3): 483-491. |
| [26] | 李桥妹, 王宗德, 谢礼文, 等. 酶法制备富含中碳链甘油二酯的山苍子核仁油工艺研究 [J]. 中国粮油学报, 2021, 36(8): 54-59. |
| Li QM, Wang ZD, Xie LW, et al. Preparation of litseacubeba kernel oil rich in medium-chain diacylglycerol byenzymaticmethod [J]. J Chin Cereals Oils Assoc, 2021, 36(8): 54-59. | |
| [27] | Liu R, Zhang Y, Xu YZ, et al. Molecular docking simulation reveals the lipase-substrate binding mechanism in the enzymatic synthesis of diacylglycerol-enriched vegetable oils [J]. Food Chem, 2025, 474: 143236. |
| [28] | Sánchez DA, Tonetto GM, Ferreira ML. Burkholderia cepacia lipase: a versatile catalyst in synthesis reactions [J]. Biotechnol Bioeng, 2018, 115(1): 6-24. |
| [29] | 秦蓁天, 王小兵, 汪晓丽, 等. 餐厨垃圾高效油脂降解菌的筛选及其降解条件研究 [J]. 扬州大学学报: 农业与生命科学版, 2023, 44(5): 118-126, 134. |
| Qin ZT, Wang XB, Wang XL, et al. Screening of high-efficiency oil-degrading bacteria from kitchen waste and study on its degradation conditions [J]. Journal of Yangzhou University: Agricultural and Life Science Edition, 2023, 44(5): 118-126, 134. | |
| [30] | Tavares M, Kozak M, Balola A, et al. Burkholderia cepacia complex bacteria: a feared contamination risk in water-based pharmaceutical products [J]. Clin Microbiol Rev, 2020, 33(3): e00139-19. |
| [31] | 李仰龙, 魏书蒙, 陈详腾, 等. 洋葱伯克霍尔德菌菌株JLS 17对Cd2+胁迫的生长及代谢响应机制 [J]. 林业科学, 2024, 60(8): 152-163. |
| [1] | Xu LL, Li JX, Zhang HJ, et al. Biological modification and industrial applications of microbial lipases: a general review [J]. Int J Biol Macromol, 2025, 302: 140486. |
| [2] | 陈善辉, 于爱霞, 王东, 等. 甘油二酯安全性、代谢及减肥降脂功能研究进展 [J]. 食品工业, 2024, 45(11): 211-217. |
| Chen SH, Yu AX, Wang D, et al. Research progress on safety, metabolism, weight loss and lipid-lowering function of diglycerides [J]. The Food Industry, 2024, 45(11): 211-217. | |
| [3] | Zeng Q, Sun MM, Xie X, et al. Lipase-entrapped colloidosomes with tunable positioning at the oil-water interface for Pickering emulsion-enhanced biocatalysis [J]. ACS Appl Mater Interfaces, 2022, 14(49): 54781-54789. |
| [4] | Akram F, Mir AS, Haq IU, et al. An appraisal on prominent industrial and biotechnological applications of bacterial lipases [J]. Mol Biotechnol, 2023, 65(4): 521-543. |
| [5] | Yan QF, Li ZY, Sun RJ, et al. Promoted expression of a lipase for its application in EPA/DHA enrichment and mechanistic insights into its substrate specificity [J]. Int J Biol Macromol, 2025, 296: 139628. |
| [6] | Wang J, Liu X, Wang XD, et al. Selective synthesis of human milk fat-style structured triglycerides from microalgal oil in a microfluidic reactor packed with immobilized lipase [J]. Bioresour Technol, 2016, 220: 132-141. |
| [7] | Zhang TT, Zhang Y, Deng CY, et al. Green and efficient synthesis of highly liposoluble and antioxidant L-ascorbyl esters by immobilized lipases [J]. J Clean Prod, 2022, 379: 134772. |
| [8] | Zhang SY, Hyatt JR, Akoh CC. Lipase-catalyzed one-step regioselective synthesis of 1, 2-dioctanoylgalloylglycerol in a solvent-free system: Optimization of reaction conditions and structural elucidation [J]. Food Chem, 2022, 382: 132302. |
| [9] | Zhang YF, Lai YD, Zheng MM. Ultrasound-assisted intensification of Pickering interfacial biocatalysis preparation of vitamin A aliphatic esters [J]. Ultrason Sonochem, 2024, 107: 106929. |
| [10] | Li DY, Chen XY, Chen ZC, et al. Directed evolution of lipase A from Bacillus subtilis for the preparation of enantiocomplementary sec-alcohols [J]. Green Synth Catal, 2021, 2(3): 290-294. |
| [11] | Alabdalall AH, Al-Anazi NA, Aldakheel LA, et al. Application and characterization of crude fungal lipases used to degrade fat and oil wastes [J]. Sci Rep, 2021, 11(1): 19670. |
| [12] | Yang XX, Zhang Y, Pang HM, et al. Codisplay of Rhizopus oryzae and Candida rugosa lipases for biodiesel production [J]. Catalysts, 2021, 11(4): 421. |
| [13] | Zulaika A, Rahman H, Ningrum SS, et al. Exploring microbial lipases: Screening and identification for biocatalyst potential in bioethanol synthesis from glycerol-based biodiesel waste [J]. Results Eng, 2024, 23: 102427. |
| [14] | Ismail AR, Kashtoh H, Baek KH. Temperature-resistant and solvent-tolerant lipases as industrial biocatalysts: Biotechnological approaches and applications [J]. Int J Biol Macromol, 2021, 187: 127-142. |
| [31] | Li YL, Wei SM, Chen XT, et al. Growth and metabolic response mechanism of Burkholderia cepacia strain JLS17 to Cd2+ stress [J]. Scientia Silvae Sinicae, 2024, 60(8): 152-163. |
| [32] | Chen G, Zhang QP, Chen HT, et al. In situ and real-time insight into Rhizopus chinensis lipase under high pressure and temperature: Conformational traits and biobehavioural analysis [J]. Int J Biol Macromol, 2020, 154: 1314-1323. |
| [33] | Abd Razak NN, Cognet P, Pérès Y, et al. Kinetics and hydrodynamics of Candida antartica lipase-catalyzed synthesis of glycerol dioleate (GDO) in a continuous flow packed-bed millireactor [J]. J Clean Prod, 2022, 373: 133816. |
| [34] | Rasmussen HØ, Wollenberg DTW, Wang HB, et al. The changing face of SDS denaturation: complexes of Thermomyces lanuginosus lipase with SDS at pH 4.0, 6.0 and 8.0 [J]. J Colloid Interface Sci, 2022, 614: 214-232. |
| [35] | Karaman F, Incekara U, Arslan NP, et al. A new enzyme for biodiesel production and food applications: lipase of Bacillus megateriumF25 isolated from an aquatic insect Rhantus suturalis [J]. GCB Bioenergy, 2024, 16(12): e70009. |
| [36] | Karabulut I, Durmaz G, Hayaloglu AA. Fatty acid selectivity of lipases during acidolysis reaction between triolein and saturated fatty acids varying from caproic to behenic acids [J]. J Agric Food Chem, 2009, 57(16): 7584-7590. |
| [37] | Cong YX, Liu YW, Yuan MY, et al. Efficient preparation of diglycerides from rapeseed oil sediment using phospholipase C in a solvent-free phospholipid hydrolysis system [J]. Food Biosci, 2024, 62: 105175. |
| [38] | Liu YS, Yang ZH, Zhou XM, et al. Diacylglycerol kinases and its role in lipid metabolism and related diseases [J]. Int J Mol Sci, 2024, 25(23): 13207. |
| [1] | 苏秀敏, 韩文清, 王佼, 李鹏, 王秋兰, 李万星, 曹晋军. 哈茨木霉M408的分离鉴定、生物学特性及对番茄早疫病的生防效果[J]. 生物技术通报, 2025, 41(9): 277-288. |
| [2] | 廉少杰, 唐胜硕, 康传利, 刘磊, 郑德强, 杜帅, 汤丽伟, 张美霞, 刘蔷. 高产银耳多糖酶菌株的分离、鉴定、发酵条件优化及其酶的特性分析[J]. 生物技术通报, 2025, 41(9): 302-313. |
| [3] | 闫梦阳, 梁晓阳, 戴君昂, 张妍, 关团, 张辉, 刘良波, 孙志华. 阿莫西林降解菌的筛选及降解机制研究[J]. 生物技术通报, 2025, 41(9): 314-325. |
| [4] | 张茹, 李一鸣, 张桐溪, 孙占斌, 任清, 潘寒姁. 厚朴中1株高产厚朴酚与和厚朴酚菌株的分离鉴定及其“发汗”工艺优化[J]. 生物技术通报, 2025, 41(8): 322-334. |
| [5] | 项波卡, 周钻钻, 冯佳卉, 夏琛, 李奇, 陈春. 一株烟叶霉变真菌的分离鉴定及其致霉因素研究[J]. 生物技术通报, 2025, 41(2): 321-330. |
| [6] | 叶妍, 吴雨萱, 周哲敏, 崔文璟. 转氨酶新酶的挖掘、表征及在2-氨基丁酸生物转化中的应用[J]. 生物技术通报, 2025, 41(11): 134-142. |
| [7] | 张亚亚, 李盼盼, 高惠惠, 贾晨波, 徐春燕. 基于根表真菌群落与病原菌鉴定探究‘宁杞5号’枸杞根腐病的发生机制[J]. 生物技术通报, 2024, 40(9): 238-248. |
| [8] | 张曼玉, 董嘉诚, 苟福凡, 弓朝晖, 刘倩, 孙文良, 孔臻, 郝捷, 王敏, 田朝光. 嗜热毁丝霉果胶酯酶MtCE12-1的克隆表达及其酶学性质和应用研究[J]. 生物技术通报, 2024, 40(9): 291-300. |
| [9] | 王芳, 于璐, 齐泽铮, 周长军, 于吉东. 大豆镰刀菌根腐病拮抗菌的筛选及生防效果[J]. 生物技术通报, 2024, 40(7): 216-225. |
| [10] | 殷亮, 王代玮, 刘悦莹, 刘海燕, 罗光宏. 蛋白酶SpP1基因克隆、表达及酶学性质的表征[J]. 生物技术通报, 2024, 40(4): 278-286. |
| [11] | 王璐, 刘梦雨, 张富源, 纪守坤, 王云, 张英杰, 段春辉, 刘月琴, 严慧. 瘤胃源粪臭素降解菌的分离鉴定及其降解特性研究[J]. 生物技术通报, 2024, 40(3): 305-311. |
| [12] | 郑菲, 杨俊钊, 牛羽丰, 李蕊麟, 赵国柱. 嗜热毁丝菌裂解性多糖单加氧酶TtLPMO9I的酶学性质及其功能研究[J]. 生物技术通报, 2024, 40(2): 289-299. |
| [13] | 陈金行, 张逸, 张军涛, 未本美, 王宏勋, 郑明明. 固定化脂肪酶的创制及其在乙酸肉桂酯无溶剂制备中的应用[J]. 生物技术通报, 2023, 39(9): 97-104. |
| [14] | 饶紫环, 谢志雄. 一株Olivibacter jilunii 纤维素降解菌株的分离鉴定与降解能力分析[J]. 生物技术通报, 2023, 39(8): 283-290. |
| [15] | 游子娟, 陈汉林, 邓辅财. 鱼皮生物活性肽的提取及功能活性研究进展[J]. 生物技术通报, 2023, 39(7): 91-104. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||