








生物技术通报 ›› 2024, Vol. 40 ›› Issue (2): 289-299.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0877
收稿日期:2023-09-12
出版日期:2024-02-26
发布日期:2024-03-13
通讯作者:
赵国柱,男,教授,研究方向:微生物资源挖掘;E-mail: zhaogz@bjfu.edu.cn作者简介:郑菲,女,博士,研究方向:微生物代谢与酶工程;E-mail: zhengfei0718@bjfu.edu.cn
基金资助:
ZHENG Fei( ), YANG Jun-zhao, NIU Yu-feng, LI Rui-lin, ZHAO Guo-zhu(
), YANG Jun-zhao, NIU Yu-feng, LI Rui-lin, ZHAO Guo-zhu( )
)
Received:2023-09-12
Published:2024-02-26
Online:2024-03-13
摘要:
【目的】为挖掘新型裂解性多糖单加氧酶(LPMO)酶资源,探究LPMO在辅助降解纤维素过程中起到的重要作用。【方法】从Thermothelomyces thermophilus基因组中克隆表达了一个新型LPMO酶TtLPMO9I,系统地分析了其序列及结构的进化特征;采用DNS法表征了TtLPMO9I的酶学性质;在反应体系中添加不同浓度的抗坏血酸探究外部电子供体对TtLPMO9I活性的影响;以玉米秸秆和微晶纤维素为底物,通过检测还原糖的生成量计算获得TtLPMO9I与纤维素酶的协同作用效果。【结果】TtLPMO9I在60℃,pH 5.0时表现出最佳酶活力。在60℃孵育12 h后,仍能剩余54%的活性。经pH 6.0-8.0处理12 h后,酶活无损失。添加外部电子供体抗坏血酸使TtLPMO9I的活性提高至184%。在玉米秸秆和微晶纤维素降解过程中,TtLPMO9I与纤维素酶表现出良好的协同作用效果。将50-200 μg的TtLPMO9I添加至降解体系中,还原糖产量分别提高了34%-142%和6%-46%。【结论】TtLPMO9I不仅具有良好的温度稳定性和pH稳定性,在木质纤维素的降解过程中也具有突出的作用效果,为工业生产应用提供了潜在的优质酶资源。
郑菲, 杨俊钊, 牛羽丰, 李蕊麟, 赵国柱. 嗜热毁丝菌裂解性多糖单加氧酶TtLPMO9I的酶学性质及其功能研究[J]. 生物技术通报, 2024, 40(2): 289-299.
ZHENG Fei, YANG Jun-zhao, NIU Yu-feng, LI Rui-lin, ZHAO Guo-zhu. Characterization and Functional Analysis of Lytic Polysaccharide Monooxygenase TtLPMO9I from Thermothelomyces thermophilus[J]. Biotechnology Bulletin, 2024, 40(2): 289-299.
| 序号No. | 蛋白 Protein | 氨基酸数目 Number of amino acids | 分子量 Molecular weight/kD | 蛋白结构域 Protein domain | 参考文献 Reference |
|---|---|---|---|---|---|
| 1 | MYCTH_112089 | 232 | 26.1 |  | [ |
| 2 | MtLPMO9B | 323 | 32.4 |  | [ |
| 3 | MtLPMO9D | 255 | 27.0 |  | [ |
| 4 | MtLPMO9A | 225 | 24.4 |  | [ |
| 5 | AEO59955.1 | 235 | 24.4 |  | 未报道 |
| 6 | AEO54509.1 | 303 | 31.5 |  | 未报道 |
| 7 | MtLPMO9 | 342 | 34.9 |  | [ |
| 8 | TtLPMO9I | 306 | 31.4 |  | 本文研究材料 |
| 9 | MtLPMO9J | 246 | 26.0 |  | [ |
| 10 | AEO59836.1 | 227 | 24.1 |  | 未报道 |
| 11 | AEO59482.1 | 198 | 21.5 |  | 未报道 |
| 12 | AEO58921.1 | 340 | 35.8 |  | 未报道 |
| 13 | AEO58412.1 | 338 | 35.2 |  | 未报道 |
| 14 | AEO61257.1 | 241 | 25.9 |  | 未报道 |
| 15 | AEO56498.1a | 151 | 16.2 |  | 未报道 |
| 16 | AEO59823.1 | 254 | 28.4 |  | 未报道 |
| 17 | AEO55776.1 | 444 | 46.6 |  | 未报道 |
| 18 | AEO61305.1 | 230 | 25.0 |  | 未报道 |
| 19 | AEO55082.1 | 226 | 24.5 |  | 未报道 |
| 20 | MtLPMO9C | 237 | 24.9 |  | [ |
| 21 | AEO56547.1 | 245 | 26.5 |  | 未报道 |
| 22 | AEO55652.1 | 242 | 25.5 |  | 未报道 |
表1 嗜热毁丝菌AA9家族LPMO蛋白性质预测
Table 1 Protein property prediction of the LPMO AA9 family from T. thermophilus
| 序号No. | 蛋白 Protein | 氨基酸数目 Number of amino acids | 分子量 Molecular weight/kD | 蛋白结构域 Protein domain | 参考文献 Reference |
|---|---|---|---|---|---|
| 1 | MYCTH_112089 | 232 | 26.1 |  | [ |
| 2 | MtLPMO9B | 323 | 32.4 |  | [ |
| 3 | MtLPMO9D | 255 | 27.0 |  | [ |
| 4 | MtLPMO9A | 225 | 24.4 |  | [ |
| 5 | AEO59955.1 | 235 | 24.4 |  | 未报道 |
| 6 | AEO54509.1 | 303 | 31.5 |  | 未报道 |
| 7 | MtLPMO9 | 342 | 34.9 |  | [ |
| 8 | TtLPMO9I | 306 | 31.4 |  | 本文研究材料 |
| 9 | MtLPMO9J | 246 | 26.0 |  | [ |
| 10 | AEO59836.1 | 227 | 24.1 |  | 未报道 |
| 11 | AEO59482.1 | 198 | 21.5 |  | 未报道 |
| 12 | AEO58921.1 | 340 | 35.8 |  | 未报道 |
| 13 | AEO58412.1 | 338 | 35.2 |  | 未报道 |
| 14 | AEO61257.1 | 241 | 25.9 |  | 未报道 |
| 15 | AEO56498.1a | 151 | 16.2 |  | 未报道 |
| 16 | AEO59823.1 | 254 | 28.4 |  | 未报道 |
| 17 | AEO55776.1 | 444 | 46.6 |  | 未报道 |
| 18 | AEO61305.1 | 230 | 25.0 |  | 未报道 |
| 19 | AEO55082.1 | 226 | 24.5 |  | 未报道 |
| 20 | MtLPMO9C | 237 | 24.9 |  | [ |
| 21 | AEO56547.1 | 245 | 26.5 |  | 未报道 |
| 22 | AEO55652.1 | 242 | 25.5 |  | 未报道 |
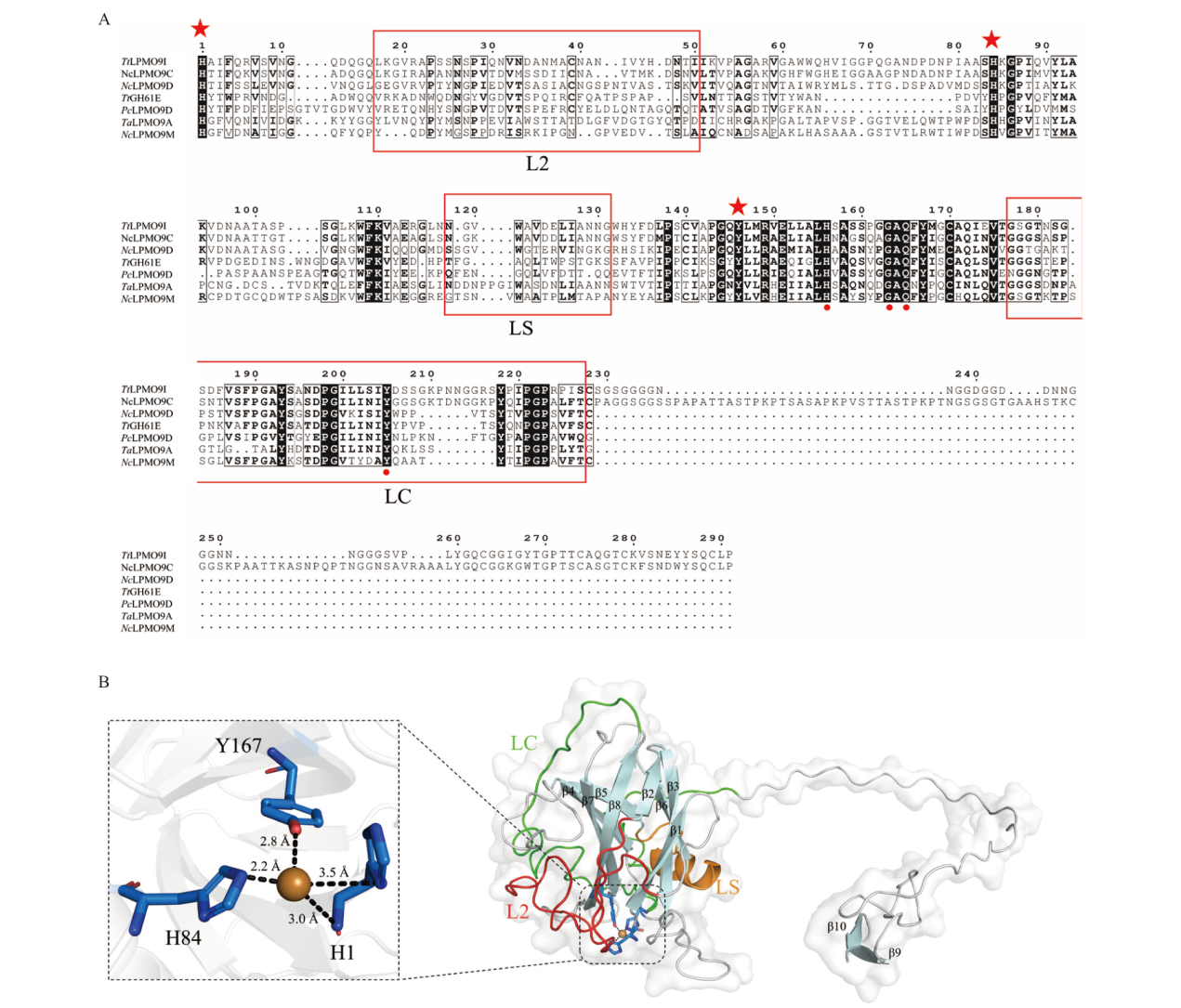
图2 TtLPMO9I生物信息学分析 A:TtLPMO9I与已解析出晶体结构LPMO的多序列比对;B:TtLPMO9I三维结构模拟图
Fig. 2 Bioinformatics analysis of TtLPMO9I A: Multiple sequence alignment of TtLPMO9I with LPMO in crystal structure. B: 3D structural simulation diagram of TtLPMO9I
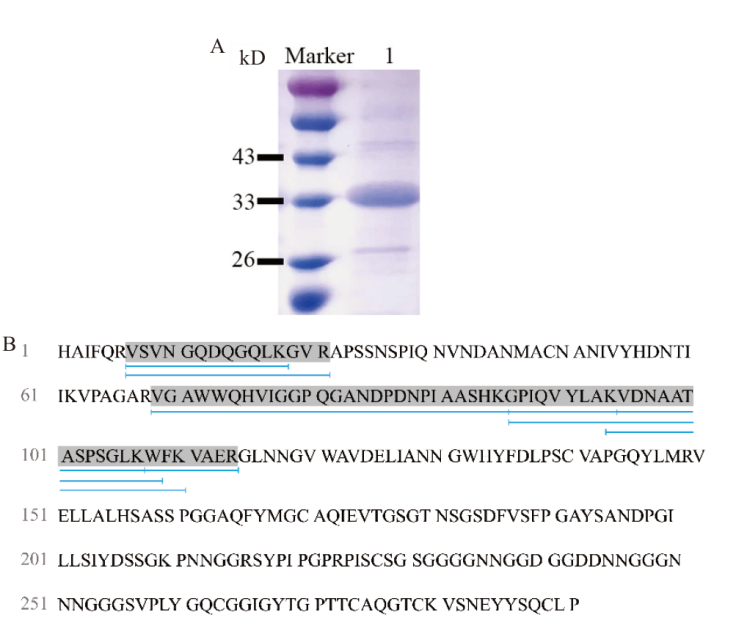
图3 重组蛋白TtLPMO9I的验证 A:TtLPMO9I的SDS-PAGE图;B:TtLPMO9I MALDI-TOF质谱分析结果图。 Marker:蛋白分子质量标准;1:TtLPMO9I纯化蛋白
Fig. 3 Validation of the recombinant protein TtLPMO9I A: SDS-PAGE analysis of TtLPMO9I. B: MALDI-TOF mass spectrometry analysis results diagram for TtLPMO9I. Marker: Protein molecular weight standard. 1: Purified protein of TtLPMO9I

图4 抗坏血酸对TtLPMO9I酶活性的影响 * P<0.05; ** P<0.01, *** P<0.001; **** P<0.000 1;ns: 差异不显著,下同
Fig. 4 Effect of ascorbic acid concentration on the enzyme activity of TtLPMO9I ns: Not significance, the same below

图5 TtLPMO9I的酶学性质 A:温度对TtLPMO9I酶活力的影响;B:温度对TtLPMO9I酶活力稳定性的影响;C:pH对TtLPMO9I酶活力的影响;D:pH对TtLPMO9I酶活力稳定性的影响
Fig. 5 Enzymatic properties of TtLPMO9I A: Effect of temperature on the cellulase activity of TtLPMO9I. B: Effect of temperature on the stability of TtLPMO9I. C: Effect of pH on the cellulase activity of TtLPMO9I. D: Effect of pH on the viability stability of TtLPMO9I

图6 TtLPMO9I与纤维素酶(Novozymes 188)降解玉米秸秆、微晶纤维素的协同反应 A:以玉米秸秆为底物反应12 h;B:以玉米秸秆为底物反应24 h;C:以微晶纤维素为底物反应12 h;D:以微晶纤维素为底物反应24 h。上图中蓝色均代表分别添加50、100、200 μg的TtLPMO9I所产生的还原糖的量;绿色均代表添加100 μL纤维素酶所产生的还原糖的量;橙色均代表50 μg TtLPMO9I+100 μL纤维素酶、100 μg TtLPMO9I+100 μL纤维素酶、200 μg TtLPMO9I+100 μL纤维素酶所产生的还原糖的量
Fig. 6 Synergistic reaction between TtLPMO9I and cellulase(Novozymes 188)for the degradation of pretreated corn straw and Avicel A: Pretreated corn straw was used as substrate for 12 h. B: Pretreated corn straw was used as substrate for 24 h. C: Reaction with Avicel as substrate for 12 h. D: Reaction with Avicel as substrate for 24 h. The blue block in the figure above indicates the amount of reducing sugar produced by adding 50, 100, and 200 μg TtLPMO9I, respectively. The green block indicates the amount of reducing sugar produced by adding 100 μL cellulase. The orange block indicates the amount of reducing sugar produced by 50 μg TtLPMO9I+100 μL cellulase, 100 μg TtLPMO9I+100 μL cellulase, and 200 μg TtLPMO9I+100 μL cellulase
| [1] | 宫秀杰, 钱春荣, 于洋, 等. 近年纤维素降解菌株筛选研究进展[J]. 纤维素科学与技术, 2021, 29(2): 68-77. |
| Gong XJ, Qian CR, Yu Y, et al. Progress on screening of cellulose degrading strains in recent years[J]. J Cellul Sci Technol, 2021, 29(2): 68-77. | |
| [2] |
Levasseur A, Drula E, Lombard V, et al. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes[J]. Biotechnol Biofuels, 2013, 6(1): 41.
doi: 10.1186/1754-6834-6-41 pmid: 23514094 |
| [3] |
Karkehabadi S, Hansson H, et al. The first structure of a glycoside hydrolase family 61 member, Cel61B from Hypocrea jecorina, at 1.6 A resolution[J]. J Mol Biol, 2008, 383(1): 144-154.
doi: 10.1016/j.jmb.2008.08.016 pmid: 18723026 |
| [4] | 李欣, 张丽丽, 田莉, 等. 裂解多糖单加氧酶高效催化的研究进展[J]. 生物化学与生物物理进展, 2016, 43(10): 970-979. |
| Li X, Zhang LL, Tian L, et al. The advance of efficient catalysis of lytic polysaccharide monooxygenases[J]. Prog Biochem Biophys, 2016, 43(10): 970-979. | |
| [5] |
Sun PC, Valenzuela SV, et al. Oxidized product profiles of AA9 lytic polysaccharide monooxygenases depend on the type of cellulose[J]. ACS Sustain Chem Eng, 2021, 9(42): 14124-14133.
doi: 10.1021/acssuschemeng.1c04100 pmid: 34722005 |
| [6] |
Couturier M, Ladevèze S, Sulzenbacher G, et al. Lytic xylan oxidases from wood-decay fungi unlock biomass degradation[J]. Nat Chem Biol, 2018, 14(3): 306-310.
doi: 10.1038/nchembio.2558 pmid: 29377002 |
| [7] |
Sabbadin F, Hemsworth GR, Ciano L, et al. An ancient family of lytic polysaccharide monooxygenases with roles in arthropod development and biomass digestion[J]. Nat Commun, 2018, 9(1): 756.
doi: 10.1038/s41467-018-03142-x pmid: 29472725 |
| [8] |
Filiatrault-Chastel C, Navarro D, Haon M, et al. AA16, a new lytic polysaccharide monooxygenase family identified in fungal secretomes[J]. Biotechnol Biofuels, 2019, 12: 55.
doi: 10.1186/s13068-019-1394-y pmid: 30923563 |
| [9] |
Sabbadin F, Urresti S, Henrissat B, et al. Secreted pectin monooxygenases drive plant infection by pathogenic oomycetes[J]. Science, 2021, 373(6556): 774-779.
doi: 10.1126/science.abj1342 pmid: 34385392 |
| [10] |
Calderaro F, Keser M, Akeroyd M, et al. Characterization of an AA9 LPMO from Thielavia australiensis, TausLPMO9B, under industrially relevant lignocellulose saccharification conditions[J]. Biotechnol Biofuels, 2020, 13(1): 195.
doi: 10.1186/s13068-020-01836-3 pmid: 33292403 |
| [11] | Hegnar OA, Petrovic DM, Bissaro B, et al. pH-dependent relationship between catalytic activity and hydrogen peroxide production shown via characterization of a lytic polysaccharide monooxygenase from Gloeophyllum trabeum[J]. Appl Environ Microbiol, 2019, 85(5): e02612-e02618. |
| [12] |
Agrawal D, Kaur B, Kaur Brar K, et al. An innovative approach of priming lignocellulosics with lytic polysaccharide mono-oxygenases prior to saccharification with glycosyl hydrolases can economize second generation ethanol process[J]. Bioresour Technol, 2020, 308: 123257.
doi: 10.1016/j.biortech.2020.123257 URL |
| [13] |
Müller G, Várnai A, Johansen KS, et al. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions[J]. Biotechnol Biofuels, 2015, 8: 187.
doi: 10.1186/s13068-015-0376-y pmid: 26609322 |
| [14] | 吴帅帅, 田娟, 谢宁, 等. AA9家族裂解多糖单加氧酶研究进展[J]. 纤维素科学与技术, 2020, 28(3): 70-79. |
| Wu SS, Tian J, Xie N, et al. Progress of AA9 family lytic polysaccharide monooxygenase[J]. J Cellul Sci Technol, 2020, 28(3): 70-79. | |
| [15] |
Frommhagen M, Westphal AH, et al. Distinct substrate specificities and electron-donating systems of fungal lytic polysaccharide monooxygenases[J]. Front Microbiol, 2018, 9: 1080.
doi: 10.3389/fmicb.2018.01080 pmid: 29896168 |
| [16] |
Chen C, Chen JY, Geng ZG, et al. Regioselectivity of oxidation by a polysaccharide monooxygenase from Chaetomium thermophi-lum[J]. Biotechnol Biofuels, 2018, 11: 155.
doi: 10.1186/s13068-018-1156-2 pmid: 29991963 |
| [17] |
Beeson WT, Vu VV, Span EA, et al. Cellulose degradation by polysaccharide monooxygenases[J]. Annu Rev Biochem, 2015, 84: 923-946.
doi: 10.1146/annurev-biochem-060614-034439 pmid: 25784051 |
| [18] |
Danneels B, Tanghe M, Joosten HJ, et al. A quantitative indicator diagram for lytic polysaccharide monooxygenases reveals the role of aromatic surface residues in HjLPMO9A regioselectivity[J]. PLoS One, 2017, 12(5): e0178446.
doi: 10.1371/journal.pone.0178446 URL |
| [19] |
Vu VV, Beeson WT, et al. Determinants of regioselective hydroxylation in the fungal polysaccharide monooxygenases[J]. J Am Chem Soc, 2014, 136(2): 562-565.
doi: 10.1021/ja409384b pmid: 24350607 |
| [20] |
Frommhagen M, Koetsier MJ, Westphal AH, et al. Lytic polysaccharide monooxygenases from Myceliophthora thermophila C1 differ in substrate preference and reducing agent specificity[J]. Biotechnol Biofuels, 2016, 9(1): 186.
doi: 10.1186/s13068-016-0594-y pmid: 27588039 |
| [21] |
Frommhagen M, Westphal AH, Hilgers R, et al. Quantification of the catalytic performance of C1-cellulose-specific lytic polysaccharide monooxygenases[J]. Appl Microbiol Biotechnol, 2018, 102(3): 1281-1295.
doi: 10.1007/s00253-017-8541-9 pmid: 29196788 |
| [22] |
Frommhagen M, Sforza S, Westphal AH, et al. Discovery of the combined oxidative cleavage of plant xylan and cellulose by a new fungal polysaccharide monooxygenase[J]. Biotechnol Biofuels, 2015, 8: 101.
doi: 10.1186/s13068-015-0284-1 pmid: 26185526 |
| [23] |
Karnaouri A, Muraleedharan MN, Dimarogona M, et al. Recombinant expression of thermostable processive Mt EG5 endoglucanase and its synergism with Mt LPMO from Myceliophthora thermophila during the hydrolysis of lignocellulosic substrates[J]. Biotechnol Biofuels, 2017, 10: 126.
doi: 10.1186/s13068-017-0813-1 pmid: 28515785 |
| [24] |
Kadowaki MAS, Várnai A, Jameson JK, et al. Functional characterization of a lytic polysaccharide monooxygenase from the thermophilic fungus Myceliophthora thermophila[J]. PLoS One, 2018, 13(8): e0202148.
doi: 10.1371/journal.pone.0202148 URL |
| [25] |
Hangasky JA, Marletta MA. A random-sequential kinetic mechanism for polysaccharide monooxygenases[J]. Biochemistry, 2018, 57(22): 3191-3199.
doi: 10.1021/acs.biochem.8b00129 pmid: 29683313 |
| [26] |
Berka RM, Grigoriev IV, Otillar R, et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Mycelioph-thora thermophila and Thielavia terrestris[J]. Nat Biotechnol, 2011, 29(10): 922-927.
doi: 10.1038/nbt.1976 |
| [27] |
Galagan JE, Calvo SE, Cuomo C, et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. ory-zae[J]. Nature, 2005, 438(7071): 1105-1115.
doi: 10.1038/nature04341 |
| [28] |
Espagne E, Lespinet O, Malagnac F, et al. The genome sequence of the model ascomycete fungus Podospora anserina[J]. Genome Biol, 2008, 9(5): R77.
doi: 10.1186/gb-2008-9-5-r77 URL |
| [29] |
Galagan JE, Calvo SE, Borkovich KA, et al. The genome sequence of the filamentous fungus Neurospora crassa[J]. Nature, 2003, 422(6934): 859-868.
doi: 10.1038/nature01554 URL |
| [30] |
Book AJ, Yennamalli RM, Takasuka TE, et al. Evolution of substrate specificity in bacterial AA10 lytic polysaccharide monooxygenases[J]. Biotechnol Biofuels, 2014, 7: 109.
doi: 10.1186/1754-6834-7-109 pmid: 25161697 |
| [31] |
Hervé C, Rogowski A, Blake AW, et al. Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects[J]. Proc Natl Acad Sci USA, 2010, 107(34): 15293-15298.
doi: 10.1073/pnas.1005732107 pmid: 20696902 |
| [32] |
Forsberg Z, Courtade G. On the impact of carbohydrate-binding modules(CBMs)in lytic polysaccharide monooxygenases(LPMOs)[J]. Essays Biochem, 2023, 67(3): 561-574.
doi: 10.1042/EBC20220162 URL |
| [33] |
马玉倩, 孙东辉, 岳浩峰, 等. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-0996 |
| Ma YQ, Sun DH, et al. Identification, heterologous expression and functional analysis of a GH61 family glycoside hydrolase from Setosphaeria turcica with the assisting function in degrading cellulose[J]. Biotechnol Bull, 2023, 39(4): 124-135. | |
| [34] |
Li YL, Li TB, Guo JT, et al. Expression and characterization of a novel lytic polysaccharide monooxygenase, PdLPMO9A, from the edible fungus Pleurotus djamor and its synergistic interactions with cellulase in corn straw biomass saccharification[J]. Bioresour Technol, 2022, 348: 126792.
doi: 10.1016/j.biortech.2022.126792 URL |
| [35] |
Srivastava A, Nagar P, Rathore S, et al. The linker region promotes activity and binding efficiency of modular LPMO towards polymeric substrate[J]. Microbiol Spectr, 2022, 10(1): e0269721.
doi: 10.1128/spectrum.02697-21 URL |
| [36] |
Dimarogona M, Topakas E, Olsson L, et al. Lignin boosts the cellulase performance of a GH-61 enzyme from Sporotrichum thermo-phile[J]. Bioresour Technol, 2012, 110: 480-487.
doi: 10.1016/j.biortech.2012.01.116 URL |
| [37] | Bulakhov AG, Gusakov AV, Chekushina AV, et al. Isolation of homogeneous polysaccharide monooxygenases from fungal sources and investigation of their synergism with cellulases when acting on cellulose[J]. Biochemistry, 2016, 81(5): 530-537. |
| [38] |
Harris PV, Welner D, McFarland KC, et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family[J]. Biochemistry, 2010, 49(15): 3305-3316.
doi: 10.1021/bi100009p pmid: 20230050 |
| [39] |
Rodríguez-Zúñiga UF, Cannella D, de Campos Giordano R, et al. Lignocellulose pretreatment technologies affect the level of enzymatic cellulose oxidation by LPMO[J]. Green Chem, 2015, 17(5): 2896-2903.
doi: 10.1039/C4GC02179G URL |
| [1] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [2] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [3] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| [4] | 陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267. |
| [5] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [6] | 魏婷柳, 苗华彪, 吴倩, 黄遵锡. 漆酶BmLac的异源表达、酶学特性及棉酚降解的研究[J]. 生物技术通报, 2023, 39(12): 320-328. |
| [7] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [8] | 牛馨, 张莹, 王茂军, 刘文龙, 路福平, 李玉. 解淀粉芽胞杆菌不同整合位点对外源碱性蛋白酶表达的影响[J]. 生物技术通报, 2022, 38(4): 253-260. |
| [9] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [10] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| [11] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [12] | 王博雅, 姜勇, 黄艳, 曹颖, 胡尚连. 慈竹纤维素合酶BeCesA4的克隆及功能分析[J]. 生物技术通报, 2022, 38(11): 185-193. |
| [13] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [14] | 张彤彤, 郑登俞, 吴忠义, 张中保, 于荣. 玉米NF-Y转录因子基因ZmNF-YB13响应干旱和盐胁迫的功能分析[J]. 生物技术通报, 2022, 38(10): 115-123. |
| [15] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||