








生物技术通报 ›› 2024, Vol. 40 ›› Issue (4): 278-286.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0813
殷亮1( ), 王代玮1,2, 刘悦莹1,2, 刘海燕1, 罗光宏1(
), 王代玮1,2, 刘悦莹1,2, 刘海燕1, 罗光宏1( )
)
收稿日期:2023-08-20
出版日期:2024-04-26
发布日期:2024-04-30
通讯作者:
罗光宏,男,教授,研究方向:藻类生物技术;E-mail: kyluo@hxu.edu.cn作者简介:殷亮,男,博士,副研究员,研究方向:酶工程及合成生物学;E-mail: yinl03@163.com
基金资助:
YIN Liang1( ), WANG Dai-wei1,2, LIU Yue-ying1,2, LIU Hai-yan1, LUO Guang-hong1(
), WANG Dai-wei1,2, LIU Yue-ying1,2, LIU Hai-yan1, LUO Guang-hong1( )
)
Received:2023-08-20
Published:2024-04-26
Online:2024-04-30
摘要:
【目的】对螺旋藻来源的一个蛋白酶基因进行克隆、表达,并探究重组酶酶学性质,为藻类蛋白酶的深入研究奠定基础。【方法】从钝顶螺旋藻(Spirulina platensis)基因组扩增获得了蛋白酶基因SpP1,进而构建 pET28a-SpP1重组质粒,将其转入Escherichia coli BL21(DE3)实现了异源表达,利用镍柱分离纯化重组蛋白酶并研究其酶学性质。【结果】螺旋藻蛋白酶SpP1属于丝氨酸蛋白酶家族成员,分子量为47.04 kD,最适温度和pH值分别为50℃和8.0。其热稳定性较差,在pH=8.0-9.0的范围内具有良好的酸碱稳定性。以酪蛋白为底物时,最大反应速度 Vmax=8.237 U/mL,米氏常数Km=16.369 μg/mL。Mn2+对其具有较强的激活作用,添加0.1 mol/L Mn2+后其酶活提高了18倍,同时0.1 mol/L 的Fe3+、Zn2+、Ca2+、乙二胺四乙酸(ethylenediaminetetraacetic acid,EDTA)等对酶活也具有明显的促进作用。【结论】螺旋藻来源的蛋白酶SpP1具有丝氨酸蛋白酶家族成员的典型结构和性质特征,具有较好的酸碱稳定性,添加金属锰离子可有效提升其催化活性。
殷亮, 王代玮, 刘悦莹, 刘海燕, 罗光宏. 蛋白酶SpP1基因克隆、表达及酶学性质的表征[J]. 生物技术通报, 2024, 40(4): 278-286.
YIN Liang, WANG Dai-wei, LIU Yue-ying, LIU Hai-yan, LUO Guang-hong. Cloning and Expression of Protease SpP1 Gene and Characterization of Enzymatic Properties[J]. Biotechnology Bulletin, 2024, 40(4): 278-286.
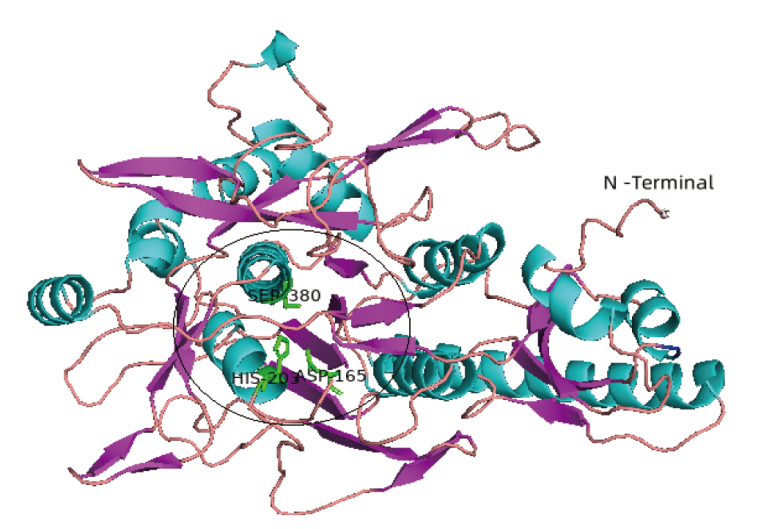
图1 蛋白酶SpP1的三维结构 Swiss-Model预测的SpP1三级结构:粉色表示 β-sheet;青色表示 α-helix;橙色为coil。黑色圆圈内为催化残基D165H203S380及底物结合口袋
Fig. 1 3-D structure of peptidase SpP1 The 3D structure of SpP1 predicted by Swiss-Model. Pink indicates β-sheet; cyan indicates α-helix; orange indicates coil. The black circle shows the catalytic residues D165H203S380 and the substrate binding pocket
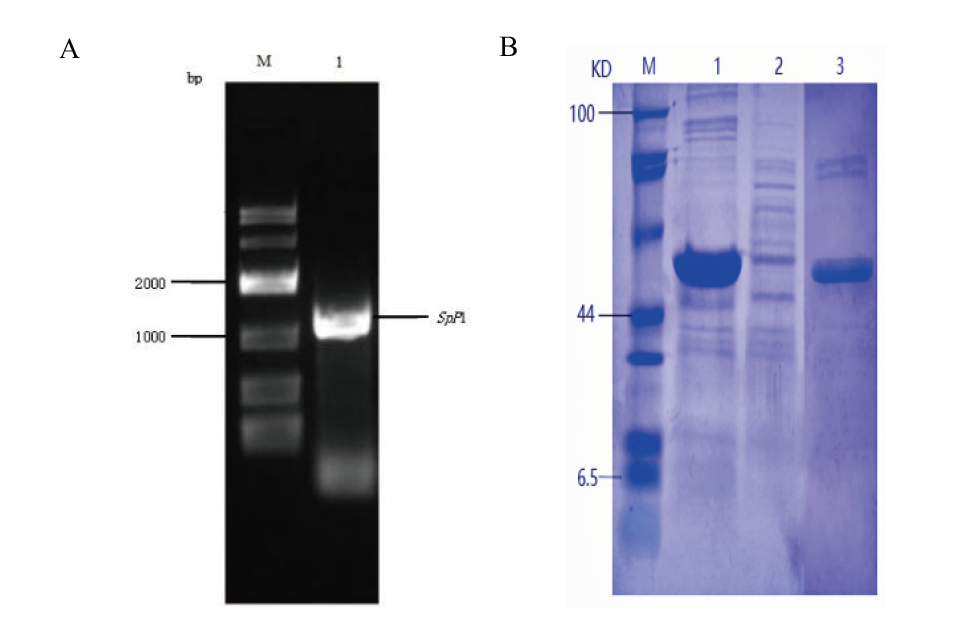
图2 SpP1基因的凝胶电泳(A)和SpP1的SDS-PAGE(B) A: SpP1基因凝胶电泳图(M:DL10000 DNA marker;1:PCR扩增的SpP1基因目的条带);B: SpP1蛋白的SDS-PAGE(M:蛋白marker;1:沉淀;2:上清;3:纯化的SpP1)
Fig. 2 SpP1 gel electrophoresis(A)and SDS - PAGE of SpP1(B) A: Gel electrophoresis map of SpP1 gene(M: DL10000 DNA marker. 1: PCR-amplified band of SpP1 gene). B: SDS-PAGE of SpP1protein(M: protein marker. 1: Precipitation. 2: Supernatant. 3: Purified SpP1 protein band)
| [1] | Ellaiah P, Srinivasulu B, Adinarayana K. A review on microbial alkaline proteases[J]. J Sci Ind Res, 2002, 61: 690-704. |
| [2] |
Gupta R, Beg QK, Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications[J]. Appl Microbiol Biotechnol, 2002, 59(1): 15-32.
doi: 10.1007/s00253-002-0975-y pmid: 12073127 |
| [3] | Matkawala F, Nighojkar S, Kumar A, et al. Microbial alkaline serine proteases: production, properties and applications[J]. World J Microbiol Biotechnol, 2021, 37(4): 63. |
| [4] |
Gurumallesh P, Alagu K, Ramakrishnan B, et al. A systematic reconsideration on proteases[J]. Int J Biol Macromol, 2019, 128: 254-267.
doi: S0141-8130(18)35199-7 pmid: 30664968 |
| [5] |
Contesini FJ, Melo RR, Sato HH. An overview of Bacillus proteases: from production to application[J]. Crit Rev Biotechnol, 2018, 38(3): 321-334.
doi: 10.1080/07388551.2017.1354354 pmid: 28789570 |
| [6] |
Yu P, Huang XX, Ren Q, et al. Purification and characterization of a H2O2-tolerant alkaline protease from Bacillus sp. ZJ1502, a newly isolated strain from fermented bean curd[J]. Food Chem, 2019, 274: 510-517.
doi: 10.1016/j.foodchem.2018.09.013 URL |
| [7] |
Dorra G, Ines K, Imen BS, et al. Purification and characterization of a novel high molecular weight alkaline protease produced by an endophytic Bacillus halotolerans strain CT2[J]. Int J Biol Macromol, 2018, 111: 342-351.
doi: 10.1016/j.ijbiomac.2018.01.024 URL |
| [8] |
Omrane Benmrad M, Moujehed E, Ben Elhoul M, et al. Production, purification, and biochemical characterization of serine alkaline protease from Penicillium chrysogenium strain X5 used as excellent bio-additive for textile processing[J]. Int J Biol Macromol, 2018, 119: 1002-1016.
doi: S0141-8130(18)33429-9 pmid: 30081129 |
| [9] |
Pérez-Lloréns JL, Benítez E, Vergara JJ, et al. Characterization of proteolytic enzyme activities in macroalgae[J]. Eur J Phycol, 2003, 38(1): 55-64.
doi: 10.1080/0967026031000096254 URL |
| [10] |
Lockau W, Massalsky B, Dirmeier A. Purification and partial characterization of a calcium-stimulated protease from the cyanobacterium, Anabaena variabilis[J]. Eur J Biochem, 1988, 172(2): 433-438.
pmid: 3127208 |
| [11] |
Strohmeier U, Gerdes C, Lockau W. Proteolysis in heterocyst-forming cyanobacteria: characterization of a further enzyme with trypsin-like specificity, and of a prolyl endopeptidase from Anabaena variabilis[J]. Z Naturforsch C J Biosci, 1994, 49(1-2): 70-78.
doi: 10.1515/znc-1994-1-212 pmid: 8148011 |
| [12] |
Niven GW. The characterization of two aminopeptidase activities from the cyanobacterium Anabaena flos-aquae[J]. Biochim Biophys Acta, 1995, 1253(2): 193-198.
pmid: 8519802 |
| [13] |
Nanni B, Balestreri E, Dainese E, et al. Characterisation of a specific phycocyanin-hydrolysing protease purified from Spirulina platensis[J]. Microbiol Res, 2001, 156(3): 259-266.
pmid: 11716214 |
| [14] |
Yada E, Nagata H, Noguchi Y, et al. An arginine specific protease from Spirulina platensis[J]. Mar Biotechnol, 2005, 7(5): 474-480.
doi: 10.1007/s10126-004-4115-9 URL |
| [15] | 吕冰心, 常蓉, 李博生. 基于蛋白质组学对螺旋藻在高温胁迫下响应机制的初步研究[J]. 植物生理学报, 2018, 54(5): 904-916. |
|
Lyu BX, Chang R, Li BS. Study on the response mechanism of Spirulina platensis to high temperature stress based on proteomics[J]. Plant Physiol J, 2018, 54(5): 904-916.
doi: 10.1104/pp.54.6.904 URL |
|
| [16] |
王娜, 向清豪, 赵肖荣, 等. 螺旋藻全蛋白与家族分类[J]. 食品科学, 2018, 39(16): 201-207.
doi: 10.7506/spkx1002-6630-201816029 |
| Wang N, Xiang QH, Zhao XR, et al. Family classification of the whole proteins of Spirulina[J]. Food Sci, 2018, 39(16): 201-207. | |
| [17] |
Gunes S, Tamburaci S, Dalay MC, et al. In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities[J]. Pharm Biol, 2017, 55(1): 1824-1832.
doi: 10.1080/13880209.2017.1331249 pmid: 28552036 |
| [18] | Stanier RY, Kunizawa MM, Cohen-Bazire G. Purification and property of unicellular blue-green algae (Order Chroococales)[J]. Bacteriol Rev, 1971, 35(2):120-171. |
| [19] |
Singh Chauhan R, Mani Mishra R. Characterization of alkaline protease producing Bacillus halodurans RSCVS-PF21 isolated from poultry farm soil[J]. Biosci, Biotech Res Asia, 2020, 17(2): 385-392.
doi: 10.13005/bbra/ URL |
| [20] |
Wen Y, Qiang J, Zhou G, et al. Characterization of redox and salinity-tolerant alkaline protease from Bacillus halotolerans strain DS5[J]. Front Microbiol, 2022, 13:935072.
doi: 10.3389/fmicb.2022.935072 URL |
| [21] | 李怡欣, 付刚, 马媛媛, 等. 碱性蛋白酶SubC在枯草芽孢杆菌中的高效异源表达[J]. 微生物学通报, 2021, 48(10): 3409-3420. |
| Li YX, Fu G, Ma YY, et al. Efficient heterologous expression of alkaline protease SubC in Bacillus subtilis[J]. Microbiol China, 2021, 48(10): 3409-3420. | |
| [22] | 周魏, 曾嵩玉, 余金凤, 等. 一株地衣芽胞杆菌产碱性蛋白酶条件优化[J]. 微生物学通报, 2022, 49(7): 2753-2766. |
| Zhou W, Zeng SY, Yu JF, et al. Optimization of alkaline protease production by a strain of Bacillus licheniformis[J]. Microbiol China, 2022, 49(7): 2753-2766. | |
| [23] | Hammami A, Bayoudh A, Hadrich B, et al. Response-surface methodology for the production and the purification of a new H2 O2-tolerant alkaline protease from Bacillus invictae AH1 strain[J]. Biotechnol Prog, 2020, 36(3): e2965. |
| [24] |
Meena P, Tripathi AD, Srivastava SK, et al. Utilization of agro-industrial waste(wheat bran)for alkaline protease production by Pseudomonas aeruginosa in SSF using Taguchi(DOE)methodology[J]. Biocatal Agric Biotechnol, 2013, 2(3): 210-216.
doi: 10.1016/j.bcab.2013.05.003 URL |
| [25] |
Boulkour Touioui S, Zaraî Jaouadi N, Bouacem K, et al. Biochemical and molecular characterization of a novel metalloprotease from Pseudomonas fluorescens strain TBS09[J]. Int J Biol Macromol, 2018, 107(Pt B): 2351-2363.
doi: S0141-8130(17)33230-0 pmid: 29055705 |
| [26] | Jenitta XJ, Priya S, Gnanadoss JJ. Optimization of culture conditions and inducers for improved protease production by Penicillium griseofulvum LCJ231 under submerged fermentation[J]. Int J Adv Biotechnol Res, 2015, 6(2):152-160. |
| [27] |
de Souza PM, Bittencourt ML, Caprara CC, et al. A biotechnology perspective of fungal proteases[J]. Braz J Microbiol, 2015, 46(2): 337-346.
doi: 10.1590/S1517-838246220140359 pmid: 26273247 |
| [28] |
Sattar H, Bibi Z, Kamran A, et al. Degradation of complex casein polymer: production and optimization of a novel serine metalloprotease from Aspergillus niger KIBGE-IB36[J]. Biocatal Agric Biotechnol, 2019, 21: 101256.
doi: 10.1016/j.bcab.2019.101256 URL |
| [29] |
Azrin NAM, Ali MSM, Rahman RNZRA, et al. Versatility of subtilisin: a review on structure, characteristics, and applications[J]. Biotechnol Appl Biochem, 2022, 69(6): 2599-2616.
doi: 10.1002/bab.v69.6 URL |
| [30] | 万明铼. 高产碱性蛋白酶菌株的筛选及酶学性质的研究[D]. 兰州: 兰州理工大学, 2023. |
| Wan ML. Screening of the bacterial strain producing high level of alkaline protease and study on its enzymatic properties[D]. Lanzhou: Lanzhou University of Technology, 2023. | |
| [31] |
Grøn H, Bech LM, Sørensen SB, et al. Studies of binding sites in the subtilisin from Bacillus lentus by means of site directed mutagenesis and kinetic investigations[J]. Adv Exp Med Biol, 1996, 379: 105-112.
pmid: 8796314 |
| [32] |
Kumar CG, Joo HS, Koo YM, et al. Thermostable alkaline protease from a novel marine haloalkalophilic Bacillus clausii isolate[J]. World J Microbiol Biotechnol, 2004, 20(4): 351-357.
doi: 10.1023/B:WIBI.0000033057.28828.a7 URL |
| [33] | Akel H, Yousef T. Characterization of a purified thermostable protease from hyperthermophilic Bacillus strain HUTBS71[J]. Eur J Sci Res, 2009, 31(2):280-288. |
| [34] |
Mei CF, Jiang XL. A novel surfactant- and oxidation-stable alkaline protease from Vibrio metschnikovii DL 33-51[J]. Process Biochem, 2005, 40(6): 2167-2172.
doi: 10.1016/j.procbio.2004.08.007 URL |
| [35] |
Jang JS, Kang DO, Chun MJ, et al. Molecular cloning of a subtilisin J gene from Bacillus stearothermophilus and its expression in Bacillus subtilis[J]. Biochem Biophys Res Commun, 1992, 184(1): 277-282.
doi: 10.1016/0006-291X(92)91189-W URL |
| [36] |
Neidhart DJ, Petsko GA. The refined crystal structure of subtilisin Carlsberg at 2.5 A resolution[J]. Protein Eng, 1988, 2(4): 271-276.
pmid: 3150541 |
| [37] |
Nonaka T, Suzuki T, Tanaka N, et al. Structure and function of subtilisin BPN'as studied through crystallographic studies on a series of its complexes with genetically engineered proteinaceous inhibitor SSI[J]. Adv Exp Med Biol, 1996, 379: 21-27.
pmid: 8796307 |
| [38] |
Gaur S, Agrahari S, Wadhwa N. Purification of protease from Pseudomonas thermaerum GW1 isolated from poultry waste site[J]. Open Microbiol J, 2010, 4: 67-74.
doi: 10.2174/1874285801004010067 URL |
| [39] |
Jellouli K, Ghorbel-Bellaaj O, Ben Ayed H, et al. Alkaline-protease from Bacillus licheniformis MP1: purification, characterization and potential application as a detergent additive and for shrimp waste deproteinization[J]. Process Biochem, 2011, 46(6): 1248-1256.
doi: 10.1016/j.procbio.2011.02.012 URL |
| [40] |
Sousa F, Jus S, Erbel A, et al. A novel metalloprotease from Bacillus cereus for protein fibre processing[J]. Enzyme Microb Technol, 2007, 40(7): 1772-1781.
doi: 10.1016/j.enzmictec.2006.12.017 URL |
| [1] | 郑菲, 杨俊钊, 牛羽丰, 李蕊麟, 赵国柱. 嗜热毁丝菌裂解性多糖单加氧酶TtLPMO9I的酶学性质及其功能研究[J]. 生物技术通报, 2024, 40(2): 289-299. |
| [2] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [3] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [4] | 马玉倩, 孙东辉, 岳浩峰, 辛佳瑜, 刘宁, 曹志艳. 具有辅助降解纤维素功能的大斑刚毛座腔菌糖苷水解酶GH61的鉴定、异源表达及功能分析[J]. 生物技术通报, 2023, 39(4): 124-135. |
| [5] | 陈楠楠, 王春来, 蒋振忠, 焦鹏, 关淑艳, 马义勇. 玉米ZmDHN15基因在烟草中的遗传转化及抗冷性分析[J]. 生物技术通报, 2023, 39(4): 259-267. |
| [6] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [7] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| [8] | 魏婷柳, 苗华彪, 吴倩, 黄遵锡. 漆酶BmLac的异源表达、酶学特性及棉酚降解的研究[J]. 生物技术通报, 2023, 39(12): 320-328. |
| [9] | 邢媛, 宋健, 李俊怡, 郑婷婷, 刘思辰, 乔治军. 谷子AP基因家族鉴定及其对非生物胁迫的响应分析[J]. 生物技术通报, 2023, 39(11): 238-251. |
| [10] | 尹国英, 刘畅, 常永春, 羽王洁, 王兵, 张盼, 郭玉双. 烟草半胱氨酸蛋白酶家族和相应miRNAs的鉴定及其对PVY的响应[J]. 生物技术通报, 2023, 39(10): 184-196. |
| [11] | 王翠翠, 傅达奇. 泛素-蛋白酶体系统影响植物农艺性状的研究进展[J]. 生物技术通报, 2023, 39(1): 72-83. |
| [12] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [13] | 牛馨, 张莹, 王茂军, 刘文龙, 路福平, 李玉. 解淀粉芽胞杆菌不同整合位点对外源碱性蛋白酶表达的影响[J]. 生物技术通报, 2022, 38(4): 253-260. |
| [14] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [15] | 王玥, 高庆华, 董聪, 罗同阳, 王庆庆. 密码子优化的吡喃糖氧化酶基因在毕赤酵母中的表达[J]. 生物技术通报, 2022, 38(4): 269-277. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||