








生物技术通报 ›› 2025, Vol. 41 ›› Issue (9): 139-146.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0190
• 研究报告 • 上一篇
张永艳1( ), 郭思健1, 李晶1, 郝思怡1, 李瑞得1, 刘嘉鹏2, 程春振1(
), 郭思健1, 李晶1, 郝思怡1, 李瑞得1, 刘嘉鹏2, 程春振1( )
)
收稿日期:2025-02-23
出版日期:2025-09-26
发布日期:2025-09-24
通讯作者:
程春振,男,博士,副教授,研究方向 :园艺植物生物技术;E-mail: ld0532cheng@126.com作者简介:张永艳,女,博士,研究方向 :果树生物技术;E-mail: zhyy0425@126.com基金资助:
ZHANG Yong-yan1( ), GUO Si-jian1, LI Jing1, HAO Si-yi1, LI Rui-de1, LIU Jia-peng2, CHENG Chun-zhen1(
), GUO Si-jian1, LI Jing1, HAO Si-yi1, LI Rui-de1, LIU Jia-peng2, CHENG Chun-zhen1( )
)
Received:2025-02-23
Published:2025-09-26
Online:2025-09-24
摘要:
目的 Phi亚家族谷胱甘肽转移酶(GSTF)在多种植物花青素积累和转运中发挥关键作用。为研究蓝莓GSTF的功能,对蓝莓花青素相关GSTF19基因进行克隆和功能研究。 方法 利用反转录PCR克隆获得蓝莓花青素相关GSTF19的编码序列并构建过表达载体,基于蓝莓果实瞬时转化和拟南芥tt19突变体互补实验研究其功能。 结果 VcGSTF19的瞬时过表达可以显著促进蓝莓果皮中花青素的积累。过表达VcGSTF19的蓝莓果皮花青素含量达到空载对照的6.61倍。其瞬时过表达显著提高了蓝莓果皮中VcCHS、VcCHI、VcF3H、VcDFR、VcANS 和 VcUFGT等花青素合成结构基因的表达水平。此外,VcGSTF19异源转化拟南芥tt19突变体恢复了突变体花青素积累,转基因莲座叶中花青素含量约为突变体的6.21倍。 结论 蓝莓VcGSTF19基因在花青素积累和转运中发挥着重要作用。
张永艳, 郭思健, 李晶, 郝思怡, 李瑞得, 刘嘉鹏, 程春振. 蓝莓花青素相关VcGSTF19基因的克隆及功能研究[J]. 生物技术通报, 2025, 41(9): 139-146.
ZHANG Yong-yan, GUO Si-jian, LI Jing, HAO Si-yi, LI Rui-de, LIU Jia-peng, CHENG Chun-zhen. Gene Cloning and Functional Analysis of the Anthocyanin-related VcGSTF19 Gene in Blueberry (Vaccinium corymbosum L.)[J]. Biotechnology Bulletin, 2025, 41(9): 139-146.
| 引物名称 | 引物序列 | 退火温度 | 用途 |
|---|---|---|---|
| Primer name | Primer sequence (5′-3′) | Annealing temperature (℃) | Applications |
| VcGSTF19F | ATGGTGGTCAAAGTTTATGGTCCAATTAGAG | 63 | 基因克隆 Gene cloning |
| VcGSTF19R | CTAATCCATAAGCTTCATTATTTTCTTCCAAGCAG | ||
| VcGSTF19-SF | CTCTACAAATCTATCTCTGGATCCATGGTGGTCAAAGTTTATGGTCCAATTAGAG | 68 | 载体构建 |
| VcGST19F-SR | GATTTTTGCGGACTCTAGGAGCTCCTAATCCATAAGCTTCATTATTTTCTTCCAAGCAG | Vector construction | |
| VcGSTF19-qF | GGAGAGAGCCCTTGTTGACC | 60 | 实时荧光定量 |
| VcGSTF19-qR | GCTCTTCGACAACCTCTGCT | RT-qPCR | |
| VcCHS-qF | CGCATGTGTGACAAATCCCA | 60 | |
| VcCHS-qR | ACCATATCCTGCCTAGCGTC | ||
| VcCHI-qF | TCTTTCCTCCGTCGGTCAAA | 60 | |
| VcCHI-qR | ACTCTACGGCGCTAACTTGT | ||
| VcF3H-qF | GTGGACGGAGCTTTTGTTGT | 60 | |
| VcF3H-qR | GTGATTGGCTCGTCGAGAAC | ||
| VcDFR-qF | GCTTCTTGAACGGGGCTATG | 60 | |
| VcDFR-qR | CTTCAATGGCCTCGTCGAAG | ||
| VcANS-qF | ACCTGAGAGCCCTAACAACC | 60 | |
| VcANS-qR | CTGTGATCCATTTGCCCTCG | ||
| VcUFGT-qF | ATTGGTGTGAGAGTGGAGGG | 60 | |
| VcUFGT-qR | GTCCAACAGCCTTCAAAGCA | ||
| VcGAPDH-qF | ACTACCATCCACTCTATCACCG | 60 | |
| VcGAPDH-qR | AACACCTTACCAACAGCCTTG |
表1 本研究所用引物信息
Table 1 Information for the primers used in this study
| 引物名称 | 引物序列 | 退火温度 | 用途 |
|---|---|---|---|
| Primer name | Primer sequence (5′-3′) | Annealing temperature (℃) | Applications |
| VcGSTF19F | ATGGTGGTCAAAGTTTATGGTCCAATTAGAG | 63 | 基因克隆 Gene cloning |
| VcGSTF19R | CTAATCCATAAGCTTCATTATTTTCTTCCAAGCAG | ||
| VcGSTF19-SF | CTCTACAAATCTATCTCTGGATCCATGGTGGTCAAAGTTTATGGTCCAATTAGAG | 68 | 载体构建 |
| VcGST19F-SR | GATTTTTGCGGACTCTAGGAGCTCCTAATCCATAAGCTTCATTATTTTCTTCCAAGCAG | Vector construction | |
| VcGSTF19-qF | GGAGAGAGCCCTTGTTGACC | 60 | 实时荧光定量 |
| VcGSTF19-qR | GCTCTTCGACAACCTCTGCT | RT-qPCR | |
| VcCHS-qF | CGCATGTGTGACAAATCCCA | 60 | |
| VcCHS-qR | ACCATATCCTGCCTAGCGTC | ||
| VcCHI-qF | TCTTTCCTCCGTCGGTCAAA | 60 | |
| VcCHI-qR | ACTCTACGGCGCTAACTTGT | ||
| VcF3H-qF | GTGGACGGAGCTTTTGTTGT | 60 | |
| VcF3H-qR | GTGATTGGCTCGTCGAGAAC | ||
| VcDFR-qF | GCTTCTTGAACGGGGCTATG | 60 | |
| VcDFR-qR | CTTCAATGGCCTCGTCGAAG | ||
| VcANS-qF | ACCTGAGAGCCCTAACAACC | 60 | |
| VcANS-qR | CTGTGATCCATTTGCCCTCG | ||
| VcUFGT-qF | ATTGGTGTGAGAGTGGAGGG | 60 | |
| VcUFGT-qR | GTCCAACAGCCTTCAAAGCA | ||
| VcGAPDH-qF | ACTACCATCCACTCTATCACCG | 60 | |
| VcGAPDH-qR | AACACCTTACCAACAGCCTTG |
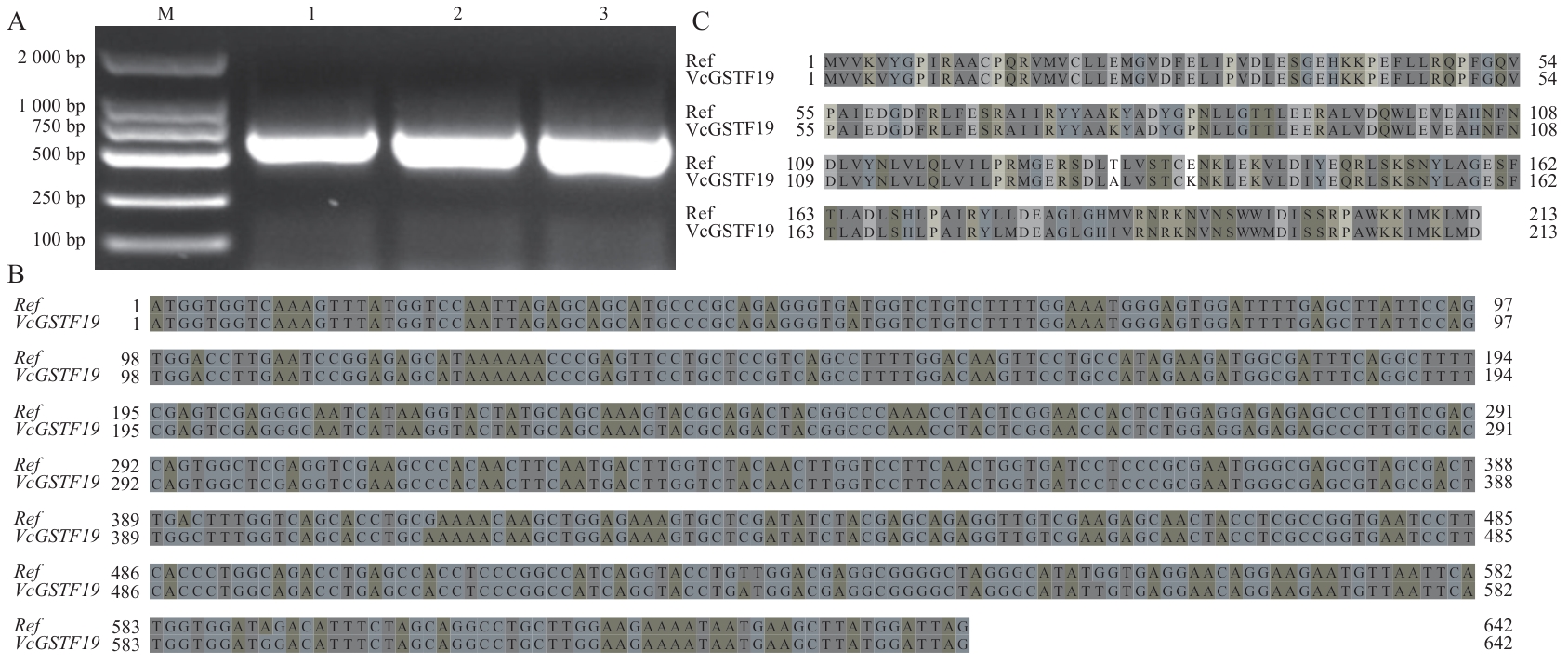
图1 蓝莓VcGSTF19基因的克隆和序列分析A:VcGSTF19全长CDS扩增产物电泳检测结果。M:DL2000 Marker;1-3代表3个重复。B:VcGSTF19与参考核苷酸序列比对结果。C:VcGSTF19与参考序列蛋白序列比对结果。Ref:参考序列
Fig. 1 Gene cloning and sequence analysis of blueberry VcGSTF19 geneA: Electrophoresis detection results of amplified VcGSTF19. M: DL2000 marker; 1-3 indicate three replications. B: Nucleotide acid sequences alignment results of amplified VcGSTF19 and reference VcGSTF19. C: Protein sequences alignment result of protein sequences encoded by amplified VcGSTF19 with reference VcGSTF19. Ref: Reference sequences

图2 基于拟南芥蛋白数据库的VcGSTF19互作蛋白预测TT8:透明种皮8;DFRA:二氢黄酮醇4-还原酶;LDOX:无色花青素双加氧酶; A3G2XYLT:花青素3-O-葡萄糖苷2'″-O-木糖基转移酶;CYP75B1:类黄酮3'-单加氧酶;5MTA:丙二酰辅酶A:花青素5-O-葡萄糖苷-6''-O-丙二酰转移酶;AHA10:拟南芥H(+)-ATP酶质子泵10;DTX41:解毒蛋白41;UGT75C1:UDP-糖基转移酶75C1
Fig. 2 Predicted interacting proteins of VcGSTF19 based on the Arabidopsis protein databaseTT8: Transparent testa 8. DFRA: Dihydroflavonol 4-reductase. LDOX: Leucoanthocyanidin dioxygenase. A3G2XYLT: Anthocyanidin 3-O-glucoside 2'″- O-xylosyltransferase. CYP75B1: Flavonoid 3'-monooxygenase. AHA10: Arabidopsis H (+)-ATPase proton pump 10. DTX41: detoxification 41. UGT75C1: UDP-glycosyltransferase 75C1
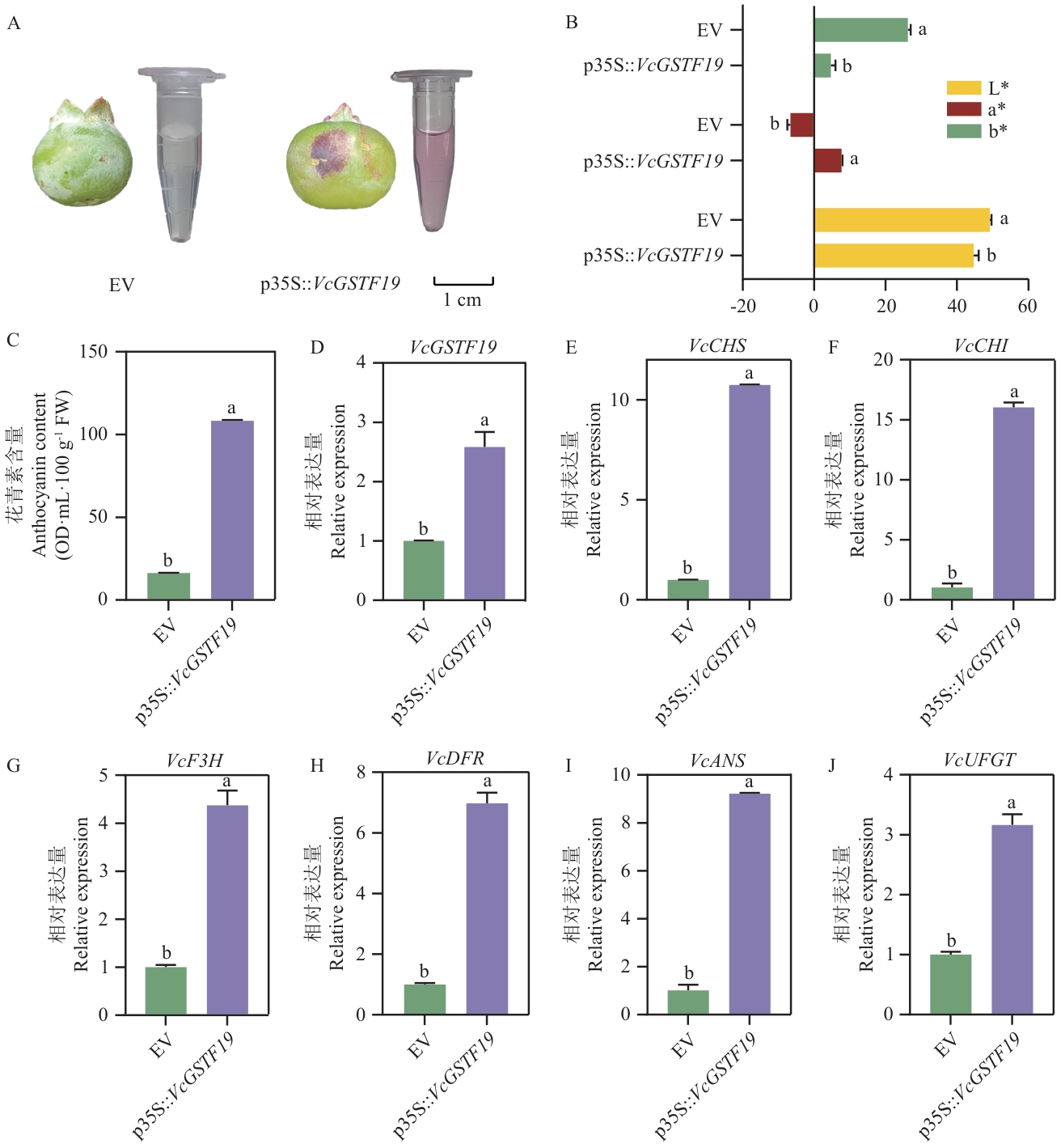
图3 VcGSTF19瞬时过表达对蓝莓果皮花青素代谢的影响A:果实表型及花青素提取溶液;B:瞬时过表达VcGSTF19对蓝莓果皮色差指标的影响;C:瞬时过表达VcGSTF19对蓝莓果皮花青素含量的影响。D-J:分别为瞬时过表达VcGSTF19的蓝莓果皮中VcGSTF19、VcCHS、VcCHI、VcF3H、VcDFR、VcANS和VcUFGT基因表达分析结果。柱上不同小写字母表示差异显著(P<0.05)。EV:空载对照;FW:鲜重;GSTF:F亚家族谷胱甘肽转移酶;CHS:查尔酮合酶;CHI:查尔酮异构酶;F3H:黄酮3-羟化酶;DFR:二氢黄酮醇4-还原酶;ANS:花青素合酶;UFGT:UDP葡萄糖:类黄酮3-糖基转移酶
Fig. 3 Influences of VcGSTF19 transient overexpression on the anthocyanin metabolism in blueberry fruit peelsA: Fruit phenotype and extracted anthocyanin solution; B: Influences of VcGSTF19 transient overexpression on the blueberry fruit color parameters. C: Influences of VcGSTF19 transient overexpression on anthocyanin contents in blueberry fruit peels. D-J: Influences of VcGSTF19 overexpression on the relative expressions of VcGSTF19, VcCHS, VcCHI, VcF3H, VcDFR, VcANS and VcUFGT in blueberry fruit peels. Different letters above columns indicate significant differences between samples at P<0.05 level. EV: Empty vector. FW: Fresh weight. GSTF: F subfamily glutathione S- transferase. CHS: Chalcone synthase. CHI: Chalcone isomerase. F3H: Flavonoid 3-hydroxylase. DFR: dihydroflavonol 4-reductase. ANS: anthocyanin synthase. UFGT: UDP Glucose. flavonoid 3-glycosyltransferase
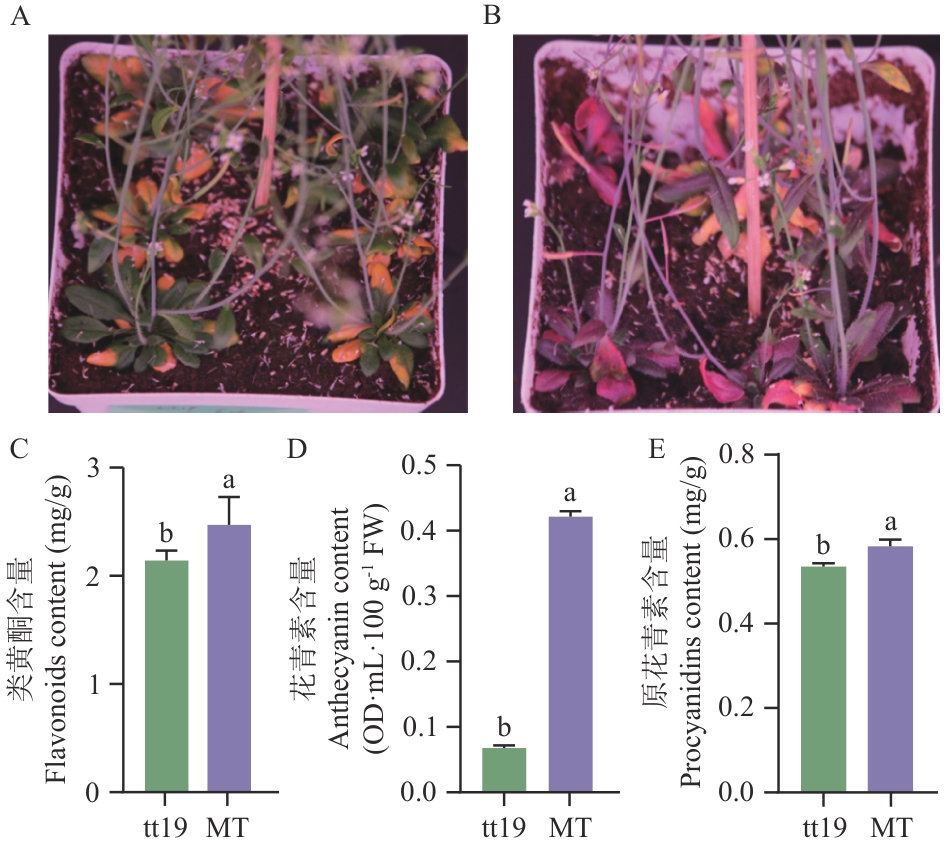
图4 VcGSTF19过表达对tt19突变体莲座叶的影响A:拟南芥tt19突变体;B:过表达VcGSTF19的转基因植株;C-E:VcGSTF19过表达对拟南芥莲座叶类黄酮、花青素和原花青素含量的影响;MT:过表达VcGSTF19的tt19转基因植株
Fig. 4 Influences of VcGSTF19 overexpression on the rosette leaves of Arabidopsistt19 mutantsA: Arabidopsistt19 mutant. B: Transgenicplants overexpressing VcGSTF19. C-E: Influences of VcGSTF19 overexpression on the contents of flavonoids, anthocyanin and procyanidins in Arabidopsis rosette leaves, respectively. MT: Transgenic tt19 plants overexpressing VcGSTF19
| [1] | Wang L, Lan W, Chen D. Blueberry (Vaccinium spp.) anthocyanins and their functions, stability, bioavailability, and applications [J]. Foods, 2024, 13(17): 2851. |
| [2] | Zhang ZN, Qu PY, Hao SY, et al. Characterization and functional analysis of Chalcone synthase genes in highbush blueberry (Vaccinium corymbosum) [J]. Int J Mol Sci, 2023, 24(18): 13882. |
| [3] | 杨艳, 胡洋, 刘霓如, 等. '红满堂'苹果MbbZIP43基因的克隆与功能研究 [J]. 生物技术通报, 2024, 40(2): 146-159. |
| Yang Y, Hu Y, Liu NR, et al. Cloning and functional analysis of MbbZIP43 gene in 'Hongmantang' red-flesh apple [J]. Biotechnol Bull, 2024, 40(2): 146-159. | |
| [4] | Zhang HB, Wang L, Deroles S, et al. New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals [J]. BMC Plant Biol, 2006, 6: 29. |
| [5] | Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays [J]. Plant Cell, 2004, 16(7): 1812-1826. |
| [6] | Saad KR, Kumar G, Puthusseri B, et al. Genome-wide identification of MATE, functional analysis and molecular dynamics of DcMATE21 involved in anthocyanin accumulation in Daucus carota [J]. Phytochemistry, 2023, 210: 113676. |
| [7] | Passamonti S, Cocolo A, Braidot E, et al. Characterization of electrogenic bromosulfophthalein transport in carnation petal microsomes and its inhibition by antibodies against bilitranslocase [J]. FEBS J, 2005, 272(13): 3282-3296. |
| [8] | Lipka V, Kwon C, Panstruga R. SNARE-ware: the role of SNARE-domain proteins in plant biology [J]. Annu Rev Cell Dev Biol, 2007, 23: 147-174. |
| [9] | Samkumar A, Jones D, Karppinen K, et al. Red and blue light treatments of ripening bilberry fruits reveal differences in signalling through abscisic acid-regulated anthocyanin biosynthesis [J]. Plant Cell Environ, 2021, 44(10): 3227-3245. |
| [10] | 尹雨钦, 徐欢欢, 唐丽萍, 等. 不结球白菜GST基因家族的全基因组鉴定及花青素相关基因BcGSTF6的功能分析 [J]. 中国农业科学, 2024, 57(16): 3234-3249. |
| Yin YQ, Xu HH, Tang LP, et al. Genome-wide identification of GST gene family and functional analysis of the BcGSTF6 gene related to anthocyanin in pak choi [J]. Sci Agric Sin, 2024, 57(16): 3234-3249. | |
| [11] | 金雪花, 洪艳, 黄河, 等. 瓜叶菊谷胱甘肽转移酶基因GST的分离及表达分析 [J]. 园艺学报, 2013, 40(6): 1129-1138. |
| Jin XH, Hong Y, Huang H, et al. Isolation and expression analysis of GST gene encoding glutathione S-transferase from Senecio cruentus [J]. Acta Hortic Sin, 2013, 40(6): 1129-1138. | |
| [12] | Islam MS, Choudhury M, Majlish AK, et al. Comprehensive genome-wide analysis of Glutathione S-transferase gene family in potato (Solanum tuberosum L.) and their expression profiling in various anatomical tissues and perturbation conditions [J]. Gene, 2018, 639: 149-162. |
| [13] | Frear DS, Swanson HR. Biosynthesis of S-(4-ethylamino-6-isopropylamino- 2-s-triazino) glutathione: partial purification and properties of a glutathione S-transferase from corn [J]. Phytochemistry, 1970, 9(10): 2123-2132. |
| [14] | Abdul Kayum M, Nath UK, Park JI, et al. Genome-wide identification, characterization, and expression profiling of glutathione S-transferase (GST) family in pumpkin reveals likely role in cold-stress tolerance [J]. Genes, 2018, 9(2): 84. |
| [15] | Aloke C, Onisuru OO, Achilonu I. Glutathione S-transferase: a versatile and dynamic enzyme [J]. Biochem Biophys Res Commun, 2024, 734: 150774. |
| [16] | Micic N, Rønager AH, Sørensen M, et al. Overlooked and misunderstood: can glutathione conjugates be clues to understanding plant glutathione transferases? [J]. Philos Trans R Soc Lond B Biol Sci, 2024, 379(1914): 20230365. |
| [17] | Zhang YY, Zhang ZN, Guo SJ, et al. Characterization of blueberry glutathione S-transferase (GST) genes and functional analysis of VcGSTF8 reveal the role of 'MYB/bHLH-GSTF' module in anthocyanin accumulation [J]. Ind Crops Prod, 2024, 218: 119006. |
| [18] | Sun Y, Li H, Huang JR. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts [J]. Mol Plant, 2012, 5(2): 387-400. |
| [19] | Luo HF, Dai C, Li YP, et al. Reduced Anthocyanins in Petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry [J]. J Exp Bot, 2018, 69(10): 2595-2608. |
| [20] | Tasaki K, Yoshida M, Nakajima M, et al. Molecular characterization of an anthocyanin-related glutathione S-transferase gene in Japanese gentian with the CRISPR/Cas9 system [J]. BMC Plant Biol, 2020, 20(1): 370. |
| [21] | Hu B, Zhao JT, Lai B, et al. LcGST4 is an anthocyanin-related glutathione S-transferase gene in Litchi chinensis Sonn [J]. Plant Cell Rep, 2016, 35(4): 831-843. |
| [22] | Jiang SH, Chen M, He NB, et al. MdGSTF6, activated by MdMYB1, plays an essential role in anthocyanin accumulation in apple [J]. Hortic Res, 2019, 6: 40. |
| [23] | Liu YF, Qi YW, Zhang AL, et al. Molecular cloning and functional characterization of AcGST1, an anthocyanin-related glutathione S-transferase gene in kiwifruit (Actinidia chinensis) [J]. Plant Mol Biol, 2019, 100(4-5): 451-465. |
| [24] | Zhao YW, Wang CK, Huang XY, et al. Genome-wide analysis of the glutathione S-transferase (GST) genes and functional identification of MdGSTU12 reveals the involvement in the regulation of anthocyanin accumulation in apple [J]. Genes, 2021, 12(11): 1733. |
| [25] | Yuan SQ, Yang CK, Zheng B, et al. Genome-wide identification and expression analysis of GST genes during light-induced anthocyanin biosynthesis in mango (Mangifera indica L.) [J]. Plants, 2024, 13(19): 2726. |
| [26] | Kalt W, Cassidy A, Howard LR, et al. Recent research on the health benefits of blueberries and their anthocyanins [J]. Adv Nutr, 2020, 11(2): 224-236. |
| [27] | Lv W, Zhu LY, Tan LF, et al. Genome-wide identification analysis of GST gene family in wild blueberry Vaccinium duclouxii and their impact on anthocyanin accumulation [J]. Plants, 2024, 13(11): 1497. |
| [28] | Wang XX, Dong JJ, Hu YT, et al. Identification and characterization of the glutathione S-transferase gene family in blueberry (Vaccinium corymbosum) and their potential roles in anthocyanin intracellular transportation [J]. Plants, 2024, 13(10): 1316. |
| [29] | Zhang YY, Huang DQ, Wang B, et al. Characterization of highbush blueberry (Vaccinium corymbosum L.) anthocyanin biosynthesis related MYBs and functional analysis of VcMYB gene [J]. Curr Issues Mol Biol, 2023, 45(1): 379-399. |
| [30] | Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana [J]. Plant J, 1998, 16(6): 735-743. |
| [31] | Vaish S, Gupta D, Mehrotra R, et al. Glutathione S-transferase: a versatile protein family [J]. 3 Biotech, 2020, 10(7): 321. |
| [32] | Cao YW, Xu LF, Xu H, et al. LhGST is an anthocyanin-related glutathione S-transferase gene in Asiatic hybrid lilies (Lilium spp.) [J]. Plant Cell Rep, 2021, 40(1): 85-95. |
| [33] | Cui YM, Fan JW, Lu CF, et al. ScGST3 and multiple R2R3-MYB transcription factors function in anthocyanin accumulation in Senecio cruentus [J]. Plant Sci, 2021, 313: 111094. |
| [34] | Duan AQ, Deng YJ, Tan SS, et al. DcGST1, encoding a glutathione S-transferase activated by DcMYB7, is the main contributor to anthocyanin pigmentation in purple carrot [J]. Plant J, 2024, 117(4): 1069-1083. |
| [35] | Qiu LK, Chen K, Pan J, et al. Genome-wide analysis of glutathione S-transferase genes in four Prunus species and the function of PmGSTF2, activated by PmMYBa1, in regulating anthocyanin accumulation in Prunus mume [J]. Int J Biol Macromol, 2024, 281: 136506. |
| [36] | Zhou HP, He JX, Zhang YY, et al. RHA2b-mediated MYB30 degradation facilitates MYB75-regulated, sucrose-induced anthocyanin biosynthesis in Arabidopsis seedlings [J]. Plant Commun, 2024, 5(3): 100744. |
| [37] | Li SN, Wang WY, Gao JL, et al. MYB75 phosphorylation by MPK4 is required for light-induced anthocyanin accumulation in Arabidopsis [J]. Plant Cell, 2016, 28(11): 2866-2883. |
| [38] | Wang JH, Xu R, Qiu SP, et al. CsTT8 regulates anthocyanin accumulation in blood orange through alternative splicing transcription [J]. Hortic Res, 2023, 10(10): uhad190. |
| [39] | Deng J, Li JJ, Su MY, et al. A bHLH gene NnTT8 of Nelumbo nucifera regulates anthocyanin biosynthesis [J]. Plant Physiol Biochem, 2021, 158: 518-523. |
| [1] | 董向向, 缪百灵, 许贺娟, 陈娟娟, 李亮杰, 龚守富, 朱庆松. 森林草莓FveBBX20基因的生物信息学分析及开花调控功能[J]. 生物技术通报, 2025, 41(9): 115-123. |
| [2] | 刘佳丽, 宋经荣, 赵文宇, 张馨元, 赵子洋, 曹一博, 张凌云. 蓝莓R2R3-MYB基因鉴定及类黄酮调控基因表达分析[J]. 生物技术通报, 2025, 41(9): 124-138. |
| [3] | 康琴, 汪霞, 谌明洋, 徐静天, 陈诗兰, 廖平杨, 许文志, 吴卫, 徐东北. 薄荷UV-B受体基因McUVR8的克隆与表达分析[J]. 生物技术通报, 2025, 41(8): 255-266. |
| [4] | 李开杰, 吴瑶, 李丹丹. 红花CtbHLH128基因克隆及调控干旱胁迫应答功能研究[J]. 生物技术通报, 2025, 41(8): 234-241. |
| [5] | 魏雨佳, 李岩, 康语涵, 弓晓楠, 杜敏, 涂岚, 石鹏, 于子涵, 孙彦, 张昆. 白颖苔草CrMYB4基因的克隆和表达分析[J]. 生物技术通报, 2025, 41(7): 248-260. |
| [6] | 李锐, 胡婷, 陈树溦, 王尧, 王计平. 紫苏PfMYB80转录因子正向调控花青素的生物合成[J]. 生物技术通报, 2025, 41(6): 243-255. |
| [7] | 许慧珍, SHANTWANA Ghimire, RAJU Kharel, 岳云, 司怀军, 唐勋. 马铃薯SUMO E3连接酶基因家族分析及StSIZ1基因的克隆与表达模式分析[J]. 生物技术通报, 2025, 41(6): 119-129. |
| [8] | 裴景琪, 赵梦然, 黄晨阳, 邬向丽. 一个影响糙皮侧耳生长发育的功能基因的发现与验证[J]. 生物技术通报, 2025, 41(6): 327-334. |
| [9] | 刘鑫, 王嘉雯, 李进伟, 牟策, 杨盼盼, 明军, 徐雷锋. 兰州百合三个LdBBXs基因的克隆与表达分析[J]. 生物技术通报, 2025, 41(5): 186-196. |
| [10] | 杨朝结, 张兰, 陈红, 黄娟, 石桃雄, 朱丽伟, 陈庆富, 李洪有, 邓娇. 苦荞转录因子基因FtbHLH3调控类黄酮生物合成的功能鉴定[J]. 生物技术通报, 2025, 41(4): 134-144. |
| [11] | 班秋艳, 赵鑫月, 迟文静, 黎俊生, 王琼, 夏瑶, 梁丽云, 贺巍, 李叶云, 赵广山. 茶树光敏色素互作因子CsPIF3a的克隆及其与光温逆境的响应[J]. 生物技术通报, 2025, 41(4): 256-265. |
| [12] | 田琴, 刘奎, 吴翔纬, 纪媛媛, 曹一博, 张凌云. 转录因子VcMYB17调控蓝莓抗旱性的功能研究[J]. 生物技术通报, 2025, 41(4): 198-210. |
| [13] | 彭婷, 林颖, 谭圆圆, 饶英, 黄覃, 张文娥, 汪波, 田瑞丰, 刘国锋. 多星韭AwANSs基因的克隆与表达分析[J]. 生物技术通报, 2025, 41(3): 230-239. |
| [14] | 许圆梦, 毛娇, 王梦瑶, 王数, 任江陵, 刘宇涵, 刘思辰, 乔治军, 王瑞云, 曹晓宁. 糜子PmDEP1和PmEP3基因的克隆与表达特征分析[J]. 生物技术通报, 2025, 41(2): 150-162. |
| [15] | 焦小雨, 吴琼, 刘丹丹, 孙明慧, 阮旭, 王雷刚, 王文杰. 茶树CsWAK8克隆及其在响应冷胁迫过程中的功能分析[J]. 生物技术通报, 2025, 41(2): 210-220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||