








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (8): 95-110.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1398
Previous Articles Next Articles
MA Xiao-xiang1( ), LIU Ya-yue1,2,3, NIE Ying-ying2, LI Yan-mei1, WANG Yuan1, XUE Xin-yi1, HONG Peng-zhi1,2,3, ZHANG Yi1,2,3(
), LIU Ya-yue1,2,3, NIE Ying-ying2, LI Yan-mei1, WANG Yuan1, XUE Xin-yi1, HONG Peng-zhi1,2,3, ZHANG Yi1,2,3( )
)
Received:2020-11-18
Online:2021-08-26
Published:2021-09-10
Contact:
ZHANG Yi
E-mail:ma.xiao.xiang@163.com;hubeizhangyi@163.com
MA Xiao-xiang, LIU Ya-yue, NIE Ying-ying, LI Yan-mei, WANG Yuan, XUE Xin-yi, HONG Peng-zhi, ZHANG Yi. LC-MS/MS Based Molecular Network Analysis of the Effects of Chemical Regulation on the Secondary Metabolites and Biological Activities of a Fungal Strain Aspergillus terreus C23-3[J]. Biotechnology Bulletin, 2021, 37(8): 95-110.

Fig.1 Growth morphology of strain C23-3 before and after chemical regulation(cultured for 28 days) A:The left column shows the cultures in seawater potato liquid medium,and the right column is for brown rice medium;the photos from top to bottom are experimental groups added 3-amino-L-tyrosine,3-hydroxy-L-tyrosine,3-nitro-L-tyrosine,3,4-dihydroxyphenylpyruvate,farnesol,MSB and H2O2,respectively. B:The top is for the seawater potato liquid medium,and the bottom is for the brown rice medium;the 3 columns on the left are the experimental groups with FPP,and the last column on the right is the experimental group with L-selenomethionine. C:The top is for the seawater potato liquid medium,and the bottom is for the brown rice medium;the photos from left to right are the solvent control groups supplemented with sterile water,DMSO and 5% HCl and blank control groups. The labels below the picture are the concentrations of the inducers added. For each of the above groups,two repeats were set
| 实验组/空白组和试剂对照组 Experimental group vs. blank group and reagent control group | 变化组分的Rf值范围 Rf value ranges of variant components | 波长 Wavelength 254 nm | 波长 Wavelength 365 nm | 茴香醛显色 Anisaldehyde colorization | DPPH自由基清除活性DPPH free radical scavenging activity | 铁氰化钾-三氯化铁显色K3[Fe(CN)6]-FeCl3 colorization | 乙酰胆碱酯酶抑制活性(Rf 0.67-0.90)Acetylcholinesterase inhibitory activity(Rf 0.67-0.90) |
|---|---|---|---|---|---|---|---|
| 3-氨基-L-酪氨酸(1 mmol/L)组/空白组和5%HCl组 3-Amino-L-tyrosine(1 mmol/L)group vs. blank group and 5%HCl group | 0.14-0.50 | - | + | - | + | + | + |
| 3-羟基-L-酪氨酸(10 mmol/L)组/空白组和5%HCl组 3-Hydroxy-L-tyrosine(10 mmol/L)group vs. blank group and 5%HCl group | 0.11-0.46 | + | + | - | + | + | + |
| 3-硝基-L-酪氨酸(10 mmol/L)组/空白组和5%HCl组 3-Nitro-L-tyrosine(10 mmol/L)group vs. blank group and 5%HCl group | 0.17-0.53 | / | + | + | + | + | + |
| FPP(5 µmol/L)组/空白组 FPP(5 µmol/L)group vs. blank group | 0.16-0.55 | - | / | + | / | / | + |
| 3,4-二羟基苯丙酮酸(1 mmol/L)组/空白组和DMSO组 3,4-Dihydroxyphenylpyruvate(1 mmol/L)group vs. blank group and DMSO group | 0.16-0.53 | + | + | / | + | + | + |
| 金合欢醇(1 µmol/L)组/空白组和DMSO组 Farnesol(1 µmol/L)group vs. blank group and DMSO group | 0.15-0.53 | + | + | / | + | / | + |
| MSB(10 µmol/L)组/空白组和无菌水组MSB(10 µmol/L)group vs. blank group and sterile water group | 0.13-0.45 | / | / | / | + | + | / |
| H2O2(1 mmol/L)组/空白组和无菌水组(14 d)H2O2(1 mmol/L)group vs. blank group and sterile water group(14 d) | 0.20-0.53 | / | / | / | + | + | / |
| L-硒代蛋氨酸(61 mmol/L)组/空白组和无菌水组 L-selenomethionine(61 mmol/L)group vs. blank group and sterile water group | 0.15-0.38 | / | + | / | / | + | / |
Table 1 The comparison of TLC fingerprints of strain C23-3 metabolic products before and after chemical regulation
| 实验组/空白组和试剂对照组 Experimental group vs. blank group and reagent control group | 变化组分的Rf值范围 Rf value ranges of variant components | 波长 Wavelength 254 nm | 波长 Wavelength 365 nm | 茴香醛显色 Anisaldehyde colorization | DPPH自由基清除活性DPPH free radical scavenging activity | 铁氰化钾-三氯化铁显色K3[Fe(CN)6]-FeCl3 colorization | 乙酰胆碱酯酶抑制活性(Rf 0.67-0.90)Acetylcholinesterase inhibitory activity(Rf 0.67-0.90) |
|---|---|---|---|---|---|---|---|
| 3-氨基-L-酪氨酸(1 mmol/L)组/空白组和5%HCl组 3-Amino-L-tyrosine(1 mmol/L)group vs. blank group and 5%HCl group | 0.14-0.50 | - | + | - | + | + | + |
| 3-羟基-L-酪氨酸(10 mmol/L)组/空白组和5%HCl组 3-Hydroxy-L-tyrosine(10 mmol/L)group vs. blank group and 5%HCl group | 0.11-0.46 | + | + | - | + | + | + |
| 3-硝基-L-酪氨酸(10 mmol/L)组/空白组和5%HCl组 3-Nitro-L-tyrosine(10 mmol/L)group vs. blank group and 5%HCl group | 0.17-0.53 | / | + | + | + | + | + |
| FPP(5 µmol/L)组/空白组 FPP(5 µmol/L)group vs. blank group | 0.16-0.55 | - | / | + | / | / | + |
| 3,4-二羟基苯丙酮酸(1 mmol/L)组/空白组和DMSO组 3,4-Dihydroxyphenylpyruvate(1 mmol/L)group vs. blank group and DMSO group | 0.16-0.53 | + | + | / | + | + | + |
| 金合欢醇(1 µmol/L)组/空白组和DMSO组 Farnesol(1 µmol/L)group vs. blank group and DMSO group | 0.15-0.53 | + | + | / | + | / | + |
| MSB(10 µmol/L)组/空白组和无菌水组MSB(10 µmol/L)group vs. blank group and sterile water group | 0.13-0.45 | / | / | / | + | + | / |
| H2O2(1 mmol/L)组/空白组和无菌水组(14 d)H2O2(1 mmol/L)group vs. blank group and sterile water group(14 d) | 0.20-0.53 | / | / | / | + | + | / |
| L-硒代蛋氨酸(61 mmol/L)组/空白组和无菌水组 L-selenomethionine(61 mmol/L)group vs. blank group and sterile water group | 0.15-0.38 | / | + | / | / | + | / |

Fig.2 TLC fingerprints of strain C23-3 fermentation products before and after chemical regulation(brown rice medium) A1-C1:UV images under 254 nm;A2-C2:fluorescence images under 365 nm;A3-C3:images of colorization by anisaldehyde-sulfuric acid reagent;A4-C4:images of 1,1-Diphenyl-2-picryl-hydrazyl(DPPH)free radical scavenging bioautography;A5-C5:images of colorization by potassium ferricyanide-ferric chloride reagent;A6-C6:images of acetylcholinesterase inhibitory bioautography. Abbreviations:Farnesyl pyrophosphate ammonium salt to FPP,menadione sodium bisulfite to MSB

Fig.3 Molecular network and its partial enlarged diagram of the metabolites of strain C23-3 under different cultural condi-tions based on MS2 relationships A1 and A2:Enlarged images of ion clusters of butyrolactone I congeners;B:enlarged images of ion clusters of territrem B congeners;C:enlarged images of ion clusters of lovastatin congeners. Compounds 1-4:butyrolactone I,territrem B,lovastatin,and lovastatin hydroxy acid. Compounds 5-13:Possible derivatives of known compounds,i.e.,[M(butyrolactoneⅠisomer)+H]+,[M(butyrolactoneⅠ-2H)+H]+,[M(butyrolactoneⅠisomer+O)+H]+,[M(butyrolactone I-H2O)+H]+,[M(territrem B-O)+H]+,[M(territrem B-CH2)+H]+,[M(lovastatin-H2O)+H]+,[M(lovastatin-O)+H]+,and[M(lovastatin+2H)+H]+,respectively. The nodes in the molecular network are marked in 15 colors, ,sequentially representing the 15 sample sources of compounds:induction groups with the addition of 3-amino-L-tyrosine(1 mmol/L),3-hydroxy-L-tyrosine(10 mmol/L),3-nitro-L-tyrosine(10 mmol/L),3,4-dihydroxyphenylpyruvate(1 mmol/L),farnesol(1 µmol/L),FPP(5 μmol/L),MSB(10 µmol/L),H2O2(1 mmol /L)and selenomethionine(61 mmol/L);blank groups cultured for 14 d and 28 d;reagent control groups supplemented separately with 5% HCl,DMSO,and sterile water(the sterile water group cultures for 14 d and 28 d,respectively)
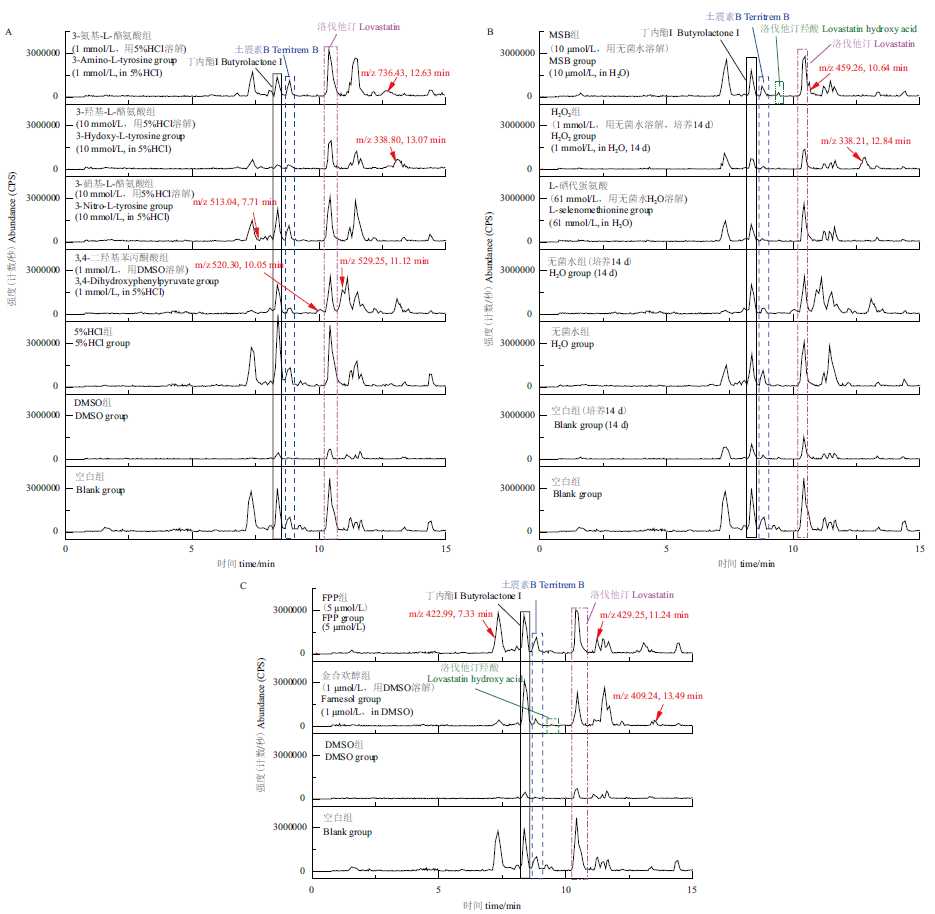
Fig.4 Base peak chromatograms of the metabolites of strain C23-3 under different culture conditions A-C are the LC-MS base peak chromatograms of the induction groups added with analogs of small molecule precursor tyrosine/4-hydroxyphenylpyruvate(3-amino-L-tyrosine,3-hydroxy-L-tyrosine,3-nitro -L-tyrosine and 3,4-dihydroxyphenylpyruvate),oxidative stress agents MSB and H2O2),L-selenomethionine,and analogs of natural terpenoid side chain donor dimethylallyl diphosphate FPPand farnesol),their corresponding blank groups and solvent control groups. Except for the specified “14 d” groups,which are for the 14 d culture extracts,the other groups were cultured for 28 d. The group name is marked in the upper left corner of each base peak chromatogram. The black frames label butyrolactone I,the blue ones label territrem B,the purple ones label lovastatin,and the green ones label lovastatin hydroxy acid;the arrows indicate variant metabolites

Fig. 5 MS2 spectra of compound 1 and its possible derivatives The labeling of Compounds 1 and 5-8 follows the same numbers as in Fig.3. The similarity values represent the cosine values of the similarity comparison between detected compounds in samples and the pure compounds recorded in GNPS database or the annotated known compound 1 or compound 5 by GNPS molecular networking(the red circles in Fig.5 mark the corresponding series of fragment ions tween the two compared compounds).(The m/z values of the parent ions in this figure and the following Fig.6 and Fig.7,showed small errors less than 0.5 Da compared with the values of the corresponding nodes in Fig.3. This difference was produced because the m/z values marked on the nodes in the molecular network are the average m/z values of the precursor ions in a set of mass spectra,and the values in Figs.5-7 are specific values from specific mass spectra). Compound 5 does not match with butyrolactone I in the molecular network and has no cosine value. However,it is speculated to be an isomer of butyrolactone I through the manual comparison of MS2 spectra

Fig.8 Contents of the characteristic products and their derivatives in the metabolites of strain C23-3 under different chemically regulated conditions Groups 1-9:Induction groups added with 3-amino-L-tyrosine(1 mmol/L),3-hydroxy-L-tyrosine(10 mmol/L),3-nitro-L-tyrosine(10 mmol/L),FPP(5 μmol/L),3,4-dihydroxyphenylpyruvate(1 mmol/L),farnesol(1 µmol/L),MSB(10 µmol /L),H2O2(1 mmol/L),and L-selenomethionine(61 mmol/L)respectively. The labeling of compounds 1-13 follows the same numbers as in Fig.3. The unit of peak area(area)is counts,counts = CPS*s
| [1] | 张偲, 张长生, 田新朋, 等. 中国海洋微生物多样性研究[J]. 生物多样性保护, 2010, 25(6):652-658. |
| Zhang S, Zhang CS, Tian XP, et al. The study of diversities of marine microbes in China[J]. Biodiversity Conservation, 2010, 25(6):652-658. | |
| [2] |
Blunt JW, Carroll AR, Copp BR, et al. Marine natural products[J]. Natural Product Reports, 2018, 35(1):8-53.
doi: 10.1039/c7np00052a pmid: 29335692 |
| [3] |
Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010[J]. Journal of Natural Products, 2012, 75(3):311-335.
doi: 10.1021/np200906s pmid: 22316239 |
| [4] | 赵成英, 朱统汉, 朱伟明. 2010~2013之海洋微生物新天然产物[J]. 有机化学, 2013, 33(6):1195-1234. |
| Zhao CY, Zhu TH, Zhu WM. New marine natural products of microbial origin from 2010 to 2013[J]. Chinese Journal of Organic Chemistry, 2013, 33(6):1195-1234. | |
| [5] |
Keller NP. Fungal secondary metabolism:regulation, function and drug discovery[J]. Nature Reviews Microbiology, 2019, 17(3):167-180.
doi: 10.1038/s41579-018-0121-1 pmid: 30531948 |
| [6] |
Bode HB, Bethe B, Höfs R, et al. Big effects from small changes:possible ways to explore nature’s chemical diversity[J]. ChemBioChem, 2002, 3(7):619-627.
doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9 URL |
| [7] |
Yang JY, Sanchez LM, Rath CM, et al. Molecular networking as a dereplication strategy[J]. Journal of Natural Products, 2013, 76(9):1686-1699.
doi: 10.1021/np400413s URL |
| [8] |
Wang M, Carver JJ, Phelan VV, et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking[J]. Nature Biotechnology, 2016, 34(8):828-837.
doi: 10.1038/nbt.3597 URL |
| [9] | Nie YY, Yang WC, Liu YY, et al. Acetylcholinesterase inhibitors and antioxidants mining from marine fungi:bioassays, bioactivity coupled LC-MS/MS analyses and molecular networking[J]. Marine Life Science & Technology, 2020, 2(4):386-397. |
| [10] | 梁金月, 李文卿, 杨静明, 等. 海洋土曲霉C23-3与不同类型海洋微生物共培养对其次生代谢产物的影响[J]. 广东海洋大学学报, 2020, 40(1):44-54. |
| Liang JY, Li WQ, Yang JM, et al. Effects of co-culture of marine fungus Aspergillus terreus C23-3 and different types of marine microorganisms on secondary metabolites[J]. Journal of Guangdong Ocean University, 2020, 40(1):44-54. | |
| [11] |
Haroon MH, Premaratne SR, Choudhry MI, et al. A new β-glucuronidase inhibiting butyrolactone from the marine endophytic fungus Aspergillus terreus[J]. Natural Product Research, 2013, 27(12):1060-1066.
doi: 10.1080/14786419.2012.708659 pmid: 22913423 |
| [12] |
Ma XH, Zhu TJ, Gu QQ, et al. Structures and Antiviral Activities of Butyrolactone Derivatives Isolated from Aspergillus terreus MXH-23[J]. Journal of Ocean University of China, 2014, 13(6):1067-1070.
doi: 10.1007/s11802-014-2324-z URL |
| [13] |
Ibrahim SRM, Mohamed GA, Khedr AIM. γ-Butyrolactones from Aspergillus species:structures, biosynjournal, and biological activities[J]. Natural Product Communications, 2017, 12(5):791-800.
pmid: 30496667 |
| [14] |
Quintanilla RA, Orellana DI, González-Billault C, et al. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway[J]. Experimental Cell Research, 2004, 295(1):245-57.
pmid: 15051507 |
| [15] |
Wei W, Wang X, Kusiak J W. Signaling events in amyloid β-peptide-induced neuronal death and insulin-like growth factor I protection[J]. Journal of Biological Chemistry, 2002, 277(20):17649-17656.
doi: 10.1074/jbc.M111704200 URL |
| [16] |
Dobashi Y, Shoji M, Kitagawa M, et al. Simultaneous suppression of cdc2 and cdk2 activities induces neuronal differentiation of PC12 cells[J]. Journal of Biological Chemistry, 2000, 275(17):12572-12580.
pmid: 10777547 |
| [17] |
Zhang YY, Zhang Y, Yao YB, et al. Butyrolactone-I from coral-derived fungus Aspergillus terreus attenuates neuro-inflammatory response via suppression of NF-κB pathway in BV-2 Cells[J]. Marine Drugs, 2018, 16(6):202.
doi: 10.3390/md16060202 URL |
| [18] |
Peng FC. Acetylcholinesterase inhibition by territrem B derivatives[J]. Journal of Natural Products, 1995, 58(6):857-862.
pmid: 7673929 |
| [19] | 杨静明, 杨文聪, 刘亚月, 等. 化学诱导对一株海洋来源土曲霉C23-3次生代谢产物及其生物活性的影响[J]. 微生物学通报, 2019, 46(3):441-452. |
| Yang JM, Yang WC, Liu YY, et al. Influence of chemical induction on the secondary metabolites and biological activities of a marine-derived fungal strain Aspergillus terreus C23-3[J]. Microbiology China, 2019, 46(3):441-452. | |
| [20] |
Guo CJ, Knox BP, Sanchez JF, et al. Application of an efficient gene targeting system linking secondary metabolites to their biosynthetic genes in Aspergillus terreus[J]. Organic Letters, 2013, 15(14):3562-3565.
doi: 10.1021/ol401384v URL |
| [21] |
Zhang LH, Feng BM, Zhao YQ, et al. Polyketide butenolide, diphenyl ether, and benzophenone derivatives from the fungus Aspergillus flavipes PJ03-11[J]. Bioorganic & Medicinal Chemistry Letters, 2016, 26(2):346-350.
doi: 10.1016/j.bmcl.2015.12.009 URL |
| [22] | 张翼, 鲍海燕, 聂影影, 等. 海洋真菌抗老年痴呆相关活性成分的筛选与追踪研究[J]. 现代食品科技, 2016, 32(11):63-71. |
| Zhang Y, Bao HY, Nie YY, et al. Screening and tracing of anti-Alzheimer’s related bioactive constituents from marine fungi[J]. Modern Food Science and Technology, 2016, 32(11):63-71. | |
| [23] |
Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes[J]. Biotechnology Advances, 2004, 22(3):189-259.
pmid: 14665401 |
| [24] | Schimmel TG, Coffman AD, Parsons SJ. Effect of butyrolactone I on the producing fungus, Aspergillus terreus[J]. Applied & Environmental Microbiology, 1998, 64(10):3707-3712. |
| [25] |
Rutledge PJ, Challis GL. Discovery of microbial natural products by activation of silent biosynthetic gene clusters[J]. Nature Reviews Microbiology, 2015, 13(8):509-523.
doi: 10.1038/nrmicro3496 pmid: 26119570 |
| [26] | 黄婷婷. 环酯肽抗生素吡啶霉素生物合成机制研究[D]. 上海:上海交通大学, 2011. |
| Huang TT. Deciphering the mechanism of the pyridomycin biosynthesis[D]. Shanghai:Shanghai Jiao Tong University, 2011. | |
| [27] | 李亚伟, 赵林, 郝永伟, 等. 诱导纤维堆囊菌高产埃博霉素的种间微生物筛选及鉴定[J]. 生物技术通报, 2013, 10:137-141. |
| Li YW, Zhao L, Hao YW, et al. Screening and identification of interspecies microorganisms inducing overproduction of epothiones in Sorangium cellulosum[J]. Biotechnology Bulletin, 2013, 10:137-141. |
| [1] | SUN Shu-fang, LUO Yong-li, LI Chun-hui, JIN Min, XU Qian. Determination of Lignin Monomer Crosslinking Structures in Wheat Stems by UPLC-MS/MS [J]. Biotechnology Bulletin, 2022, 38(10): 66-72. |
| [2] | LIU Shan, YE Wei, ZHU Mu-zi, LI Sai-ni, DENG Zhang-shuang, ZHANG Wei-min. Cloning,Expression and Characterization of a Novel Acyltransferase GPAT [J]. Biotechnology Bulletin, 2021, 37(11): 257-266. |
| [3] | MENG Li-ná, PENG Chun-ying, LI Tie-dong, LI Bo-sheng. Proteomic ánálysis of Spiruliná plátensis in Response to ársenic Stress [J]. Biotechnology Bulletin, 2020, 36(4): 107-116. |
| [4] | GAN Chong-kun, ZHOU Hui-wen, CHEN Rong-fa, FAN Ye-geng, QIU Li-hang, HUANG Xing, LI Yang-rui, LU Xing-gao, WU Jian-ming. Application of Chemical Regulating Technology in Sugarcane Production [J]. Biotechnology Bulletin, 2019, 35(2): 163-170. |
| [5] | HUANG Zi-lei, ZHANG Wei-min, YE Wei, LI Sai-ni, LI Hao-hua, ZHU Mu-zi. Cloning and Identification of Gene Promoter for Gliotoxin Biosynthesis from Deep-Sea-Derived Fungus Dichotomomyces cejpii [J]. Biotechnology Bulletin, 2018, 34(4): 144-150. |
| [6] | Li Haohua, Chen Yuchan, Wang Lei, Zhang Weimin. Study on Antimicrobial and Antitumor Activities of Marine-derived Fungus Penicillium herquei FS83 [J]. Biotechnology Bulletin, 2013, 0(1): 151-155. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||