








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (12): 265-273.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1511
Previous Articles Next Articles
WANG Huan-yu( ), CHANG Hao-wan, ZHANG Chong-qi, JIN Wei-lin, WEI Fang(
), CHANG Hao-wan, ZHANG Chong-qi, JIN Wei-lin, WEI Fang( )
)
Received:2020-12-13
Online:2021-12-26
Published:2022-01-19
Contact:
WEI Fang
E-mail:Huanyu_Wang@sjtu.edu.cn;fangwei@sjtu.edu.cn
WANG Huan-yu, CHANG Hao-wan, ZHANG Chong-qi, JIN Wei-lin, WEI Fang. Comparison of 5 Methods of Evaluating the Expressions of Chimeric Antigen Receptors[J]. Biotechnology Bulletin, 2021, 37(12): 265-273.
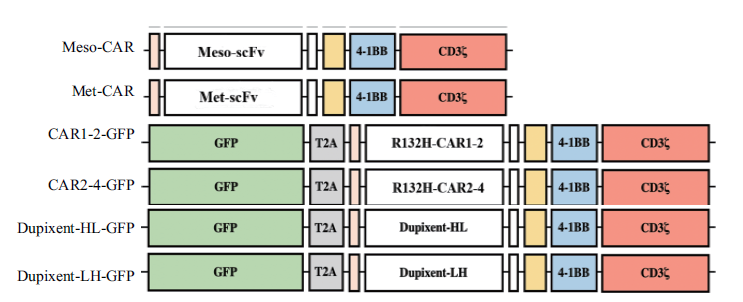
Fig. 1 Schematic diagram of CAR(chimeric antigen rec-eptor)vectors 4-1BB and CD3ζ are intracellular costimulatory signals of CAR molecules,and scFv is single-chain antibody fragment targeting specific antigens. The CAR molecules in the latest 4 vectors co-express with green fluorescent protein by the T2A system.(The scFv in Dupixent-HL-GFP is arranged in an order of the heavy chain followed by the light chain,and the scFv in Dupixent-LH-GFP is arranged in an order of the light chain followed by the heavy chain)

Fig. 2 Map of restriction enzyme digestion of CAR vectors The upper band is the vector backbone,and the lower band is the CAR DNA schemed in Fig. 1 M:Marker. 1:Meso-CAR. 2:Met-CAR. 3:CAR1-2-GFP. 4:CAR2-4-GFP. 5:Dupixent-HL-GFP. 6:Dupixent-LH-GFP
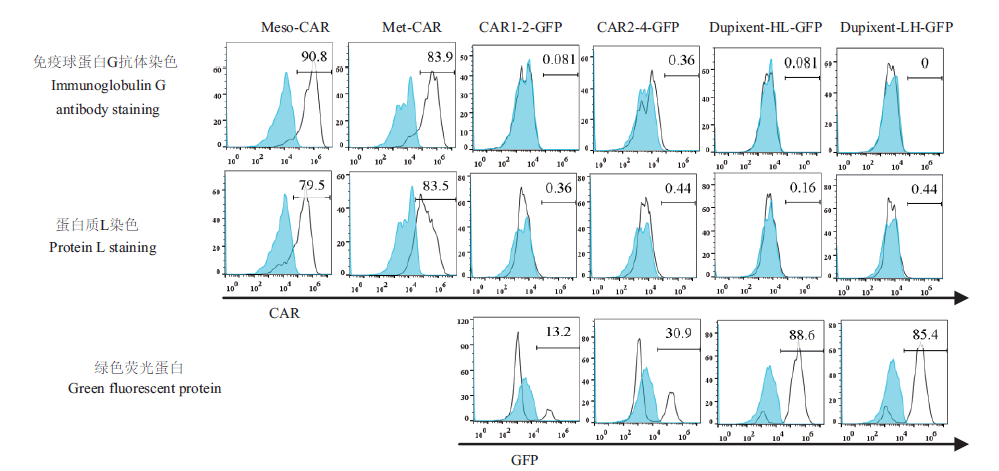
Fig. 4 Results of CAR expression detected by flow cytometry The primary antibody used for Met-CAR,Dupixent-HL-GFP and Dupixent-HL-GFP cells in the immunoglobulin G antibody staining assay is Biotin-SP Rabbit anti-Human IgG,while that for Meso-CAR,CAR1-2-GFP and CAR2-4-GFP is Biotin-SP Goat Anti-Mouse IgG. In protein L staining assay,the primary antibody used for the six types of cells is Biotin-protein L. The secondary antibody used in both staining methods is PE-Streptavidin. In the first two rows of data,the experimental group for the primary antibody and fluorescent secondary antibody is represented as a solid black line,and control with only fluorescent secondary antibody is represented as sky blue shade. In the third row of data,the Jurkat cell with lentiviral infection group is represented as a solid black line and the 4 types of cells co-expressed by GFP and CAR are represented as sky blue shade. The X-axis represents the number of cells,and Y-axis represents the fluorescence intensity. The data shows the positive efficiency of CAR molecules
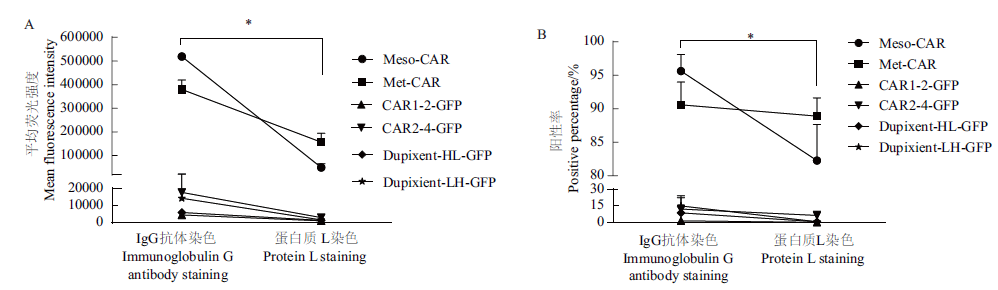
Fig. 5 Comparison of two staining methods shown as average fluorescence intensity and positive percentage The data measured by the flow cytometry in Fig. 4 are presented as the average fluorescence intensity(the value of each sample - the value of the control sample with only the fluorescent secondary antibody)and the positive percentage. The final data are the average of results from 3 independent experiments
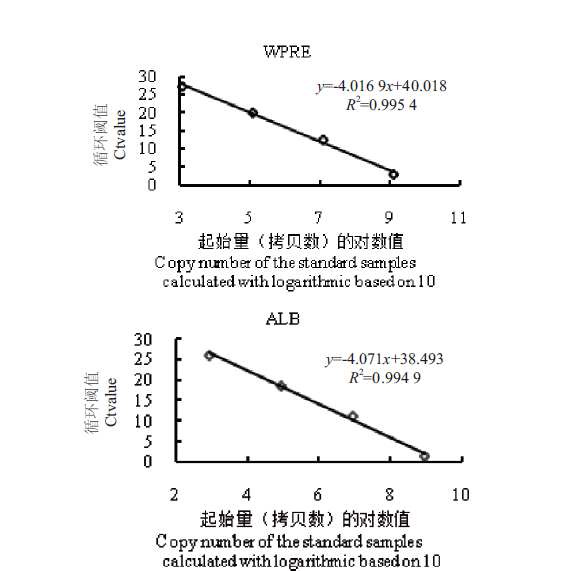
Fig. 7 Standard curve diagrams of standard samples(absolute quantitative method) The gene fragments of standard samples with 4 different copy number gradients(standard WPRE:1.23×103,1.23×105,1.23×107,1.23×109;standard ALB:8.64×102,8.64×104,8.64106 and 8.64×108)as templates were in fluorescent quantitative PCR. The copy number of the standard samples was calculated with logarithmic based on 10,shown in X-axis,and the Y-axis is is the cycle threshold(Ct value)in the real-time quantitative PCR
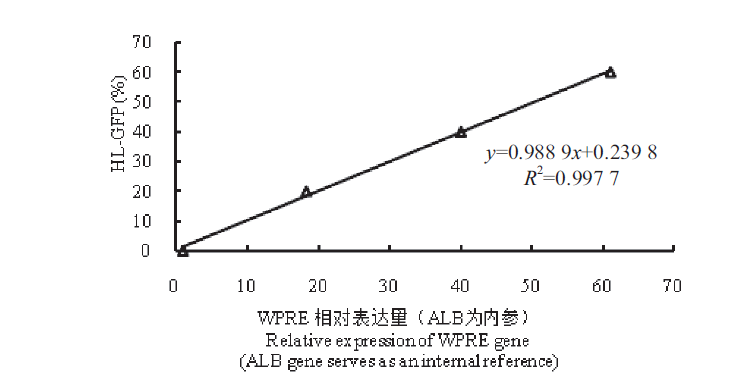
Fig. 8 Standard curve of the percentage of HL-GFP and the relative expression of WPRE(relative quantitative method) The percentage of Jurkat-Dupixent-HL-GFP positive cells was determined by flow cytometry. Uninfected Jurkat cells were mixed with Jurkat-Dupixent-HL-GFP positive cells to obtain the gradients of GFP positive cells:0(Jurkat),20%,40% and 60%,respectively. The genome DNA was extracted for quantitative PCR detection,and a standard curve was depicted based on the relative expression of WPRE gene(ALB gene serves as an internal reference)and the percentage of Jurkat-Dupixent-HL-GFP positive population

Fig. 9 Comparison of 3 different methods in detecting 4 novel CAR molecules The GFP co-expression refers to the percentage of GFP positive population measured by flow cytometry as shown in Fig. 4. The relative quantitative method refers to calculation via relative quantitative method as shown in Fig. 8. The absolute quantitative method refers to the average copy number of CAR molecules integrated per cell by substituting the standard curve of Fig. 7
| 适用性Applicability | 直观性Intuitiveness | 缺点Disadvantages | 应用Application | |
|---|---|---|---|---|
| GFP流式检测 GFP flow cytometry | 普适 Universal | 间接 Indirect | 不可用于临床 Cannot be used in clinical cases | 无法进行CAR分子染色情况下, 检测临床前CAR表达水平 Preclinical CAR expression levels can be detected when CAR molecular staining is not possible |
| IgG抗体染色 IgG antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| Protein L染色 Protein L antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| 相对定量法(推测CAR阳性率) Relative quantitative method(calculating CAR expression positive rate) | 普适 Universal | 间接 Indirect | 需同时构建含GFP和不含GFP的两种CAR T细胞 Two kinds of CAR T cells with and without GFP need to be constructed at the same time | 无法进行CAR分子染色情况下, 获得大致CAR表达水平 In the absence of CAR molecular staining, approximate CAR expression levels can be obtained |
| 绝对定量法(每个细胞整合CAR拷贝数) Absolute quantitative method (average copy number of CAR molecules integrated per cell) | 普适 Universal | 间接 Indirect | 每个细胞整合CAR分子的拷贝数是一个均值,与实际CAR分子的表达比例存在一定误差 The number of copies of each cell integrated CAR molecule is an average, with errors in the proportion of actual CAR molecule expression | 临床中监测CAR T细胞的扩增,持久性 Clinical monitoring of the amplification and persistence of CAR T cells |
Table 1 Comparison of the five methods in detecting CAR moleculars
| 适用性Applicability | 直观性Intuitiveness | 缺点Disadvantages | 应用Application | |
|---|---|---|---|---|
| GFP流式检测 GFP flow cytometry | 普适 Universal | 间接 Indirect | 不可用于临床 Cannot be used in clinical cases | 无法进行CAR分子染色情况下, 检测临床前CAR表达水平 Preclinical CAR expression levels can be detected when CAR molecular staining is not possible |
| IgG抗体染色 IgG antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| Protein L染色 Protein L antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| 相对定量法(推测CAR阳性率) Relative quantitative method(calculating CAR expression positive rate) | 普适 Universal | 间接 Indirect | 需同时构建含GFP和不含GFP的两种CAR T细胞 Two kinds of CAR T cells with and without GFP need to be constructed at the same time | 无法进行CAR分子染色情况下, 获得大致CAR表达水平 In the absence of CAR molecular staining, approximate CAR expression levels can be obtained |
| 绝对定量法(每个细胞整合CAR拷贝数) Absolute quantitative method (average copy number of CAR molecules integrated per cell) | 普适 Universal | 间接 Indirect | 每个细胞整合CAR分子的拷贝数是一个均值,与实际CAR分子的表达比例存在一定误差 The number of copies of each cell integrated CAR molecule is an average, with errors in the proportion of actual CAR molecule expression | 临床中监测CAR T细胞的扩增,持久性 Clinical monitoring of the amplification and persistence of CAR T cells |
| [1] | June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy:a race to the finish line[J]. Sci Transl Med, 2015, 7(280):280ps7. |
| [2] |
Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia[J]. N Engl J Med, 2013, 368(16):1509-1518.
doi: 10.1056/NEJMoa1215134 URL |
| [3] |
Panagopoulou TI, Rafiq QA. CAR-T immunotherapies:Biotechnological strategies to improve safety, efficacy and clinical outcome through CAR engineering[J]. Biotechnol Adv, 2019, 37(7):107411.
doi: S0734-9750(19)30101-6 pmid: 31251969 |
| [4] |
Martinez M, Moon EK. CAR T cells for solid tumors:new strategies for finding, infiltrating, and surviving in the tumor microenvironment[J]. Front Immunol, 2019, 10:128.
doi: 10.3389/fimmu.2019.00128 pmid: 30804938 |
| [5] |
Chen D, Yang J. Development of novel antigen receptors for CAR T-cell therapy directed toward solid malignancies[J]. Transl Res, 2017, 187:11-21.
doi: 10.1016/j.trsl.2017.05.006 URL |
| [6] |
Lonez C, Hendlisz A, Shaza L, et al. Celyad’s novel CAR T-cell therapy for solid malignancies[J]. Curr Res Transl Med, 2018, 66(2):53-56.
doi: 10.1016/j.retram.2018.03.001 URL |
| [7] |
Zheng Z, Chinnasamy N, Morgan RA. Protein L:a novel reagent for the detection of chimeric antigen receptor(CAR)expression by flow cytometry[J]. J Transl Med, 2012, 10:29.
doi: 10.1186/1479-5876-10-29 URL |
| [8] |
Lv J, Zhao R, Wu D, et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer[J]. J Hematol Oncol, 2019, 12(1):18.
doi: 10.1186/s13045-019-0704-y URL |
| [9] |
Wang H, Du X, Chen WH, et al. Establishment of a quantitative polymerase chain reaction assay for monitoring chimeric antigen receptor T cells in peripheral blood[J]. Transplant Proc, 2018, 50(1):104-109.
doi: 10.1016/j.transproceed.2017.11.028 URL |
| [10] | Bunse L, Schumacher T, Sahm F, et al. Proximity ligation assay evaluates IDH1R132H presentation in gliomas[J]. J Clin Invest, 2015, 125(2):593-606. |
| [11] |
Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma[J]. The Oncol, 2007, 12(5):569-576.
doi: 10.1634/theoncologist.12-5-569 URL |
| [12] |
Wang C, Zhu C, Wei F, et al. Constitutive activation of interleukin-13/STAT6 contributes to Kaposi’s sarcoma-associated herpesvirus-related primary effusion lymphoma cell proliferation and survival[J]. J Virol, 2015, 89(20):10416-10426.
doi: 10.1128/JVI.01525-15 URL |
| [13] | 张飞飞, 孙文, 耿琦, 等. 一种检测慢病毒滴度的实时荧光定量PCR方法[J]. 生物技术通讯, 2019, 30(4):523-527, 588. |
| Zhang FF, Sun W, Geng Q, et al. A real-time fluorescence quantitative PCR method for detecting Lentivirus titer[J]. Lett Biotechnol, 2019, 30(4):523-527, 588. | |
| [14] |
Hu Y, Huang J. The chimeric antigen receptor detection toolkit[J]. Front Immunol, 2020, 11:1770.
doi: 10.3389/fimmu.2020.01770 URL |
| [15] |
Fehse B, Badbaran A, Berger C, et al. Digital PCR assays for precise quantification of CD19-CAR-T cells after treatment with axicabtagene ciloleucel[J]. Mol Ther Methods Clin Dev, 2020, 16:172-178.
doi: 10.1016/j.omtm.2019.12.018 URL |
| [16] |
Sheih A, Voillet V, Hanafi LA, et al. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy[J]. Nat Commun, 2020, 11(1):219.
doi: 10.1038/s41467-019-13880-1 URL |
| [17] |
Davenport AJ, Cross RS, Watson KA, et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity[J]. PNAS, 2018, 115(9):E2068-E2076.
doi: 10.1073/pnas.1716266115 URL |
| [1] | CAO Ying-fang, ZHAO Xin, LIU Shuang, LI Rui-huan, LIU Na, XU Shi-yong, GAO Fang-rui, MA Hui, LAN Qing-kuo, TAN Jian-xin, WANG Yong. Establishment of Real-time Fluorescent Quantitative PCR Detection Method for Genetically Modified Herbicide-tolerant Soybean GE-J12 [J]. Biotechnology Bulletin, 2022, 38(7): 146-152. |
| [2] | WU Xin-yuan, WANG Guang-chao, LIN Jin-xing, JING Yan-ping. Correlative Light and Electron Microscopy and Its Application in Botanical Research [J]. Biotechnology Bulletin, 2022, 38(1): 278-288. |
| [3] | CHEN Nan, YU Fei, HE Yan-liu, BU Ning. Labeling and Tracing of Green Fluorescent Protein in Fungal Endophyte with Growth-promoting Activity to Rice Seedlings [J]. Biotechnology Bulletin, 2017, 33(3): 100-105. |
| [4] | LIU Xiao-yu, MA Yu-chao. Green Fluorescent Protein Marker of Biocontrol Streptomyces SSD49 and Its Colonization on the Populus tomentosa Somaclone [J]. Biotechnology Bulletin, 2016, 32(9): 197-202. |
| [5] | WANG Hai-tao,JIN Bo,LI Shu-wei. Eukaryotic Expression of MSTN Gene from Hetian Sheep,Carla Kul Sheep,and Duolang Sheep in the Southern Xinjiang [J]. Biotechnology Bulletin, 2016, 32(5): 82-90. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||