








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (9): 274-284.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1440
Previous Articles Next Articles
SHEN Ya-li( ), PAN Yang-yang, WANG Jing-lei, MA Rui, ZHAO Gai-hong, WANG Gui-rong, ZHANG Qian, WANG Meng(
), PAN Yang-yang, WANG Jing-lei, MA Rui, ZHAO Gai-hong, WANG Gui-rong, ZHANG Qian, WANG Meng( )
)
Received:2020-11-18
Online:2021-09-26
Published:2021-10-25
Contact:
WANG Meng
E-mail:1204013099@qq.com;wangmeng@gsau.edu.cn
SHEN Ya-li, PAN Yang-yang, WANG Jing-lei, MA Rui, ZHAO Gai-hong, WANG Gui-rong, ZHANG Qian, WANG Meng. Construction and Application of a Drug Screening Method Based on PXR Promoter Reporter Gene[J]. Biotechnology Bulletin, 2021, 37(9): 274-284.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 片段大小 Fragment length/bp |
|---|---|---|
| PXRpro | F:5'-gcgtgctagcccgggctcgagAGG- AACAGGTGCAGTTTG-3' | 2 042 |
| R:5'-cagtaccggaatgccaagcttATT- ACCTGTGTACGGCAA-3' |
Table 1 List of primer sequence used in PCR
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 片段大小 Fragment length/bp |
|---|---|---|
| PXRpro | F:5'-gcgtgctagcccgggctcgagAGG- AACAGGTGCAGTTTG-3' | 2 042 |
| R:5'-cagtaccggaatgccaagcttATT- ACCTGTGTACGGCAA-3' |
| 组分 Component | 重组反应 Recombination reaction/µL | 阴性对照 Negative control/µL |
|---|---|---|
| 线性化pGL3-basic载体 Linearized pGL3-basic vector | 4 | 4 |
| DNA片段 PXRpro DNA fragment | 4 | 0 |
| 5× CE II Buffer | 4 | 0 |
| Exnase II | 2 | 0 |
| ddH2O | 6 | 16 |
Table 2 Recombination reaction system
| 组分 Component | 重组反应 Recombination reaction/µL | 阴性对照 Negative control/µL |
|---|---|---|
| 线性化pGL3-basic载体 Linearized pGL3-basic vector | 4 | 4 |
| DNA片段 PXRpro DNA fragment | 4 | 0 |
| 5× CE II Buffer | 4 | 0 |
| Exnase II | 2 | 0 |
| ddH2O | 6 | 16 |
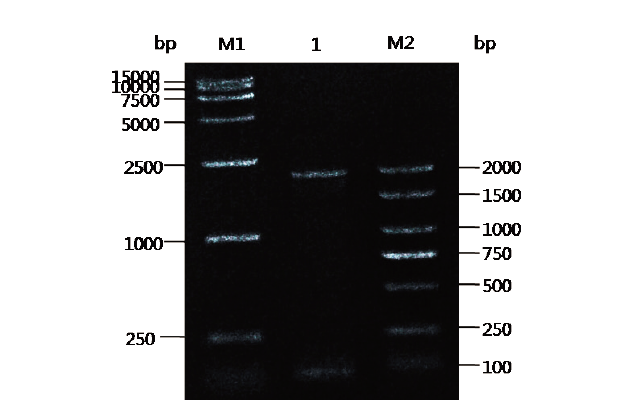
Fig.2 Gel electrophoresis of PCR amplified PXR promoter fragment M1:DL 15 000 DNA marker. 1:PXR promoter gene PCR amplification results. M2:DL 2 000 Plus DNA marker

Fig.4 Colony PCR gel electrophoresis M1:DL 15 000 DNA marker. 1 and 2:Constructed pGL3-Basic-PXRpro vector monoclonal colony PCR product. M2:DL 2 000 Plus DNA marker

Fig.5 Gel electrophoresis of pGL3-basic-PXRpro by dou-ble restriction enzymes digestion M:DL 15 000 DNA marker. 1:pGL3-basic-PXRpro double restriction enzymes digestion product

Fig.6 Partial sequencing results of pGL3-basic-PXRpro A:Sequencing result of the 5' end of the PXR promoter fragment inserted into the recombinant plasmid. B:Sequencing result of the 3' end of the PXR promoter fragment inserted into the recombinant plasmid. The letters marked in red are mutated bases
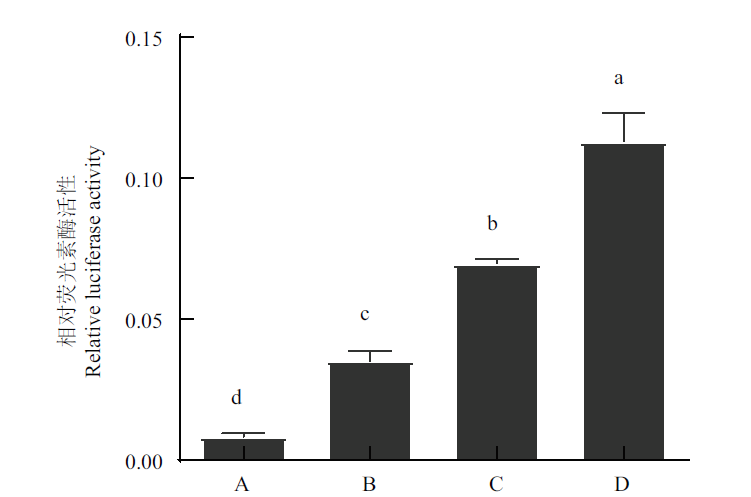
Fig.7 Validation of constructed PXR promoter reporter gene drug screening method A:Normel group. B:Empty vector group. C:Carrying reporter gene vector control group. D:A positive agonist of mPXR dexamethasone group. Different lowercase letters a,b,c,and d on the graph column indicate significant differences between groups(P<0.05). The same below
| 组别 Group | N | 相对荧光素酶活性 Relative luciferase activity | F | P |
|---|---|---|---|---|
| 1 | 3 | 0.1129± 0.0100 | 0.200 | 0.824 |
| 2 | 3 | 0.1352 ± 0.0056 | ||
| 3 | 3 | 0.1114 ± 0.0093 |
Table 3 Relative luciferase activity of dexamethasone in three independent repeated experiments
| 组别 Group | N | 相对荧光素酶活性 Relative luciferase activity | F | P |
|---|---|---|---|---|
| 1 | 3 | 0.1129± 0.0100 | 0.200 | 0.824 |
| 2 | 3 | 0.1352 ± 0.0056 | ||
| 3 | 3 | 0.1114 ± 0.0093 |
| [1] |
Pinne M, Raucy JL. Advantages of cell-based high-volume screening assays to assess nuclear receptor activation during drug discovery[J]. Expert Opinion on Drug Discovery, 2014, 9(6):669-686.
doi: 10.1517/17460441.2014.913019 URL |
| [2] |
Gregory N, Kwon PS, Dordick JS, et al. Cell-based assay design for high-content screening of drug candidates[J]. Journal of Microbiology and Biotechnology, 2016, 26(2):213-225.
doi: 10.4014/jmb.1508.08007 pmid: 26428732 |
| [3] |
Michelini E, Cevenini L, Mezzanotte L, et al. Cell-based assays:fuelling drug discovery[J]. Analytical and Bioanalytical Chemistry, 2010, 398(1):227-238.
doi: 10.1007/s00216-010-3933-z pmid: 20623273 |
| [4] |
Daujat-Chavanieu M, Gerbal-Chaloin S. Regulation of CAR and PXR expression in health and disease[J]. Cells, 2020, 9:2395.
doi: 10.3390/cells9112395 URL |
| [5] |
Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity[J]. PNAS, 2001, 98(6):3369-3374.
pmid: 11248085 |
| [6] |
Sultana H, Watanabe K, Rana MM, et al. Effects of vitamin K2 on the expression of genes involved in bile acid synjournal and glucose homeostasis in mice with humanized PXR[J]. Nutrients, 2018, 10(8):982.
doi: 10.3390/nu10080982 URL |
| [7] | 郭一婷, 邵云云, 刘俊瑾, 等. 核受体作为炎症性肠疾病治疗靶点的研究进展[J]. 中国现代应用药学, 2020, 37(4):496-500. |
| Guo YT, Shao YY, Liu JJ, et al. Progress in research on nuclear receptors as therapeutic targets for inflammatory bowel diseases[J]. Chinese Journal of Modern Applied Pharmacy, 2020, 37(4):496-500. | |
| [8] | 朱丹丹, 熊玉卿. 孕烷X受体介导的药物对转运体调控作用研究进展[J]. 中国临床药理学杂志, 2018, 34(20):2469-2472. |
| Zhu DD, Xiong YQ. Advances in regulation of pregnane X receptor-mediated drug transporter[J]. The Chinese Journal of Clinical Pharmacology, 2018, 34(20):2469-2472. | |
| [9] |
Tolson AH, Wang HB. Regulation of drug-metabolizing enzymes by xenobiotic receptors:PXR and CAR[J]. Advanced Drug Delivery Reviews, 2010, 62(13):1238-1249.
doi: 10.1016/j.addr.2010.08.006 URL |
| [10] |
Desirée B, Francesca DF, Pierangelo T, et al. Garcinoicacid is a natural and selective agonist of pregnane X receptor[J]. Journal of Medicinal Chemistry, 2020, 63(7):3701-3712.
doi: 10.1021/acs.jmedchem.0c00012 URL |
| [11] |
Punita B, Britto SS. Development of activity-based reporter gene technology for imaging of protease activity with an exquisite specificity in a single live cell[J]. ACS Chemical Biology, 2019, 14(10):2276-2285.
doi: 10.1021/acschembio.9b00614 pmid: 31498985 |
| [12] |
Monimoy B, Delira R, Chen TS. Targeting xenobiotic receptors PXR and CAR in human diseases[J]. Drug Discovery Today, 2015, 20(5):618-628.
doi: 10.1016/j.drudis.2014.11.011 URL |
| [13] |
Liu MJ, Zhang GH, Zheng CG, et al. Activating the pregnane X receptor by imperatorin attenuates dextran sulphate sodium-induced colitis in mice[J]. British Journal of Pharmacology, 2018, 175(17):3563-3580.
doi: 10.1111/bph.v175.17 URL |
| [14] |
Steven K, Timothy W. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor[J]. Journal of Lipid Research, 2002, 43(3):359-364.
doi: 10.1016/S0022-2275(20)30141-3 URL |
| [15] |
Xie W, Barwick JL, Downes M et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR[J]. Nature, 2000, 406:435-439.
doi: 10.1038/35019116 URL |
| [16] |
Leitner JM, Graninger W, Thalhammer F. Hepatotoxicity of antibacterials:pathomechanisms and clinical[J]. Infection, 2010, 38:3-11.
doi: 10.1007/s15010-009-9179-z pmid: 20107858 |
| [17] |
Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury:an update on the 2007 overview[J]. Expert Opin Drug Saf, 2014, 13:67-81.
doi: 10.1517/14740338.2013.828032 pmid: 24073714 |
| [18] | Wang JH, Bwayi M, Gee RRF et al. PXR-mediated idiosyncratic drug-induced liver injury:mechanistic insights and targeting approaches[J]. Expert Opin Drug MetabToxicol, 2020, 16:711-722. |
| [19] | Guo XW, Li WY, An R, et al. Composite ammonium glycyrrhizin has hepatoprotective effects in chicken hepatocytes with lipopolysaccharide/enrofloxacin-induced injury[J]. Exp Ther Med, 2020, 20(5):52. |
| [20] |
Han C, Wei YY, Cui YQ, et al. Florfenicol induces oxidative stress and hepatocyte apoptosis in broilers via Nrf2 pathway[J]. Ecotoxicol Environ Saf, 2020, 191:110239.
doi: 10.1016/j.ecoenv.2020.110239 URL |
| [21] |
Wang X, Wu QH, Liu AM, et al. Paracetamol:overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro[J]. Drug Metab Rev, 2017, 49:395-437.
doi: 10.1080/03602532.2017.1354014 pmid: 28766385 |
| [22] | 刘婉玉, Tadiyose GB, 赵洪霞. 恩诺沙星在鲫鱼肝微粒体中的代谢及代谢关键酶[J]. 生态毒理学报, 2020, 15(3):64-70. |
| Liu WY, Tadiyose GB, Zhao HX. Metabolism of enrofloxacin in liver microsomes of crucian carp(Carassius auratus)and its key enzymes in vitro[J]. Asian Journal of Ecotoxicology, 2020, 15(3):64-70. | |
| [23] | 刘娜. 氟苯尼考在家兔体内的代谢机制及药物间相互作用的研究[D]. 南京:南京农业大学, 2011. |
| Liu N. The metabolic mechanism of florfenicoland drug-drug interaction in rabbits[D]. Nanjing:Nanjing Agricultural University, 2011. | |
| [24] | 夏伟, 董诚明, 杨朝帆, 等. 连翘化学成分及其药理学研究进展[J]. 中国现代中药, 2016, 18(12):1670-1674. |
| Xia W, Dong CM, Yang CF, et al. Research progress on chemical constituents and pharmacology of Forsythia suspense[J]. Modern Chinese Medicine, 2016, 18(12):1670-1674. | |
| [25] | Okamura M, Shizu R, Abe T, et al. PXR functionally interacts with NF-κB and AP-1 to downregulate the inflammation-induced expression of chemokine in mice[J]. Cells, 2020, 9(10):E2296. |
| [26] |
Zhang GH, Liu MJ, Song M, et al. Patchouli alcohol activates PXR and suppresses the NF-κB-mediated intestinal inflammatory[J]. J Ethnopharmacol, 2020, 248:112302.
doi: 10.1016/j.jep.2019.112302 URL |
| [27] | 陈丽青. 连翘酯苷A抗AD模型炎症及凋亡的机制研究[D]. 太原:山西大学, 2018. |
| Chen LQ. Study on the mechanism of anti-AD model inflammation and apoptosis of Forsythiaside[D]. Taiyuan:Shanxi University, 2018. | |
| [28] |
Pan CW, Zhou GY, Chen WL, et al. Protective effect of forsythiaside a on lipopolysaccharide/d-galactosamine-induced liver injury[J]. Int Immunopharmacol, 2015, 26:80-85.
doi: 10.1016/j.intimp.2015.03.009 pmid: 25797347 |
| [29] | Kim SJ, Um JY, Lee JY, et al. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-kappaB activation in mouse peritoneal macrophages[J]. Am J Chn Med, 2011, 39(1):171-181. |
| [30] | Lee S, Shin S, Kim H, et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways[J]. J Inflamm(Lond), 2011, 8:16. |
| [31] |
Chen Y, Zeng QL, Liu XF, et al. LINE-1 ORF-1p enhances the transcription factor activity of pregnenolone X receptor and promotes sorafenib resistance in hepatocellular carcinoma cells[J]. Cancer Management and Research, 2018, 10:4421-4438.
doi: 10.2147/CMAR.S176088 pmid: 30349375 |
| [32] | 王丽敏, 贾洪岩, 张明远, 等. 金丝桃苷对小鼠S180肿瘤细胞获得性多药耐药相关因子表达的影响[J]. 中国医院药学杂志, 2015, 35(6):478-480. |
| Wang LM, Jia HY, Zhang MY, et al. Effects of hyperin on expression of correlation factor of multidrug-resistant in mouse S180 tumor cells[J]. Chinese Journal of Hospital Pharmacy, 2015, 35(6):478-480. | |
| [33] | 刘广遐, 王婷婷, 胡文静, 等. 连翘醇提物对恶性胸腹水中原代肿瘤细胞的抗肿瘤作用[J]. 实用老年医学, 2009, 23(5):359-363. |
| Liu GX, Wang TT, Hu WJ, et al. Anticancer effect of ethanol extract of Fructus Forsythiae on primary cancer cells isolated from ascites and pleural fluids[J]. Practical Geriatrics, 2009, 23(5):359-363. | |
| [34] |
Lou JS, Yao P, Tsim KWK. Cancer treatment by using traditional Chinese medicine:probing active compounds in anti-multidrug resistance during drug therapy[J]. Curr Med Chem, 2018, 25:5128-5141.
doi: 10.2174/0929867324666170920161922 URL |
| [35] | 林艳芹, 郑薇, 黄鹤光. 熊果酸对急性肝损伤动物模型作用的研究[J]. 福建医药杂志, 2014, 36(4):63-65. |
| Lin YQ, Zheng W, Huang HG. Study on the effect of ursolic acid on animal model of acute liver injury[J]. Fujian Medical Journal, 2014, 36(4):63-65. | |
| [36] | 林艳芹, 郑薇, 黄鹤光. 熊果酸对CCl4灌胃所致小鼠肝损伤的治疗作用研究[J]. 福建医药杂志, 2015, 37(1):50-53. |
| Lin YQ, ZhengW, Huang HG. Protective effects of ursolic acid on acute liver induced by CCl4 in mice[J]. Fujian Medical Journal, 2015, 37(1):50-53. | |
| [37] | 樊威洋, 吴灏, 王永刚, 等. 柚皮苷和柚皮素对HepaRG细胞核受体蛋白表达的影响[J]. 药学研究, 2019, 38(12):683-687. |
| Fan WY, Wu H, Wang YG, et al. Effects of naringin and naringenin on the protein expressions of nuclear receptors in HepaRG cells[J]. Journal of Pharmaceutical Research, 2019, 38(12):683-687. | |
| [38] |
Erika HA, Pablo M. Beneficial effects of naringenin in liver diseases:Molecular mechanisms[J]. World J Gastroenterol, 2018, 24:1679-1707.
doi: 10.3748/wjg.v24.i16.1679 URL |
| [39] | He ZH, Fan R, Zhang CH, et al. Chaihu-Shugan-San reinforces CYP3A4 expression via pregnane X receptor in depressive treatment of liver-Qi stagnation syndrome[J]. Evidence-Based Complementary and Alternative Medicine, 2019: 9781675. |
| [40] |
Rasmussen MK, Klausen CL, Ekstrand B. Regulation of cytochrome P450 mRNA expression in primary porcine hepatocytes by selected secondary plant metabolites from chicory(Cichorium intybus L.)[J]. Food Chem, 2014, 146:255-263.
doi: 10.1016/j.foodchem.2013.09.068 pmid: 24176340 |
| [1] | LIU Yu-ling, WANG Meng-yao, SUN Qi, MA Li-hua, ZHU Xin-xia. Effect of RD29A Promoter on the Stress Resistance of Transgenic Tobacco with SikCDPK1 Gene from Saussurea involucrata [J]. Biotechnology Bulletin, 2023, 39(9): 168-175. |
| [2] | GUO San-bao, SONG Mei-ling, LI Ling-xin, YAO Zi-zhao, GUI Ming-ming, HUANG Sheng-he. Cloning and Analysis of Chalcone Synthase Gene and Its Promoter from Euphorbia maculata [J]. Biotechnology Bulletin, 2023, 39(4): 148-156. |
| [3] | YANG Lan, ZHANG Chen-xi, FAN Xue-wei, WANG Yang-guang, WANG Chun-xiu, LI Wen-ting. Gene Cloning, Expression Pattern, and Promoter Activity Analysis of Chicken BMP15 [J]. Biotechnology Bulletin, 2023, 39(4): 304-312. |
| [4] | SHI Guang-zhen, WANG Zhao-ye, SUN Qi, ZHU Xin-xia. Cloning and Activity Analysis of SikCDPK1 Promoter from Saussurea involucrata [J]. Biotechnology Bulletin, 2022, 38(9): 191-197. |
| [5] | CHEN Guang, LI Jia, DU Rui-ying, WANG Xu. pOsHAK1:OsFLN2 Expression Enhances the Drought Tolerance by Altering Sugar Metabolism in Rice [J]. Biotechnology Bulletin, 2022, 38(8): 92-100. |
| [6] | NIE Li-bin, YI Ling-xin, DENG Yan, SHENG Qi, WU Xiao-yu, ZHANG Bin. Pathway Engineering Modification of Corynebacterium glutamicum for Shikimic Acid Production [J]. Biotechnology Bulletin, 2022, 38(6): 93-102. |
| [7] | HAO Qing-qing, YAO Sheng, LIU Jia-he, CHEN Pei-zhen, ZHANG Meng-yang, JI Kong-shu. Cloning and Expression Analysis of NAC Transcription Factor PmNAC8 in Pinus massoniana [J]. Biotechnology Bulletin, 2022, 38(4): 202-216. |
| [8] | YE Peng-lin, Kwasi Kyere-Yeboah, GAO E-bin. Effects on the Biosynthesis of Ethanol by Promoters PpetE and Pcpc560 in Synechocystis sp. PCC 6803 [J]. Biotechnology Bulletin, 2022, 38(2): 141-149. |
| [9] | LIU Meng-meng, HAN Li-jun, LIU Bao-ling, XUE Jin-ai, LI Run-zhi. Cloning and Expression Analysis of GhSDP1 and Its Promoter in Gossypium hirsutum [J]. Biotechnology Bulletin, 2022, 38(2): 34-43. |
| [10] | SHI Ya-qian, SHEN Ya-ru, CHEN Man-ying, HE Shu-min, LIU Yu-han, HE Tian-nan, CHEN Qing-xi, WEN Zhi-feng. Molecular Cloning and Expression Analysis of a F-box Protein Gene FnFBOX1 and Its Promoter from Fragaria nilgerrensis [J]. Biotechnology Bulletin, 2022, 38(2): 44-56. |
| [11] | CHEN Chen, HUANG Zhi-yang, YU Hai-yan, YUAN Hai-bin, TIAN Huai-xiang. Research Technology and Progress in Transcriptional Regulation in Prokaryotes [J]. Biotechnology Bulletin, 2022, 38(10): 54-65. |
| [12] | YU Jing, YANG Hui, YU Shi-zhou, ZHAO Hui-na, ZHENG Qing-xia, WANG Bing, LEI Bo. Construction of Yeast One-hybrid Bait Vector of Tobacco NtCBT Gene Promoter and Screening of Interacted Proteins [J]. Biotechnology Bulletin, 2022, 38(10): 73-79. |
| [13] | CEN You-fei, ZHU Mu-zi, YE Wei, LI Sai-ni, ZHONG Guo-hua, ZHANG Wei-min. Cloning and Functional Identification of Trichothecene Mycotoxin Biosynthesis Gene Promoter from Paramyrothecium roridum [J]. Biotechnology Bulletin, 2021, 37(8): 85-94. |
| [14] | LIN Yan-li, QIN Jian-bing, WU Xiang, WANG Yan-yan, PAN You-zhao, LIU Zhong-yu. Cloning and Activity Analysis of PcMYB1 Promoter from Polygonum cuspidatum [J]. Biotechnology Bulletin, 2021, 37(5): 48-55. |
| [15] | CUI Xiang-hua, TAO Nan, CHENG Bo-pu, ZHAO Yong-chang, CHEN Wei-min, LI Jing. Screening Promoters for Genetic Transformation of Cyclocybe aegerita [J]. Biotechnology Bulletin, 2021, 37(5): 259-266. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||