








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (3): 139-148.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0620
Previous Articles Next Articles
TANG Bin( ), LIU Wen-bin, LI Xiao-bo, WANG Ning, JIN Xiao-bao(
), LIU Wen-bin, LI Xiao-bo, WANG Ning, JIN Xiao-bao( )
)
Received:2021-05-11
Online:2022-03-26
Published:2022-04-06
Contact:
JIN Xiao-bao
E-mail:1375540061@qq.com;jinxf2001@163.com
TANG Bin, LIU Wen-bin, LI Xiao-bo, WANG Ning, JIN Xiao-bao. Screening and Identification of Strains Producing 7-β-xylosyltaxanes Glycoside Hydrolases from the Periplaneta americana Gut[J]. Biotechnology Bulletin, 2022, 38(3): 139-148.
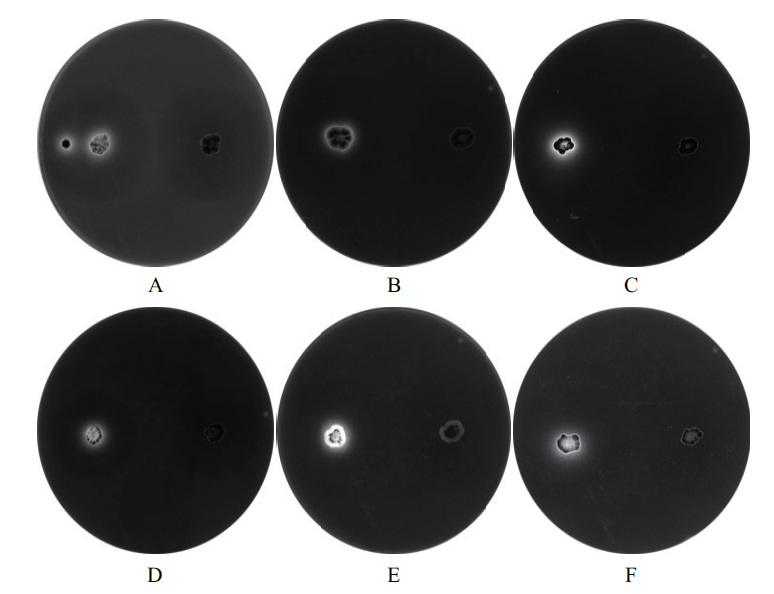
Fig. 3 Fluorescence color image of xylanase-producing strain A to F:WA1-37,WA2-17,WA3-2-36,WA4-65,WA11-2-9 and WA14-2-7. The left is fluorescent color,and the right is self-control
| 菌株编号 Strain code | 菌落直径 Diameter of colony(d/cm) | 透明圈直径 Diameter of transparent circle of clony(D/cm) | 比值 Ratio(D/d) | ||
|---|---|---|---|---|---|
| 内圈Inner circle | 外圈Outer circle | 内圈Inner circle | 外圈Outer circle | ||
| 1-37 | 0.75±0.02 | 1.67±0.12 | 3.68±0.18 | 2.21±0.11 | 4.88±0.12 |
| 2-17 | 0.83±0.05 | 1.54±0.05 | 2.66±0.04 | 1.87±0.16 | 3.23±0.17 |
| 3-2-36 | 0.64±0.06 | 1.33±0.07 | 2.89±0.02 | 2.13±0.06 | 4.63±0.17 |
| 4-65 | 0.68±0.08 | 1.72±0.12 | 3.50±0.17 | 2.56±0.16 | 5.03±0.13 |
| 11-2-9 | 0.62±0.04 | 1.33±0.05 | 3.72±0.04 | 2.07±0.12 | 5.55±0.17 |
| 14-2-7 | 0.62±0.04 | 1.50±0.03 | 2.92±0.04 | 2.44±0.10 | 4.80±0.15 |
Table 1 Colony diameters,Congo red transparent circles and their ratios of xylanase-producing strains(n=3)
| 菌株编号 Strain code | 菌落直径 Diameter of colony(d/cm) | 透明圈直径 Diameter of transparent circle of clony(D/cm) | 比值 Ratio(D/d) | ||
|---|---|---|---|---|---|
| 内圈Inner circle | 外圈Outer circle | 内圈Inner circle | 外圈Outer circle | ||
| 1-37 | 0.75±0.02 | 1.67±0.12 | 3.68±0.18 | 2.21±0.11 | 4.88±0.12 |
| 2-17 | 0.83±0.05 | 1.54±0.05 | 2.66±0.04 | 1.87±0.16 | 3.23±0.17 |
| 3-2-36 | 0.64±0.06 | 1.33±0.07 | 2.89±0.02 | 2.13±0.06 | 4.63±0.17 |
| 4-65 | 0.68±0.08 | 1.72±0.12 | 3.50±0.17 | 2.56±0.16 | 5.03±0.13 |
| 11-2-9 | 0.62±0.04 | 1.33±0.05 | 3.72±0.04 | 2.07±0.12 | 5.55±0.17 |
| 14-2-7 | 0.62±0.04 | 1.50±0.03 | 2.92±0.04 | 2.44±0.10 | 4.80±0.15 |

Fig. 4 TLC result of transformed product of strain WA11-2-9 1st to 4th are:WA 10-1-2,WA 11-2-9,WA 11-1-3 and WA 23-1-5 transformation products,the points from 5th to 7th are:7-XDT,10-DAT and 10-DAB standards
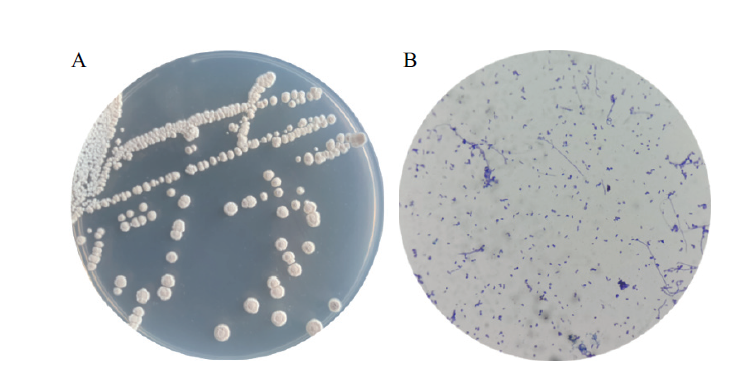
Fig. 5 Morphological characteristics of strain WA11-2-9 A:The colony morphology of strain WA11-2-9. B:The gram staining diagram of strain WA11-2-9(100×)
| 检测项目Test item | 结果Result | 检测项目Test item | 结果Result | 检测项目Test item | 结果Result | ||
|---|---|---|---|---|---|---|---|
| 淀粉水解 | + | H2S产生 | + | 葡萄糖 | + | ||
| 明胶液化 | + | 肌醇 | + | 阿拉伯糖 | + | ||
| 硝酸盐还原 | + | 果糖 | + | 生长温度范围 | 20-40℃ | ||
| 石蕊牛奶 | + | 甘露醇 | + | 生长pH范围 | 4-9 | ||
| 尿素酶 | + | 木糖 | + | 生长耐受NaCl范围 | 2%-8% |
Table 2 Physiological and biochemical characteristics of strain WA11-2-9
| 检测项目Test item | 结果Result | 检测项目Test item | 结果Result | 检测项目Test item | 结果Result | ||
|---|---|---|---|---|---|---|---|
| 淀粉水解 | + | H2S产生 | + | 葡萄糖 | + | ||
| 明胶液化 | + | 肌醇 | + | 阿拉伯糖 | + | ||
| 硝酸盐还原 | + | 果糖 | + | 生长温度范围 | 20-40℃ | ||
| 石蕊牛奶 | + | 甘露醇 | + | 生长pH范围 | 4-9 | ||
| 尿素酶 | + | 木糖 | + | 生长耐受NaCl范围 | 2%-8% |
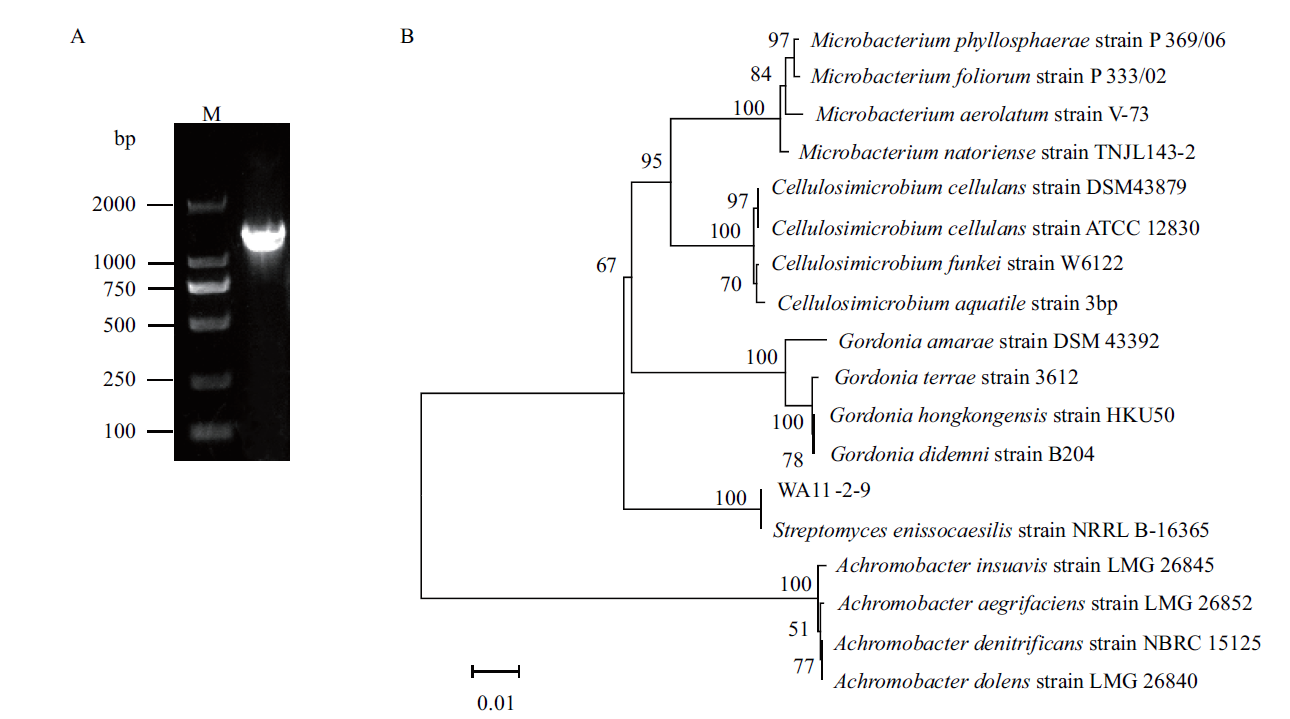
Fig. 6 Molecular biology identification of strain WA11-2-9 A:The 16S rDNA amplified products of strain WA11-2-9. B:The phylogenetic tree of strain WA11-2-9 based on 16S rDNA gene sequence

Fig. 8 HPLC analyzing spectrum of transformed product of strain WA11-2-9 A:The HPLC spectrogram of standard 7-XDT. B:The HPLC spectrogram of standard 10-DAT. C:The HPLC spectrogram of standard 10-DAB. D:The HPLC spectrogram of strain WA11-2-9. E:The HPLC spectrogram of WA11-2-9 contrast group(no 7-XDT added)
| [1] |
Yu C, Zhang C, Xu X, et al. Omic analysis of the endangered Taxaceae species Pseudotaxus chienii revealed the differences in taxol biosynjournal pathway between Pseudotaxus and Taxus yunnanensis trees[J]. BMC Plant Biol, 2021, 21(1):104.
doi: 10.1186/s12870-021-02883-0 URL |
| [2] |
邱德有, 张彬, 杨艳芳, 等. 紫杉醇生物合成研究历史、现状及展望[J]. 生物技术通报, 2015, 31(4):56-64.
doi: 10.13560/j.cnki.biotech.bull.1985.2015.03.004 |
| Qiu DY, Zhang B, Yang YF, et al. History, current status and the prospects of taxol biosynjournal research[J]. Biotechnol Bull, 2015, 31(4):56-64. | |
| [3] | Xue B, Zhao J, Fan Y, et al. Synjournal of taxol and docetaxel by using 10-deacetyl-7-xylosyltaxanes[J]. Chem Biodivers, 2020, 17(2):e1900631. |
| [4] | 熊亮斌, 唐红菊, 宋新巍, 等. 紫杉醇类抗肿瘤原料药生产的研究进展[J]. 中草药, 2020, 51(15):4042-4049. |
| Xiong LB, Tang HJ, Song XW, et al. Recent advances in synjournal of paclitaxel antitumor pharmaceutical raw materials[J]. Chin Tradit Herb Drugs, 2020, 51(15):4042-4049. | |
| [5] |
朱凤芝, 程赪, 刘祥胜, 等. 利用响应面法优化巴卡亭III生成10-DAB的工艺条件[J]. 生物技术通报, 2017, 33(4):238-246.
doi: 10.13560/j.cnki.biotech.bull.1985.2017.04.031 |
| Zhu FZ, Cheng C, Liu XS, et al. Optimization of biotransformation technology for 10-DAB production from baccatin III using response surface methodology[J]. Biotechnol Bull, 2017, 33(4):238-246. | |
| [6] |
Li BJ, Wang H, Gong T, et al. Improving 10-deacetylbaccatin III-10-β-O-acetyltransferase catalytic fitness for Taxol production[J]. Nat Commun, 2017, 8:15544.
doi: 10.1038/ncomms15544 URL |
| [7] | 赵俊宏, 樊燕鸽, 王红星, 等. 7-木糖紫杉烷类化合物合成紫杉醇的新工艺研究[J]. 河南师范大学学报:自然科学版, 2020, 48(6):92-98, 105. |
| Zhao JH, Fan YG, Wang HX, et al. New technology of synjournal of taxol from 7-xylosyltaxanes[J]. J Henan Norm Univ:Nat Sci Ed, 2020, 48(6):92-98, 105. | |
| [8] |
Hao DC, Ge GB, Yang L. Bacterial diversity of Taxus rhizosphere:culture-independent and culture-dependent approaches[J]. FEMS Microbiol Lett, 2008, 284(2):204-212.
doi: 10.1111/fml.2008.284.issue-2 URL |
| [9] |
Gales A, Chatellard L, Abadie M, et al. Screening of phytophagous and xylophagous insects guts microbiota abilities to degrade lignocellulose in bioreactor[J]. Front Microbiol, 2018, 9:2222. DOI: 10.3389/fmicb.2018.02222.
doi: 10.3389/fmicb.2018.02222 URL |
| [10] | Lin PB, Shen J, Ou PY, et al. Prodigiosin isolated from Serratia marcescens in the Periplaneta americana gut and its apoptosis-inducing activity in HeLa cells[J]. Oncol Rep, 2019, 41(6):3377-3385. |
| [11] | 曾佳佳, 陈静, 陈振, 等. 复合微生物的筛选及其发酵秸秆的应用效果[J]. 中国酿造, 2021, 40(3):124-128. |
| Zeng JJ, Chen J, Chen Z, et al. Screening of compound microorganism and application effect of straw fermentation[J]. China Brew, 2021, 40(3):124-128. | |
| [12] | 张正雨, 田继远, 于娟, 等. 黄、东海春季海水胞外酶活性水平分布特征研究[J]. 海洋学报, 2020, 42(4):1-11. |
| Zhang ZY, Tian JY, Yu J, et al. Horizontal distribution of extracellular enzyme activities in the Yellow Sea and the East China Sea in spring[J]. Acta Oceanol Sin, 2020, 42(4):1-11. | |
| [13] |
Wang XH, Zhang CH, Yang LL, et al. Screening and identification of microbial strains that secrete an extracellular C-7 xylosidase of taxanes[J]. World J Microbiol Biotechnol, 2011, 27(3):627-635.
doi: 10.1007/s11274-010-0499-z URL |
| [14] | 中国科学院微生物研究所放线菌分类组. 链霉菌鉴定手册[M]. 北京: 科学出版社, 1975. |
| Actinomycetes Classification Group, Institute of Microbiology, Chinese Academy of Sciences. Streptomyces identification handbook[M]. Beijing: Science Press, 1975. | |
| [15] | 汤勇, 丁泓皓, 蔡俊. 黑曲霉发酵产木糖苷酶工艺优化[J]. 食品科学, 2020, 41(10):172-179. |
| Tang Y, Ding HH, Cai J. Optimization of fermentation conditions for xylosidase production by Aspergillus niger[J]. Food Sci, 2020, 41(10):172-179. | |
| [16] |
Rubio MV, Terrasan CRF, Contesini FJ, et al. Redesigning N-glycosylation sites in a GH3 β-xylosidase improves the enzymatic efficiency[J]. Biotechnol Biofuels, 2019, 12:269.
doi: 10.1186/s13068-019-1609-2 URL |
| [17] |
Aftab MN, Zafar A, Awan AR. Expression of thermostable β-xylosidase in Escherichia coli for use in saccharification of plant biomass[J]. Bioengineered, 2017, 8(5):665-669.
doi: 10.1080/21655979.2016.1267884 URL |
| [18] | 李娜, 张蕊, 黄遵锡, 等. β-木糖苷酶的生物活性物质转化功能研究进展[J]. 微生物学通报, 2020, 47(7):2290-2299. |
| Li N, Zhang R, Huang ZX, et al. Research progress in bioactive substances transformation by β-xylosidases[J]. Microbiol China, 2020, 47(7):2290-2299. | |
| [19] | 张衡, 甘慧, 吴卓娜, 等. 7-木糖紫杉烷类化合物生物转化[J]. 中国新药杂志, 2013, 22(9):1029-1033. |
| Zhang H, Gan H, Wu ZN, et al. Biotransformation of 7-xylose taxanes[J]. Chin J New Drugs, 2013, 22(9):1029-1033. | |
| [20] | 区佩渝, 刘凌燕, 陈志宇, 等. 美洲大蠊肠道内生分枝杆菌的分离鉴定及其抑菌活性的初步研究[J]. 中国病原生物学杂志, 2019, 14(5):560-564, 567. |
| Ou PY, Liu LY, Chen ZY, et al. Isolation and identification of endophytic Mycobacteria from Periplaneta americana and a preliminary study of its antibacterial activity[J]. J Pathog Biol, 2019, 14(5):560-564, 567. | |
| [21] | 刘宇, 郑水, 刘汉博, 等. 利用天蓝色链霉菌转化7-木糖紫杉烷的研究[J]. 氨基酸和生物资源, 2012, 34(4):39-41. |
| Liu Y, Zheng S, Liu HB, et al. Study on Transforming 7-xyloyl-taxane by Streptomyces coelicolor[J]. Amino Acids Biotic Resour, 2012, 34(4):39-41. | |
| [22] | 李艳, 周剑, 何东贤, 等. 微生物转化在现代中药研发中的应用[J]. 中国抗生素杂志, 2020, 45(5):418-422. |
| Li Y, Zhou J, He DX, et al. Application of microbial transformation in the research of modern traditional Chinese medicine[J]. Chin J Antibiot, 2020, 45(5):418-422. | |
| [23] | 李勇超, 杨靖, 周修任, 等. 耦合培养法对10-DAB产量的影响[J]. 生物技术, 2016, 26(1):93-97. |
| Li YC, Yang J, Zhou XR, et al. Effects of coupling cultivation on the 10-DAB yield[J]. Biotechnology, 2016, 26(1):93-97. | |
| [24] | 朱凤芝. 巴卡亭Ⅲ转化生成10-DAB的微生物的筛选、鉴定和工艺优化[D]. 天津:天津科技大学, 2016. |
| Zhu FZ. The screening, identification and technology optimization of the strains with the biotransformation ability from baccatin Ⅲ to 10-DAB[D]. Tianjin:Tianjin University of Science & Technology, 2016. | |
| [25] | 李建华. 三尖杉宁碱的微生物转化与10-去乙酰紫杉醇的酶法合成[D]. 北京:中国协和医科大学, 2007. |
| Li JH. Microbial transformation of cephalomannine and the enzymatic synthesis of 10-deacetyltaxol[D]. Beijing:Peking Union Medical College, 2007. | |
| [26] | 李建华. 紫杉醇类似物的生物转化[C]. 吉林:中国植物学会, 2006. |
| Li JH. Biotransformation of paclitaxel analogues[C]. Jilin:Chinese Botany Society, 2016. | |
| [27] | 张衡. 7-木糖-10-去乙酰紫杉醇的生物转化研究[D]. 南宁:广西医科大学, 2013. |
| Zhang H. Biotransformation research of 7-β-xylosyl-10-deacetylpaclitaxel[D]. Nanning:Guangxi Medical University, 2013. | |
| [28] |
Li Q, Jiang YJ, Tong XY, et al. Cloning and characterization of the β-xylosidase from Dictyoglomus turgidum for high efficient biotransformation of 10-deacetyl-7-xylosltaxol[J]. Bioorg Chem, 2020, 94:103357.
doi: 10.1016/j.bioorg.2019.103357 URL |
| [29] |
Dou TY, Luan HW, Liu XB, et al. Enzymatic hydrolysis of 7-xylosyltaxanes by an extracellular xylosidase from Cellulosimicrobium cellulans[J]. Biotechnol Lett, 2015, 37(9):1905-1910.
doi: 10.1007/s10529-015-1867-4 URL |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||