








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (6): 245-251.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0927
Previous Articles Next Articles
FU Qiao1( ), LIN Qi-lan1, XUE Qiang1, XIONG Hai-rong1, WANG Ya-wei1,2(
), LIN Qi-lan1, XUE Qiang1, XIONG Hai-rong1, WANG Ya-wei1,2( )
)
Received:2021-07-17
Online:2022-06-26
Published:2022-07-11
Contact:
WANG Ya-wei
E-mail:1196840811@qq.com;253182556@qq.com
FU Qiao, LIN Qi-lan, XUE Qiang, XIONG Hai-rong, WANG Ya-wei. Effects of CBM41 N-terminal Truncation on the Enzymological Properties of the Pullulanase from Bacillus subtilis 168[J]. Biotechnology Bulletin, 2022, 38(6): 245-251.
| Primer name | Nucleotide sequence(5'-3') | Gene |
|---|---|---|
| WT-F(Nco I) | CATGCCATGGTCAGCATCCGCCGCAG- CTTC | PulB |
| M1-F(Nco I) | CATGCCATGGATAGCATCCGCCGCAG- CTTCGAAG | PulBΔN2 |
| M2-F(Nco I) | CATGCCATGGATCGCCGCAGCTTCGA- AGCGTATG | PulBΔN4 |
| M3-F(Nco I) | CATGCCATGGATAGCTTCGAAGC- GTATGTCGATG | PulBΔN6 |
| WT-R(Xho I) | CCGCTCGAGAGCAAAACTCTTAAGAT- CTGATGC |
Table 1 Primers for amplification of wild-type and trun-cated mutants
| Primer name | Nucleotide sequence(5'-3') | Gene |
|---|---|---|
| WT-F(Nco I) | CATGCCATGGTCAGCATCCGCCGCAG- CTTC | PulB |
| M1-F(Nco I) | CATGCCATGGATAGCATCCGCCGCAG- CTTCGAAG | PulBΔN2 |
| M2-F(Nco I) | CATGCCATGGATCGCCGCAGCTTCGA- AGCGTATG | PulBΔN4 |
| M3-F(Nco I) | CATGCCATGGATAGCTTCGAAGC- GTATGTCGATG | PulBΔN6 |
| WT-R(Xho I) | CCGCTCGAGAGCAAAACTCTTAAGAT- CTGATGC |
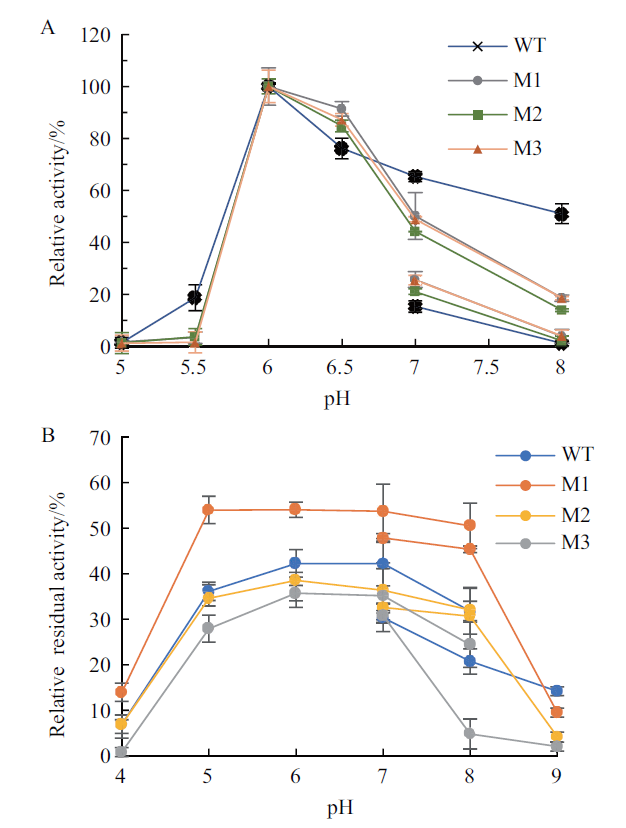
Fig.4 Optimal pH and pH stability of wild-type pullulan-ase and the truncated mutants A:Optimal pH. The reaction temperature and time:40℃ and 30 min. B:pH stability. pH:4.0-9.0;treatment time:1 h;reaction temperature:40℃. WT:Wild-type pullulanase;M1,M2 and M3:different mutants of pullulanase. The same below
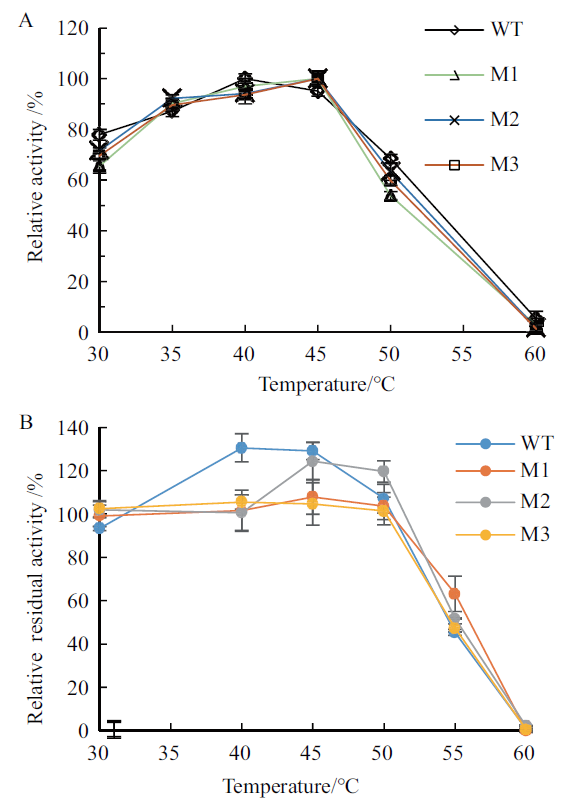
Fig.5 Optimal temperature and thermostability of wild-type pullulanase and the truncated mutants A:Optimal temperature. The reaction pH and time:6.0 and 30 min. B:Thermostability. Temperature:30-60℃;treatment time:1 h;reaction temperature:40℃
| 比酶活Specific enzyme activity/(U·mg-1) | Km/(mg·mL-1) | Vmax/(U·mg-1) | Tm/℃ | |
|---|---|---|---|---|
| WT | 2.30±0.09 | 23.89±0.66 | 4.06±0.09 | 48.57±0.42 |
| M1 | 2.72±0.08 | 29.01±1.21 | 2.35±0.12 | 50.03±0.25 |
| M2 | 3.69±0.07 | 17.29±0.73 | 3.92±0.06 | 48.43±0.22 |
| M3 | 5.62±0.06 | 19.08±0.41 | 7.24±0.11 | 49.50±0.32 |
Table 2 Comparison of specific activity,kinetic parameters and Tm of pullulanase and the truncated mutants
| 比酶活Specific enzyme activity/(U·mg-1) | Km/(mg·mL-1) | Vmax/(U·mg-1) | Tm/℃ | |
|---|---|---|---|---|
| WT | 2.30±0.09 | 23.89±0.66 | 4.06±0.09 | 48.57±0.42 |
| M1 | 2.72±0.08 | 29.01±1.21 | 2.35±0.12 | 50.03±0.25 |
| M2 | 3.69±0.07 | 17.29±0.73 | 3.92±0.06 | 48.43±0.22 |
| M3 | 5.62±0.06 | 19.08±0.41 | 7.24±0.11 | 49.50±0.32 |
| [1] |
Ali G, Rihouey C, Le Cerf D, et al. Effect of carboxymethyl groups on degradation of modified pullulan by pullulanase from Klebsiella pneumoniae[J]. Carbohydr Polym, 2013, 93(1):109-115.
doi: 10.1016/j.carbpol.2012.07.039 URL |
| [2] |
Talekar S, Pandharbale A, Ladole M, et al. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates(combi-CLEAs):a tri-enzyme biocatalyst with one pot starch hydrolytic activity[J]. Bioresour Technol, 2013, 147:269-275.
doi: 10.1016/j.biortech.2013.08.035 URL |
| [3] | Nie Y, Yan W, Xu Y, et al. High-level expression of Bacillus naganoensis pullulanase from recombinant Escherichia coli with auto-induction:effect of lac operator[J]. PLoS One, 2013, 8(10):e78416. |
| [4] |
Ram KA, Venkatasubramanian K. Enhancement of starch conversion efficiency with free and immobilized pullulanase and alpha-1, 4-glucosidase[J]. Biotechnol Bioeng, 1982, 24(2):355-369.
pmid: 18546308 |
| [5] |
Meng F, Zhu X, Nie T, et al. Enhanced expression of pullulanase in Bacillus subtilis by new strong promoters mined from transcriptome data, both alone and in combination[J]. Front Microbiol, 2018, 9:2635.
doi: 10.3389/fmicb.2018.02635 URL |
| [6] | da Silva VM, Cabral AD, Sperança MA, et al. High-resolution structure of a modular hyperthermostable endo-β-1, 4-mannanase from Thermotoga petrophila:The ancillary immunoglobulin-like module is a thermostabilizing domain[J]. Biochim Biophys Acta Proteins Proteom, 2020, 1868(8):140437. |
| [7] |
Malle D, Itoh T, Hashimoto W, et al. Overexpression, purification and preliminary X-ray analysis of pullulanase from Bacillus subtilis strain 168[J]. Acta Crystallogr Sect F Struct Biol Cryst Commun, 2006, 62(pt 4):381-384.
doi: 10.1107/S1744309106007901 URL |
| [8] | Chang MH, Chu XY, Lv J, et al. Improving the thermostability of acidic pullulanase from Bacillus naganoensis by rational design[J]. PLoS One, 2016, 11(10):e0165006. |
| [9] |
Turkenburg JP, Brzozowski AM, Svendsen A, et al. Structure of a pullulanase from Bacillus acidopullulyticus[J]. Proteins, 2009, 76(2):516-519.
doi: 10.1002/prot.22416 URL |
| [10] |
Zeng Y, Xu J, Fu X, et al. Effects of different carbohydrate-binding modules on the enzymatic properties of pullulanase[J]. Int J Biol Macromol, 2019, 137:973-981.
doi: S0141-8130(19)33084-3 pmid: 31295482 |
| [11] | 陈阿娜, 刘秀霞, 戴晓峰, 等. N端截短对嗜酸普鲁兰芽孢杆菌普鲁兰酶酶学特性及功能的影响[J]. 生物工程学报, 2016, 32(3):355-364. |
| Chen AN, Liu XX, Dai XF, et al. Effect of N-terminal truncation of Bacillus acidopullulyticus pullulanase on enzyme properties and functions[J]. Chin J Biotechnol, 2016, 32(3):355-364. | |
| [12] |
Duan X, Wu J. Enhancing the secretion efficiency and thermostability of a Bacillus deramificans pullulanase mutant(D437H/D503Y)by N-terminal domain truncation[J]. Appl Environ Microbiol, 2015, 81(6):1926-1931.
doi: 10.1128/AEM.03714-14 URL |
| [13] |
Jiao Y, Wu Y, Chen H, et al. The impact of N-terminal nonessential domains on the enzymological properties of the pullulanase from a marine Bacillus megaterium[J]. Biotechnol Lett, 2019, 41(6/7):849-857.
doi: 10.1007/s10529-019-02686-2 URL |
| [14] |
Chen A, Sun Y, Zhang W, et al. Downsizing a pullulanase to a small molecule with improved soluble expression and secretion efficiency in Escherichia coli[J]. Microb Cell Fact, 2016, 15:9.
doi: 10.1186/s12934-015-0403-5 pmid: 26762529 |
| [15] | 韩来闯, 马闪闪, 刘亚娟, 等. 构建重组质粒的二步PCR方法[J]. 河南科学, 2015, 33(8):1321-1325. |
| Han LC, Ma SS, Liu YJ, et al. Construction of recombinant plasmid by a simple step-reverse PCR method[J]. Henan Sci, 2015, 33(8):1321-1325. | |
| [16] |
Kang J, Park KM, Choi KH, et al. Molecular cloning and biochemical characterization of a heat-stable type I pullulanase from Ther-motoga neapolitana[J]. Enzym Microb Technol, 2011, 48(3):260-266.
doi: 10.1016/j.enzmictec.2010.11.006 URL |
| [17] | 邢岩, 邱爽, 聂慧慧, 等. Thermotoga petrophila普鲁兰酶基因的异源表达与酶学性质分析[J]. 河南农业大学学报, 2018, 52(3):404-411, 423. |
| Xing Y, Qiu S, Nie HH, et al. Heterologous expression and enzymatic characterization of the pullulanase from Thermotoga petrophila[J]. J Henan Agric Univ, 2018, 52(3):404-411, 423. | |
| [18] |
Ericsson UB, Hallberg BM, Detitta GT, et al. Thermofluor-based high-throughput stability optimization of proteins for structural studies[J]. Anal Biochem, 2006, 357(2):289-298.
pmid: 16962548 |
| [19] |
Janeček Š, Majzlová K, Svensson B, et al. The starch-binding domain family CBM41-An in silico analysis of evolutionary relationships[J]. Proteins, 2017, 85(8):1480-1492.
doi: 10.1002/prot.25309 URL |
| [20] | 王馨叶. 普鲁兰酶催化效率强化的分子改造及其分泌表达调控[D]. 无锡: 江南大学, 2019. |
| Wang XY. Molecular engineering of pullulanase for enhancement of catalytic efficiency and its extracellular expression regulation[D]. Wuxi: Jiangnan University, 2019. | |
| [21] |
Nisha M, Satyanarayana T. The role of N1 domain on the activity, stability, substrate specificity and raw starch binding of amylopullulanase of the extreme thermophile Geobacillus thermoleovorans[J]. Appl Microbiol Biotechnol, 2015, 99(13):5461-5474.
doi: 10.1007/s00253-014-6345-8 pmid: 25573470 |
| [22] | 叶延欣, 闫鹏飞, 胡渝, 等. F/10木聚糖酶二级结构含量对热稳定性的影响[J]. 河南农业大学学报, 2013, 47(4):446-450. |
| Ye YX, Yan PF, Hu Y, et al. Influence of secondary structure content on F/10 xylanase thermostability[J]. J Henan Agric Univ, 2013, 47(4):446-450. | |
| [23] |
Guillén D, Sánchez S, Rodríguez-Sanoja R. Carbohydrate-binding domains:multiplicity of biological roles[J]. Appl Microbiol Biotechnol, 2010, 85(5):1241-1249.
doi: 10.1007/s00253-009-2331-y pmid: 19908036 |
| [24] |
Li WF, Zhou XX, Lu P. Structural features of thermozymes[J]. Biotechnol Adv, 2005, 23(4):271-281.
pmid: 15848038 |
| [1] | YANG Jun-zhao, ZHANG Xin-rui, ZHAO Guo-zhu, ZHENG Fei. Structure and Function Analysis of Novel GH5 Multi-domain Cellulase [J]. Biotechnology Bulletin, 2023, 39(4): 71-80. |
| [2] | WANG Yu-chen, DING Zun-dan, GUAN Fei-fei, TIAN Jian, LIU Guo-an, WU Ning-feng. Identification of the Thermostable Laccase Gene ba4 and Characterization of Its Enzymatic Properties [J]. Biotechnology Bulletin, 2022, 38(8): 252-260. |
| [3] | WANG Xiao-tao, ZOU Hang, WU Yi, XIANG Shen-wei, LV Hua, LIU Chao-lan, LIN Jia-fu, WANG Xin-rong, CHU Yi-wen, SONG Tao. Heterologous Expression and Enzymatic Properties Analysis of Novel β-agarase Aga2 from Paraglaciecola hydrolytica [J]. Biotechnology Bulletin, 2022, 38(11): 258-268. |
| [4] | ZHANG Yao-xin, WANG Liang-jie, ZHENG Wen, XU Han-qin, ZHENG Lian, ZHONG Jing. Study on Enzyme Production of a Chitinase-producing Strain Achromobacter sp. ZWW8 by Fermentation and Its Enzymatic Characterization [J]. Biotechnology Bulletin, 2021, 37(4): 96-106. |
| [5] | WANG Hui-lan, WU Jin-yong, CHEN Xiang-song, YUAN Li-xia, ZHU Wei-wei, YAO Jian-ming. Immobilization of N-acetylneuraminic Acid Aldolaseand Properties of the Immobilized Enzyme [J]. Biotechnology Bulletin, 2020, 36(6): 165-173. |
| [6] | LI Cheng-ming, WANG Jia-yi, LU Lei. Extracellular Expression of Laccase Gene from Bacillus pumilus LC01 in Pichia pastoris and Characterization of the Recombinant Laccase [J]. Biotechnology Bulletin, 2018, 34(3): 185-193. |
| [7] | ZHAO Shu-mei, WANG Lin-hui, TANG Jia-qi, ZHAO Jing-yi. Cloning,Expression and Characterization of the Esterase Gene estZ from Vibrio alginolyticus [J]. Biotechnology Bulletin, 2017, 33(6): 190-196. |
| [8] | Jia Yang, Shi Yanhua, Ren Lei, Qiao Cheng, Wang Junhuan, Yan Yanchun. Cloning and Characterization of mpd and ophc2 from an Organophosphorus Pesticide-degrading Bacteria YC-YH1 [J]. Biotechnology Bulletin, 2015, 31(11): 228-235. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||