








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (7): 17-27.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0124
Previous Articles Next Articles
LI Si-bo( ), QIAN Hong-ping, XU Chang-wen, WANG Xiao, LIN Jin-xing, CUI Ya-ning(
), QIAN Hong-ping, XU Chang-wen, WANG Xiao, LIN Jin-xing, CUI Ya-ning( )
)
Received:2025-02-06
Online:2025-07-26
Published:2025-07-22
Contact:
CUI Ya-ning
E-mail:lisibo@bjfu.edu.cn;cuiyaning@bjfu.edu.cn
LI Si-bo, QIAN Hong-ping, XU Chang-wen, WANG Xiao, LIN Jin-xing, CUI Ya-ning. Research Progress in the Involvement of Intracellular Transport Regulated by Endogenous Elicitors in Plant Growth and Development and Response to Adverse Stress[J]. Biotechnology Bulletin, 2025, 41(7): 17-27.
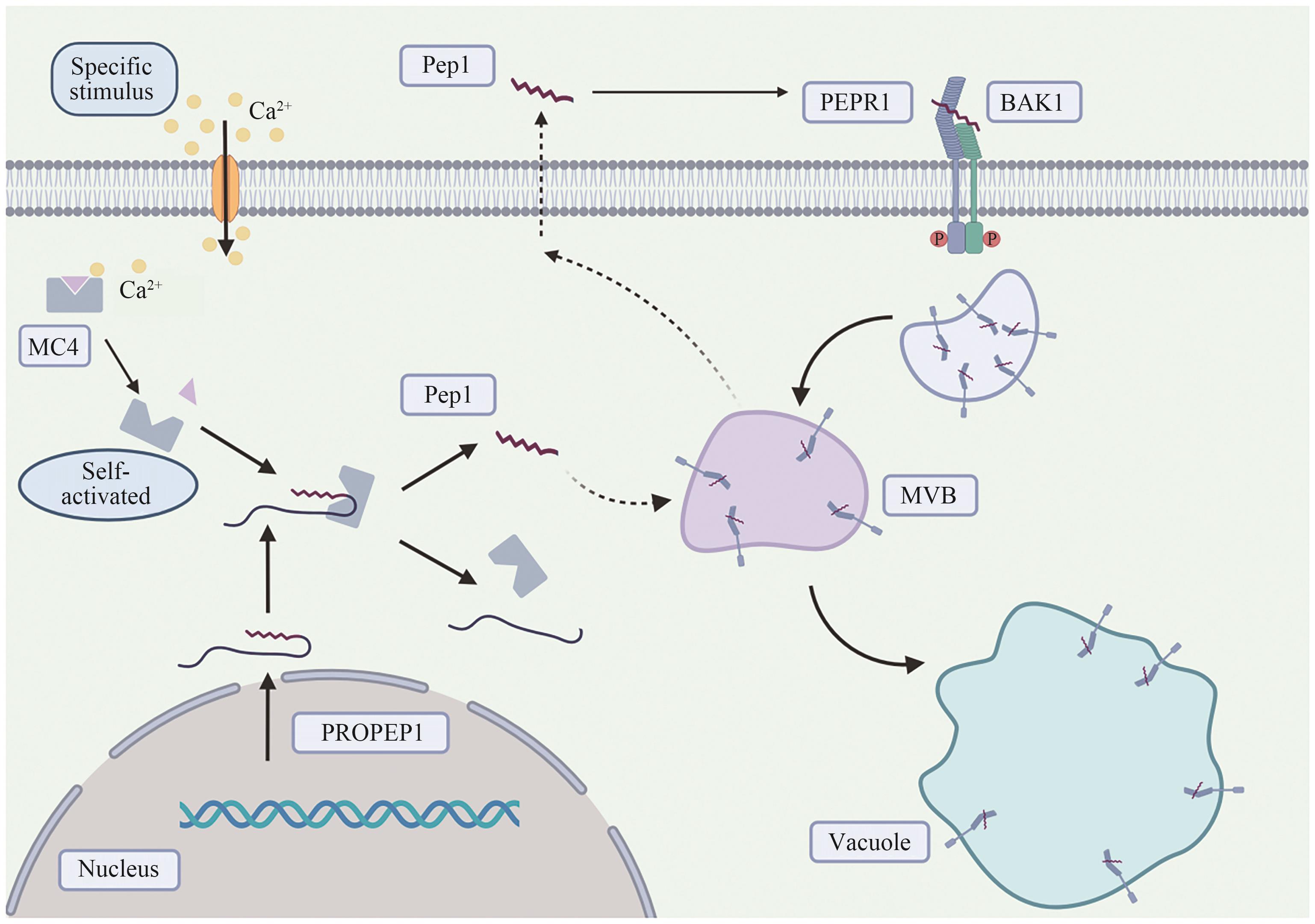
Fig. 1 Pep1 secretion and PEPR1 endocytosisExtracellular Ca2+ quickly flows into the cell when plants are subjected to specific stimuli, which in turn causes the concentration of intracellular Ca2+ to rise rapidly, activates cysteine protease MC4, thus cutting the specific site of the C terminal of AtPROPEP1 and releasing AtPep1 into the cytoplasm. Finally, AtPep1 may be secreted extracellular by MVB fusion with plasma membrane. Extracellular AtPep1 can recognize and bind to the PEPR1 receptor on the plasma membrane, induce heterodimerization of PEPR1 and co-receptor BAK1, induce endocytosis of PEPR1, and regulate immune signal transmission. The AtPep 1-PEPR1 complex may bypass TGN/EE and internalize directly to MVB. MC4: Ca2+-dependent metacaspase 4; MVB: multivesicular body; PROPEP 1: precursor of Pep 1; Pep 1: plant elicitor peptide 1; PEPR1: Pep 1 receptor; BAK1: brassinosteroid-insensitive 1-associated receptor kinase 1
| 配体 Ligand | 受体 Receptor | 生物学效应 Biological effects |
|---|---|---|
| AtPep1 | PEPR1 | 激活MAPK级联反应,促进防御相关基因的转录 迅速诱导细胞内活性氧(如超氧阴离子、过氧化氢)的产生 调控植物叶片免疫和主根生长抑制 |
| SYS | SYR1 | 激活蛋白酶抑制剂基因的表达,抑制昆虫和食草动物的消化蛋白酶 MAPK磷酸化、钙调素基因表达的上调 调节植物激素的平衡来影响植物的生长速度和形态 |
| RALF1 | FER | 抑制质子泵(H+-ATPase)的活性,引起细胞生长和扩张的减缓 影响细胞形态和细胞壁的合成 |
| PIP1 | RLK7 | 对PAMP flg22信号起放大作用 诱导SA信号而非JA信号参与植物免疫调节 |
Table 1 Biological effects of endogenous elicitors and pattern recognition receptors
| 配体 Ligand | 受体 Receptor | 生物学效应 Biological effects |
|---|---|---|
| AtPep1 | PEPR1 | 激活MAPK级联反应,促进防御相关基因的转录 迅速诱导细胞内活性氧(如超氧阴离子、过氧化氢)的产生 调控植物叶片免疫和主根生长抑制 |
| SYS | SYR1 | 激活蛋白酶抑制剂基因的表达,抑制昆虫和食草动物的消化蛋白酶 MAPK磷酸化、钙调素基因表达的上调 调节植物激素的平衡来影响植物的生长速度和形态 |
| RALF1 | FER | 抑制质子泵(H+-ATPase)的活性,引起细胞生长和扩张的减缓 影响细胞形态和细胞壁的合成 |
| PIP1 | RLK7 | 对PAMP flg22信号起放大作用 诱导SA信号而非JA信号参与植物免疫调节 |
| [1] | DeFalco TA, Zipfel C. Molecular mechanisms of early plant pattern-triggered immune signaling [J]. Mol Cell, 2021, 81(17): 3449-3467. |
| [2] | Wu Y, Zhou JM. Receptor-like kinases in plant innate immunity [J]. J Integr Plant Biol, 2013, 55(12): 1271-1286. |
| [3] | Macho AP, Zipfel C. Plant PRRs and the activation of innate immune signaling [J]. Mol Cell, 2014, 54(2): 263-272. |
| [4] | Cui HT, Tsuda K, Parker JE. Effector-triggered immunity: from pathogen perception to robust defense [J]. Annu Rev Plant Biol, 2015, 66: 487-511. |
| [5] | Lee DH, Lee HS, Belkhadir Y. Coding of plant immune signals by surface receptors [J]. Curr Opin Plant Biol, 2021, 62: 102044. |
| [6] | Brutus A, Sicilia F, Macone A, et al. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides [J]. Proc Natl Acad Sci USA, 2010, 107(20): 9452-9457. |
| [7] | Benedetti M, Pontiggia D, Raggi S, et al. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns [J]. Proc Natl Acad Sci USA, 2015, 112(17): 5533-5538. |
| [8] | Davidsson P, Broberg M, Kariola T, et al. Short oligogalacturonides induce pathogen resistance-associated gene expression in Arabidopsis thaliana [J]. BMC Plant Biol, 2017, 17(1): 19. |
| [9] | Huffaker A, Dafoe NJ, Schmelz EA. ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance [J]. Plant Physiol, 2011, 155(3): 1325-1338. |
| [10] | Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response [J]. Proc Natl Acad Sci USA, 2006, 103(26): 10098-10103. |
| [11] | Bartels S, Lori M, Mbengue M, et al. The family of peps and their precursors in Arabidopsis: differential expression and localization but similar induction of pattern-triggered immune responses [J]. J Exp Bot, 2013, 64(17): 5309-5321. |
| [12] | 荆彦平, 沈诺, 兰文智. 植物激发子肽Peps的功能研究进展 [J]. 中国科学: 生命科学, 2022, 52(3): 301-311. |
| Jing YP, Shen N, Lan WZ. Research progress on the function of plant elicitor peptides [J]. Sci Sin Vitae, 2022, 52(3): 301-311. | |
| [13] | 聂甲玥, 杨文文, 樊红霞, 等. 植物Pep短肽的研究进展 [J]. 生物技术通报, 2021, 37(9): 219-225. |
| Nie JY, Yang WW, Fan HX, et al. Recent advances in plant Pep peptide [J]. Biotechnol Bull, 2021, 37(9): 219-225. | |
| [14] | Shen WZ, Liu JE, Li JF. Type-II metacaspases mediate the processing of plant elicitor peptides in Arabidopsis [J]. Mol Plant, 2019, 12(11): 1524-1533. |
| [15] | Zhang C, Wu YY, Liu JE, et al. SUMOylation controls peptide processing to generate damage-associated molecular patterns in Arabidopsis [J]. Dev Cell, 2025, 60(5): 696-705.e4. |
| [16] | Rabouille C. Pathways of unconventional protein secretion [J]. Trends Cell Biol, 2017, 27(3): 230-240. |
| [17] | Hu S, Li Y, Shen JB. A diverse membrane interaction network for plant multivesicular bodies: roles in proteins vacuolar delivery and unconventional secretion [J]. Front Plant Sci, 2020, 11: 425. |
| [18] | Pearce G, Strydom D, Johnson S, et al. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins [J]. Science, 1991, 253(5022): 895-897. |
| [19] | Constabel CP, Yip L, Ryan CA. Prosystemin from potato, black nightshade, and bell pepper: primary structure and biological activity of predicted systemin polypeptides [J]. Plant Mol Biol, 1998, 36(1): 55-62. |
| [20] | Zhang HY, Yu PL, Zhao JH, et al. Expression of tomato prosystemin gene in Arabidopsis reveals systemic translocation of its mRNA and confers necrotrophic fungal resistance [J]. New Phytol, 2018, 217(2): 799-812. |
| [21] | Yang WT, Zhai HW, Wu FM, et al. Peptide REF1 is a local wound signal promoting plant regeneration [J]. Cell, 2024, 187(12): 3024-3038.e14. |
| [22] | Stegmann M, Monaghan J, Smakowska-Luzan E, et al. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling [J]. Science, 2017, 355(6322): 287-289. |
| [23] | Cho H, Seo D, Kim M, et al. SERKs serve as co-receptors for SYR1 to trigger systemin-mediated defense responses in tomato [J]. J Integr Plant Biol, 2024, 66(10): 2273-2287. |
| [24] | Abarca A, Franck CM, Zipfel C. Family-wide evaluation of rapid alkalinization factor peptides [J]. Plant Physiol, 2021, 187(2): 996-1010. |
| [25] | 侯书国. 拟南芥分泌肽PIP1调节植物免疫的分子机制 [D]. 济南: 山东大学, 2015. |
| Hou SG. Molecular mechanism of Arabidopsis thaliana secretory peptide PIP1 regulating plant immunity [D]. Jinan: Shandong University, 2015. | |
| [26] | Vie AK, Najafi J, Liu B, et al. The IDA/IDA-LIKE and PIP/PIP-LIKE gene families in Arabidopsis: phylogenetic relationship, expression patterns, and transcriptional effect of the PIPL3 peptide [J]. J Exp Bot, 2015, 66(17): 5351-5365. |
| [27] | Dievart A, Gottin C, Périn C, et al. Origin and diversity of plant receptor-like kinases [J]. Annu Rev Plant Biol, 2020, 71: 131-156. |
| [28] | Liu ZX, Wu Y, Yang F, et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity [J]. Proc Natl Acad Sci USA, 2013, 110(15): 6205-6210. |
| [29] | Xu SM, Liao CJ, Jaiswal N, et al. Tomato PEPR1 ORTHOLOG RECEPTOR-LIKE KINASE1 regulates responses to systemin, necrotrophic fungi, and insect herbivory [J]. Plant Cell, 2018, 30(9): 2214-2229. |
| [30] | Buonanno M, Coppola M, Di Lelio I, et al. Prosystemin, a prohormone that modulates plant defense barriers, is an intrinsically disordered protein [J]. Protein Sci, 2018, 27(3): 620-632. |
| [31] | Zhang HY, Zhang H, Lin JX. Systemin-mediated long-distance systemic defense responses [J]. New Phytol, 2020, 226(6): 1573-1582. |
| [32] | Haruta M, Sabat G, Stecker K, et al. A peptide hormone and its receptor protein kinase regulate plant cell expansion [J]. Science, 2014, 343(6169): 408-411. |
| [33] | Wang P, Clark NM, Nolan TM, et al. Integrated omics reveal novel functions and underlying mechanisms of the receptor kinase FERONIA in Arabidopsis thaliana [J]. Plant Cell, 2022, 34(7): 2594-2614. |
| [34] | Duan QH, Liu MJ, Kita D, et al. FERONIA controls pectin- and nitric oxide-mediated male-female interaction [J]. Nature, 2020, 579(7800): 561-566. |
| [35] | Chen J, Yu F, Liu Y, et al. FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis [J]. Proc Natl Acad Sci USA, 2016, 113(37): E5519-27. |
| [36] | Yu F, Qian LC, Nibau C, et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase [J]. Proc Natl Acad Sci USA, 2012, 109(36): 14693-14698. |
| [37] | Guo HQ, Nolan TM, Song GY, et al. FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana [J]. Curr Biol, 2018, 28(20): 3316-3324.e6. |
| [38] | Yu M, Li RL, Cui YN, et al. The RALF1-FERONIA interaction modulates endocytosis to mediate control of root growth in Arabidopsis [J]. Development, 2020, 147(13): dev189902. |
| [39] | Liu LN, Liu X, Bai ZK, et al. Small but powerful: RALF peptides in plant adaptive and developmental responses [J]. Plant Sci, 2024, 343: 112085. |
| [40] | Beck M, Zhou J, Faulkner C, et al. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting [J]. Plant Cell, 2012, 24(10): 4205-4219. |
| [41] | Ben Khaled S, Postma J, Robatzek S. A moving view: subcellular trafficking processes in pattern recognition receptor-triggered plant immunity [J]. Annu Rev Phytopathol, 2015, 53: 379-402. |
| [42] | 张建. 拟南芥PEPR1动态特征及胞吞过程的单分子研究 [D]. 北京: 北京林业大学, 2017. |
| Zhang J. Study on dynamic characteristics and endocytosis of PEPR1 in Arabidopsis thaliana by single molecule [D]. Beijing: Beijing Forestry University, 2017. | |
| [43] | Choi SW, Tamaki T, Ebine K, et al. RABA members act in distinct steps of subcellular trafficking of the FLAGELLIN SENSING2 receptor [J]. Plant Cell, 2013, 25(3): 1174-1187. |
| [44] | Ortiz-Morea FA, Savatin DV, Dejonghe W, et al. Danger-associated peptide signaling in Arabidopsis requires clathrin [J]. Proc Natl Acad Sci USA, 2016, 113(39): 11028-11033. |
| [45] | Xiao Y, Stegmann M, Han ZF, et al. Mechanisms of RALF peptide perception by a heterotypic receptor complex [J]. Nature, 2019, 572(7768): 270-274. |
| [46] | Goh LK, Huang FT, Kim W, et al. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor [J]. J Cell Biol, 2010, 189(5): 871-883. |
| [47] | Hurst CH, Turnbull D, Xhelilaj K, et al. S-acylation stabilizes ligand-induced receptor kinase complex formation during plant pattern-triggered immune signaling [J]. Curr Biol, 2023, 33(8): 1588-1596.e6. |
| [48] | Cui YN, Qian HP, Yin JH, et al. Single-molecule analysis reveals the phosphorylation of FLS2 governs its spatiotemporal dynamics and immunity [J]. eLife, 2024, 12: RP91072. |
| [49] | Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis [J]. Cold Spring Harb Perspect Biol, 2014, 6(1): a016808. |
| [50] | Ishimori M. Transcription factor binding site prediction: finding the point from many data [J]. Plant Cell Physiol, 2022, 63(10): 1324-1325. |
| [51] | Zhou BJ, Zeng LR. Conventional and unconventional ubiquitination in plant immunity [J]. Mol Plant Pathol, 2017, 18(9): 1313-1330. |
| [52] | Zhou JG, Liu DR, Wang P, et al. Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB13-mediated ubiquitination [J]. Proc Natl Acad Sci USA, 2018, 115(8): E1906-E1915. |
| [53] | Lu DP, Lin WW, Gao XQ, et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity [J]. Science, 2011, 332(6036): 1439-1442. |
| [54] | Jing YP, Zheng XJ, Zhang DL, et al. Danger-associated peptides interact with PIN-dependent local auxin distribution to inhibit root growth in Arabidopsis [J]. Plant Cell, 2019, 31(8): 1767-1787. |
| [55] | Yamaguchi Y, Huffaker A, Bryan AC, et al. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis [J]. Plant Cell, 2010, 22(2): 508-522. |
| [56] | Nakaminami K, Okamoto M, Higuchi-Takeuchi M, et al. AtPep3 is a hormone-like peptide that plays a role in the salinity stress tolerance of plants [J]. Proc Natl Acad Sci USA, 2018, 115(22): 5810-5815. |
| [57] | Zhou HP, Xiao F, Zheng Y, et al. Pamp-induced secreted peptide 3 modulates salt tolerance through receptor-like kinase 7 in plants [J]. Plant Cell, 2022, 34(2): 927-944. |
| [58] | Liu MJ, Yeh FJ, Yvon R, et al. Extracellular pectin-RALF phase separation mediates FERONIA global signaling function [J]. Cell, 2024, 187(2): 312-330.e22. |
| [59] | Bar M, Sharfman M, Ron M, et al. BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1 [J]. Plant J, 2010, 63(5): 791-800. |
| [60] | Xing JJ, Ji DC, Duan ZK, et al. Spatiotemporal dynamics of FERONIA reveal alternative endocytic pathways in response to flg22 elicitor stimuli [J]. New Phytol, 2022, 235(2): 518-532. |
| [61] | Postma J, Liebrand TWH, Bi GZ, et al. Avr4 promotes Cf-4 receptor-like protein association with the BAK1/SERK3 receptor-like kinase to initiate receptor endocytosis and plant immunity [J]. New Phytol, 2016, 210(2): 627-642. |
| [62] | Ma XY, LAN Claus, Leslie ME, et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity [J]. Nature, 2020, 581(7807): 199-203. |
| [1] | JIANG Yan-ke, LU Chong-chong, YIN Zi-yi, LI Yang, DING Xin-hua. Research Progress in Alternative Splicing in Plant Immunity [J]. Biotechnology Bulletin, 2022, 38(1): 215-227. |
| [2] | WU Xin-yuan, WANG Guang-chao, LIN Jin-xing, JING Yan-ping. Correlative Light and Electron Microscopy and Its Application in Botanical Research [J]. Biotechnology Bulletin, 2022, 38(1): 278-288. |
| [3] | WEI Ying, LUO Meng, DAI Liang-ying, PENG De-liang, LIU Jing. Research Advance of Calreticulin from Plant Parasitic Nematode [J]. Biotechnology Bulletin, 2021, 37(7): 81-87. |
| [4] | QIANG Xiao-nan, LI Xin, CHEN Jia, LIAO Hong-dong, YU Feng. Preliminary Analysis of Functional Diversity of RALF Peptide Family in Arabidopsis thaliana [J]. Biotechnology Bulletin, 2019, 35(1): 2-10. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||