








Biotechnology Bulletin ›› 2021, Vol. 37 ›› Issue (11): 14-31.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1052
Previous Articles Next Articles
LI Jing1( ), FENG Na2, WANG Sheng-yang3, LIN Zhan-xi1(
), FENG Na2, WANG Sheng-yang3, LIN Zhan-xi1( )
)
Received:2021-08-18
Online:2021-11-26
Published:2021-12-03
Contact:
LIN Zhan-xi
E-mail:13959197195@163.com;lzxjuncao@163.com
LI Jing, FENG Na, WANG Sheng-yang, LIN Zhan-xi. Research Progress in Chemical Constituents of Taiwanofungus camphoratum and Its Pharmacological Activities[J]. Biotechnology Bulletin, 2021, 37(11): 14-31.
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 1 | Methyl antcinate A[ |  | 23 | Antcamphin K[ | 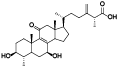 |
| 2 | Methyl antcinate G[ | 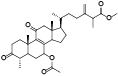 | 24 | Antcamphin L[ | 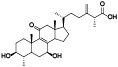 |
| 3 | Methyl antcinate H[ |  | 25 | 15-acetyldehydrosulfurenic[ |  |
| 4 | Methyl antcinate L[ | 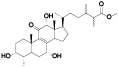 | 26 | 15α-acetyl-dehydrosulphurenic acid[ | 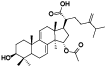 |
| 5 | Zhankuic acid B[ | 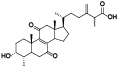 | 27 | Antcamphin E[ | 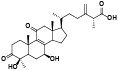 |
| 6 | Zhankuic acid D[ | 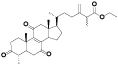 | 28 | Antcamphin F[ | 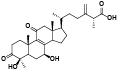 |
| 7 | Antcin A[ | 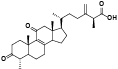 | 29 | Antcamphin G[ | 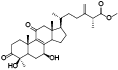 |
| 8 | Antcin B (Zhankuic acid A)[ | 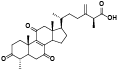 | 30 | Methyl antcinateK[ | 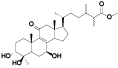 |
| 9 | Antcin C[ |  | 31 | Antcamphin H[ | 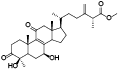 |
| 10 | Antcin D (Zhankuic acid F)[ |  | 32 | Antcin K[ | 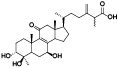 |
| 11 | Antcin E[ | 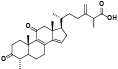 | 33 | Dehydrosulphurenic acid[ | 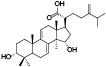 |
| 12 | Antcin F[ |  | 34 | Dehydroeburicoic acid[ | 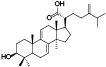 |
| 13 | Antcin G[ | 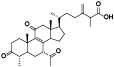 | 35 | 15-O-acetylganolucidate A[ | 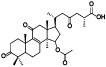 |
| 14 | Antcin H (Zhankuic acid C)[ |  | 36 | Ethyl lucidenate A[ | 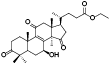 |
| 15 | Antcin M[ | 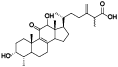 | 37 | Ethyl lucidenate F[ | 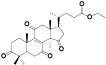 |
| 16 | Zhankuic acid E[ | 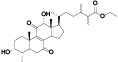 | 38 | 3, 7, 11-trioxolanosta-8, 24-dien-26-al, 26-carboxylic acid, 11a-alcohol, me ester[ |  |
| 17 | Antcamphin A[ |  | 39 | 3, 7, 11-trioxolanosta-8, 24-dien-26-oic acid[ |  |
| 18 | Antcamphin B[ | 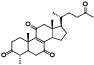 | 40 | 3, 7, 12-trihydroxy-11, 15, 23-trioxolanost-8-en-26-oic acid, 3, 7, 12-triketone, me ester[ | 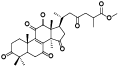 |
| 19 | Antcamphin C[ |  | 41 | 3, 7, 12-trihydroxy-11, 15, 23-trioxolanost-8-en-26-oic acid, 3, 7, 12-triketone, et ester[ | 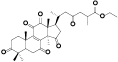 |
| 20 | Antcamphin D[ | 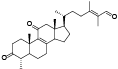 | 42 | Eburicol[ | 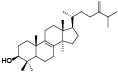 |
| 21 | Antcamphin I[ | 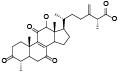 | 43 | Dehydroeburicoic acid monoucetate[ | 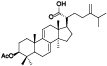 |
| 22 | Antcamphin J[ | 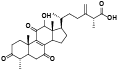 | 44 | Epi-friedelinol[ |  |
Table 1 Structures of triterpenoids
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 1 | Methyl antcinate A[ |  | 23 | Antcamphin K[ |  |
| 2 | Methyl antcinate G[ |  | 24 | Antcamphin L[ |  |
| 3 | Methyl antcinate H[ |  | 25 | 15-acetyldehydrosulfurenic[ |  |
| 4 | Methyl antcinate L[ |  | 26 | 15α-acetyl-dehydrosulphurenic acid[ |  |
| 5 | Zhankuic acid B[ |  | 27 | Antcamphin E[ |  |
| 6 | Zhankuic acid D[ |  | 28 | Antcamphin F[ |  |
| 7 | Antcin A[ |  | 29 | Antcamphin G[ |  |
| 8 | Antcin B (Zhankuic acid A)[ |  | 30 | Methyl antcinateK[ |  |
| 9 | Antcin C[ |  | 31 | Antcamphin H[ |  |
| 10 | Antcin D (Zhankuic acid F)[ |  | 32 | Antcin K[ |  |
| 11 | Antcin E[ |  | 33 | Dehydrosulphurenic acid[ |  |
| 12 | Antcin F[ |  | 34 | Dehydroeburicoic acid[ |  |
| 13 | Antcin G[ |  | 35 | 15-O-acetylganolucidate A[ |  |
| 14 | Antcin H (Zhankuic acid C)[ |  | 36 | Ethyl lucidenate A[ |  |
| 15 | Antcin M[ |  | 37 | Ethyl lucidenate F[ |  |
| 16 | Zhankuic acid E[ |  | 38 | 3, 7, 11-trioxolanosta-8, 24-dien-26-al, 26-carboxylic acid, 11a-alcohol, me ester[ |  |
| 17 | Antcamphin A[ |  | 39 | 3, 7, 11-trioxolanosta-8, 24-dien-26-oic acid[ |  |
| 18 | Antcamphin B[ |  | 40 | 3, 7, 12-trihydroxy-11, 15, 23-trioxolanost-8-en-26-oic acid, 3, 7, 12-triketone, me ester[ |  |
| 19 | Antcamphin C[ |  | 41 | 3, 7, 12-trihydroxy-11, 15, 23-trioxolanost-8-en-26-oic acid, 3, 7, 12-triketone, et ester[ |  |
| 20 | Antcamphin D[ |  | 42 | Eburicol[ |  |
| 21 | Antcamphin I[ |  | 43 | Dehydroeburicoic acid monoucetate[ |  |
| 22 | Antcamphin J[ |  | 44 | Epi-friedelinol[ |  |
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 45 | 1, 6-cyclo-1, 10-secoergosta-5, 7, 9, 22-tetraen-3-ol[ |  | 50 | Stigmasterol[ |  |
| 46 | Ergostatrien-3β-ol[ | 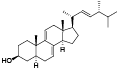 | 51 | β-sitostenone[ |  |
| 47 | Ergosta-4, 6, 8(14), 22-tetraen-3-one[ |  | 52 | β-sitosterol[ |  |
| 48 | Ergosterol peroxide[ | 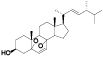 | 53 | (14α, 22E)-14-hydroxyergosta-7, 22-diene-3, 6-dione[ | 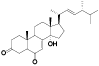 |
Table 2 Structures of steroids
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 45 | 1, 6-cyclo-1, 10-secoergosta-5, 7, 9, 22-tetraen-3-ol[ |  | 50 | Stigmasterol[ |  |
| 46 | Ergostatrien-3β-ol[ |  | 51 | β-sitostenone[ |  |
| 47 | Ergosta-4, 6, 8(14), 22-tetraen-3-one[ |  | 52 | β-sitosterol[ |  |
| 48 | Ergosterol peroxide[ |  | 53 | (14α, 22E)-14-hydroxyergosta-7, 22-diene-3, 6-dione[ |  |
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 54 | 14-dexyandrographolide[ | 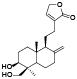 | 73 | 19-hydroxy-8(17)-labden-16, 15-olide[ |  |
| 55 | 3, 19-dihydroxy-8(17), 11-labdadien-16, 15-olide[ |  | 74 | Pinusolidic acid[ |  |
| 56 | 3, 19-dihydroxy-8(17), 11-labdadien-16, 15-olide, 13-epimer[ |  | 75 | 2, 3-dimethoxy-5-methyl-1, 4-benzoquinone[ |  |
| 57 | 14-deoxy-11, 12-didehyd-roandrographolide[ | 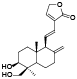 | 76 | 2, 3, 6-trimethoxy-4-methylphenol[ | 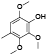 |
| 58 | Antrocin[ | 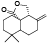 | 77 | 2, 3, 6-trimethoxy-5-methylphenol[ | 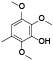 |
| 59 | Antrocamphin A[ | 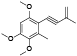 | 78 | 6-methoxy-4-methyl-7-(1-methylethenyl)-1, 3-benzodioxol-5-ol[ | 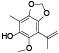 |
| 60 | Antrocamphin B[ | 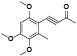 | 79 | 2-hydroxy-4, 4’-dimethoxy-3, 5'-dimethyl-2’, 3':5, 6-bis(methylenedioxy)biphenyl[ | 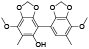 |
| 61 | Antrocamphin C[ |  | 80 | 4, 4’-dihydroxy-5, 5'-dimethoxy-6, 6’-dimethyl-2, 3:2’, 3'-bis(methylenedioxy)biphenyl[ | 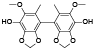 |
| 62 | Antrocamphin O[ | 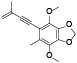 | 81 | 2-hydroxybenzeneethanol[ |  |
| 63 | Antrodioxolanone[ | 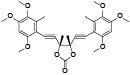 | 82 | 4, 5-dimethoxy-2, 3-(methylenedioxy)benzoic acid[ |  |
| 64 | 2-(4-hydroxyphenyl)-3-(2-methylpropyl)-2-butenedioic acid, di-me ester[ | 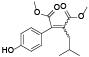 | 83 | 4-isobutylphenol[ | 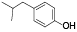 |
| 65 | 2, 3-dimethoxy-5-methyl-1, 4-benzoquinone[ | 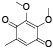 | 84 | 5-methyl-1, 3-benzodioxole-4, 7-dione[ | 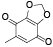 |
| 66 | 2, 3, 4, 5-tetrahydroxybenzoic acid[ | 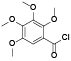 | 85 | 5-methoxy-7-methyl-1, 3-benzodioxol-4-ol[ | 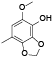 |
| 67 | 2, 3-dimethoxy-5-methyl-1, 4-benzenediol[ | 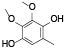 | 86 | 5-methyl-1, 3-benzodioxole-4, 7-diol[ | 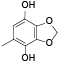 |
| 68 | 4, 7-dimethoxy-5-methyl-1, 3-benzodioxole[ |  | 87 | 7-methoxy-6-methyl-1, 3-benzodioxol-4-ol[ |  |
| 69 | 2, 4-dimethoxy-6-methyl-1, 3-benzenediol[ | 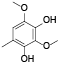 | 88 | 2-methoxy-6-methyl-1, 4-benzoquinone[ | 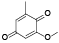 |
| 70 | 4, 4’, 7, 7’-tetramethoxy-6, 6’-dimethyl-5, 5'-bi-1, 3-benzodioxole[ |  | 89 | 4-hydroxysesamin[ | 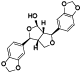 |
| 71 | (-)-sesamin[ | 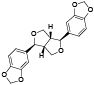 | 90 | 2-(2-hydroxyethyl)phenol[ | 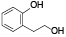 |
| 72 | (+)-sesamin[ |  | 91 | 3, 19-dihydroxy-8(17), 11-labdadien-16, 15-olide, 13-Epimer[ |  |
Table 3 Structures of diterpenes,sesquiterpenes and benzene derivatives
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 54 | 14-dexyandrographolide[ |  | 73 | 19-hydroxy-8(17)-labden-16, 15-olide[ |  |
| 55 | 3, 19-dihydroxy-8(17), 11-labdadien-16, 15-olide[ |  | 74 | Pinusolidic acid[ |  |
| 56 | 3, 19-dihydroxy-8(17), 11-labdadien-16, 15-olide, 13-epimer[ |  | 75 | 2, 3-dimethoxy-5-methyl-1, 4-benzoquinone[ |  |
| 57 | 14-deoxy-11, 12-didehyd-roandrographolide[ |  | 76 | 2, 3, 6-trimethoxy-4-methylphenol[ |  |
| 58 | Antrocin[ |  | 77 | 2, 3, 6-trimethoxy-5-methylphenol[ |  |
| 59 | Antrocamphin A[ |  | 78 | 6-methoxy-4-methyl-7-(1-methylethenyl)-1, 3-benzodioxol-5-ol[ |  |
| 60 | Antrocamphin B[ |  | 79 | 2-hydroxy-4, 4’-dimethoxy-3, 5'-dimethyl-2’, 3':5, 6-bis(methylenedioxy)biphenyl[ |  |
| 61 | Antrocamphin C[ |  | 80 | 4, 4’-dihydroxy-5, 5'-dimethoxy-6, 6’-dimethyl-2, 3:2’, 3'-bis(methylenedioxy)biphenyl[ |  |
| 62 | Antrocamphin O[ |  | 81 | 2-hydroxybenzeneethanol[ |  |
| 63 | Antrodioxolanone[ |  | 82 | 4, 5-dimethoxy-2, 3-(methylenedioxy)benzoic acid[ |  |
| 64 | 2-(4-hydroxyphenyl)-3-(2-methylpropyl)-2-butenedioic acid, di-me ester[ |  | 83 | 4-isobutylphenol[ |  |
| 65 | 2, 3-dimethoxy-5-methyl-1, 4-benzoquinone[ |  | 84 | 5-methyl-1, 3-benzodioxole-4, 7-dione[ |  |
| 66 | 2, 3, 4, 5-tetrahydroxybenzoic acid[ |  | 85 | 5-methoxy-7-methyl-1, 3-benzodioxol-4-ol[ |  |
| 67 | 2, 3-dimethoxy-5-methyl-1, 4-benzenediol[ |  | 86 | 5-methyl-1, 3-benzodioxole-4, 7-diol[ |  |
| 68 | 4, 7-dimethoxy-5-methyl-1, 3-benzodioxole[ |  | 87 | 7-methoxy-6-methyl-1, 3-benzodioxol-4-ol[ |  |
| 69 | 2, 4-dimethoxy-6-methyl-1, 3-benzenediol[ |  | 88 | 2-methoxy-6-methyl-1, 4-benzoquinone[ |  |
| 70 | 4, 4’, 7, 7’-tetramethoxy-6, 6’-dimethyl-5, 5'-bi-1, 3-benzodioxole[ |  | 89 | 4-hydroxysesamin[ |  |
| 71 | (-)-sesamin[ |  | 90 | 2-(2-hydroxyethyl)phenol[ |  |
| 72 | (+)-sesamin[ |  | 91 | 3, 19-dihydroxy-8(17), 11-labdadien-16, 15-olide, 13-Epimer[ |  |
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 92 | 4-acetyl-antroquinonol B[ |  | 98 | Antrocamol LT2[ |  |
| 93 | Antroquinonol A[ |  | 99 | Antroquinonol B[ |  |
| 94 | AC0009[ |  | 100 | Antroquinonol D[ | 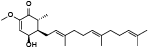 |
| 95 | Antrocamol LT1[ | 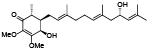 | 101 | 4-acetylantrocamol LT3[ | 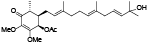 |
| 96 | Antrocamol LT3[ | 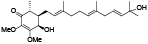 | 102 | 4-acetylantroquinonol B[ | 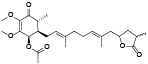 |
Table 4 Structures of antroquinonol and its derivatives
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 92 | 4-acetyl-antroquinonol B[ |  | 98 | Antrocamol LT2[ |  |
| 93 | Antroquinonol A[ |  | 99 | Antroquinonol B[ |  |
| 94 | AC0009[ |  | 100 | Antroquinonol D[ |  |
| 95 | Antrocamol LT1[ |  | 101 | 4-acetylantrocamol LT3[ |  |
| 96 | Antrocamol LT3[ |  | 102 | 4-acetylantroquinonol B[ |  |
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 103 | 2-(4-hydroxyphenyl)-3-isobutylmaleimide, 3R, 4S-dihydro, O-(3-methyl-2-butenyl)[ |  | 118 | Antrocinnamomin H[ | 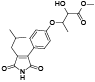 |
| 104 | 2-(4-hydroxyphenyl)-3-isobutylmaleimide, 3R*, 4S*-dihydro, N-hydroxy[ | 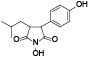 | 119 | Antrodin C (Camphorataimide C)[ | 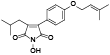 |
| 105 | 3, 4-dihydro-3-(4-hydroxyphenyl)-4-(2-methylpropyl)-2, 5-furandione[ | 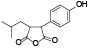 | 120 | Antrodin D (Camphorataimide E)[ |  |
| 106 | Antrodin B (Camphorataimide B)[ | 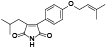 | 121 | Antrodin E (Camphorataimide D)[ |  |
| 107 | Antrocinnamomin A[ | 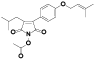 | 122 | Antrodin A[ | 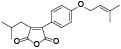 |
| 108 | Antrocinnamomin B[ | 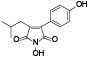 | 123 | Himanimide C[ | 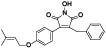 |
| 109 | Antrocinnamomin C[ |  | 124 | Himanimide A[ |  |
| 110 | Antrocinnamomin D[ | 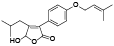 | 125 | 3-(4-hydroxyphenyl)-4- isobutylfuran-2, 5-dione[ | 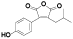 |
| 111 | Antrocinnamomin E[ | 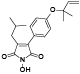 | 126 | dimethyl 2-(4-hydroxyphenyl)-3-isobutylmaleate[ |  |
| 112 | Antrocinnamomin F[ |  | 127 | 3R*, 4R*-1-hydroxy-3-isobutyl-4-[4-(3-methyl-2-butenyloxy)phenyl]pyrrolidine-2, 5-dione[ | 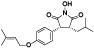 |
| 113 | Antrocinnamomin G[ | 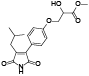 |
Table 5 Structures of succinic acid and maleic acid derivatives
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 103 | 2-(4-hydroxyphenyl)-3-isobutylmaleimide, 3R, 4S-dihydro, O-(3-methyl-2-butenyl)[ |  | 118 | Antrocinnamomin H[ |  |
| 104 | 2-(4-hydroxyphenyl)-3-isobutylmaleimide, 3R*, 4S*-dihydro, N-hydroxy[ |  | 119 | Antrodin C (Camphorataimide C)[ |  |
| 105 | 3, 4-dihydro-3-(4-hydroxyphenyl)-4-(2-methylpropyl)-2, 5-furandione[ |  | 120 | Antrodin D (Camphorataimide E)[ |  |
| 106 | Antrodin B (Camphorataimide B)[ |  | 121 | Antrodin E (Camphorataimide D)[ |  |
| 107 | Antrocinnamomin A[ |  | 122 | Antrodin A[ |  |
| 108 | Antrocinnamomin B[ |  | 123 | Himanimide C[ |  |
| 109 | Antrocinnamomin C[ |  | 124 | Himanimide A[ |  |
| 110 | Antrocinnamomin D[ |  | 125 | 3-(4-hydroxyphenyl)-4- isobutylfuran-2, 5-dione[ |  |
| 111 | Antrocinnamomin E[ |  | 126 | dimethyl 2-(4-hydroxyphenyl)-3-isobutylmaleate[ |  |
| 112 | Antrocinnamomin F[ |  | 127 | 3R*, 4R*-1-hydroxy-3-isobutyl-4-[4-(3-methyl-2-butenyloxy)phenyl]pyrrolidine-2, 5-dione[ |  |
| 113 | Antrocinnamomin G[ |  |
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 128 | 4-methoxy-2-oxo-2H-pyran-6-propanoic acid[ | 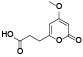 | 133 | 5-hydroxy-5-(methoxymethyl)-4-methyl-2(5H)-furanone[ | 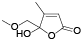 |
| 129 | 11-hydroxy-4-dodecanolide[ | 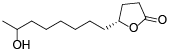 | 134 | Dihydro-5-octyl-2(3H)-furanone[ | 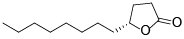 |
| 130 | Dihydro-5-(6-hydroxyoctyl)-2(3H)-furanone[ | 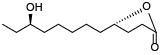 | 135 | Camphosterol A[ | 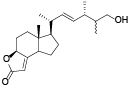 |
| 131 | α-tocospiro B[ | 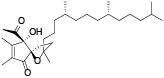 | 136 | 10-hydroxy-γ-dodecalactone[ | 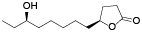 |
| 132 | 12-hydroxydodecanoic acid methyl ester[ | 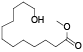 |
Table 6 Structures of meroterpenoids
| 序号No. | 名称Name | 结构图Structure chart | 序号No. | 名称Name | 结构图Structure chart |
|---|---|---|---|---|---|
| 128 | 4-methoxy-2-oxo-2H-pyran-6-propanoic acid[ |  | 133 | 5-hydroxy-5-(methoxymethyl)-4-methyl-2(5H)-furanone[ |  |
| 129 | 11-hydroxy-4-dodecanolide[ |  | 134 | Dihydro-5-octyl-2(3H)-furanone[ |  |
| 130 | Dihydro-5-(6-hydroxyoctyl)-2(3H)-furanone[ |  | 135 | Camphosterol A[ |  |
| 131 | α-tocospiro B[ |  | 136 | 10-hydroxy-γ-dodecalactone[ |  |
| 132 | 12-hydroxydodecanoic acid methyl ester[ |  |
| [1] |
Chang TT, Chou WN. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan[J]. Mycol Res, 1995, 99(6): 756-758.
doi: 10.1016/S0953-7562(09)80541-8 URL |
| [2] | Chang TT, Chou WN. Antrodia cinnamomea Reconsidered and A. salmonea sp. nov. on Cunninghamia konishii in Taiwan[J]. Bot Bull Acad Sin, 2004, 45(4): 347-352. |
| [3] | 吴声华. 药用真菌樟芝拉丁学名的定案[J]. 食药用菌, 2019, 27(4): 253-256. |
| Wu SH. Resolution of scientific binomial of the medicinal fungus “Niuchangchih”[J]. Edible Med Mushrooms, 2019, 27(4): 253-256. | |
| [4] | Wu SH, Yu ZH, Dai YC, et al. Taiwanofungus, a polypore new genus[J]. Fungal Science, 2004, 19(3&4): 109-116. |
| [5] | Yu ZH, Wu SH, Wang DM, et al. Phylogenetic relationships of Antrodia species and related taxa based on analyses of nuclear large subunit ribosomal DNA sequences[J]. Botanical Studies, 2010, 51(1), 53-60. |
| [6] |
May TW. Report of the nomenclature committee for fungi - 20[J]. IMA Fungus, 2017, 8(1): 189-203.
doi: 10.5598/imafungus.2017.08.01.12 URL |
| [7] | 臧穆, 苏庆华. 我国台湾产灵芝属一新种——樟芝[J]. 云南植物研究, 1990, 12(4): 395-396. |
| Zang M, Su QH. Ganoderma comphoratum, a new taxon in genus Ganoderma from Taiwan, China[J]. Acta Bot Yunnanica, 1990, 12(4): 395-396. | |
| [8] | 李春如, 程文明. 我国台湾牛樟芝研究进展[C]// 首届药用真菌产业发展暨学术研讨会论文集. 南通, 2005:42-46. |
| Li CR, Cheng WM. Research development on Antrodia camphorate [C]// Proceedings of first symposium on development of China’s medicinal Fungi Industry. Nantong, 2005:42-46. | |
| [9] | 王升陽, 林進忠. 我国台湾森林红宝石牛樟芝-从森林、实验室到国家认证保健食品[C]// 中国菌物学会第六届会员代表大会(2014年学术年会)暨贵州省食用菌产业发展高峰论坛会议. 贵州, 2014. |
| Wang SY, Lin JZ. Antrodia cinnamomea, the Ruby of Taiwan forest - from forest, laboratory to the functional food[C]// The sixth member congress of the Chinese Mycological Society(2014 academic annual conference)and Guizhou Edible Mushroom Industry Development Summit Forum. Guizhou, 2014. | |
| [10] |
Lu MC, El-Shazly M, Wu TY, et al. Recent research and development of Antrodia cinnamomea[J]. Pharmacol Ther, 2013, 139(2): 124-156.
doi: 10.1016/j.pharmthera.2013.04.001 URL |
| [11] |
Senthil Kumar KJ, Gokila Vani M, et al. A mechanistic and empirical review of antcins, a new class of phytosterols of Formosan fungi origin[J]. J Food Drug Anal, 2020, 28(1): 38-59.
doi: S1021-9498(19)30085-7 pmid: 31883608 |
| [12] |
Tsai WC, Rao YK, Lin SS, et al. Methylantcinate A induces tumor specific growth inhibition in oral cancer cells via Bax-mediated mitochondrial apoptotic pathway[J]. Bioorg Med Chem Lett, 2010, 20(20): 6145-6148.
doi: 10.1016/j.bmcl.2010.08.006 URL |
| [13] |
Cherng IH, Wu DP, Chiang HC. Triterpenoids from Antrodia cinnamomea[J]. Phytochemistry, 1996, 41(1): 263-267.
doi: 10.1016/0031-9422(95)00541-2 URL |
| [14] |
Shen CC, Wang YH, Chang TT, et al. Anti-inflammatory ergostanes from the basidiomata of Antrodia Salmonea[J]. Planta Med, 2007, 73(11): 1208-1213.
doi: 10.1055/s-2007-981591 URL |
| [15] |
Chen CH, Yang SW, Shen YC. New steroid acids from Antrodia cinnamomea, a fungal parasite of Cinnamomum micranthum[J]. J Nat Prod, 1995, 58(11): 1655-1661.
pmid: 8594142 |
| [16] |
Yang SW, Shen YC, Chen CH. Steroids and triterpenoids of Antodia cinnamomea—A fungus parasitic on Cinnamomum micranthum[J]. Phytochemistry, 1996, 41(5): 1389-1392.
doi: 10.1016/0031-9422(95)00767-9 URL |
| [17] |
Cherng IH, Chiang HC, et al. Three new triterpenoids from Antrodia cinnamomea[J]. J Nat Prod, 1995, 58(3): 365-371.
doi: 10.1021/np50117a004 URL |
| [18] |
Du YC, Wu TY, Chang FR, et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata[J]. J Pharm Biomed Anal, 2012, 58: 182-192.
doi: 10.1016/j.jpba.2011.09.007 URL |
| [19] |
Lin TY, Chen CY, Chien SC, et al. Metabolite profiles for Antrodia cinnamomea fruiting bodies harvested at different culture ages and from different wood substrates[J]. J Agric Food Chem, 2011, 59(14): 7626-7635.
doi: 10.1021/jf201632w URL |
| [20] |
Huang Y, Lin X, Qiao X, et al. Antcamphins A-L, ergostanoids from Antrodia camphorata[J]. J Nat Prod, 2014, 77(1): 118-124.
doi: 10.1021/np400741s pmid: 24387703 |
| [21] |
Liaw CC, Chen YC, Huang GJ, et al. Anti-inflammatory lanostanoids and lactone derivatives from Antrodia camphorata[J]. J Nat Prod, 2013, 76(4): 489-494.
doi: 10.1021/np300443p URL |
| [22] |
Huang HC, Liaw CC, Yang HL, et al. Lanostane triterpenoids and sterols from Antrodia camphorata[J]. Phytochemistry, 2012, 84: 177-183.
doi: 10.1016/j.phytochem.2012.08.011 URL |
| [23] |
Wu DP, Chiang HC. Constituents of Antrodia cinnamomea[J]. Jnl Chin Chemical Soc, 1995, 42(5): 797-800.
doi: 10.1002/jccs.v42.5 URL |
| [24] |
Chen JJ, Lin WJ, et al. Anti-inflammatory benzenoids from Antrodia camphorata[J]. J Nat Prod, 2007, 70(6): 989-992.
doi: 10.1021/np070045e URL |
| [25] |
Shu CH, Wu HJ, Ko YH, et al. Effects of red light and addition of monoterpenes and tangerine oil on the production of biomass and triterpenoids of Antrodia cinnamomea in submerged cultures[J]. J Taiwan Inst Chem Eng, 2016, 67: 140-147.
doi: 10.1016/j.jtice.2016.08.023 URL |
| [26] | Li J, Lin XJ, Lin DM, et al. Improved mycelium biomass by submerged culture of Antrodia cinnamomea using plackett-burman design and response surface methodology[C]// Biotechnology and Medical Science. Nanjing, Jiangsu, China. World Scientific, 2016. |
| [27] | Emke A, Midgley JM, Whalley WB. Unsaturated steroids. Part 10. The mechanism of the anthrasteroid rearrangement:the conformation of anthrasteroids[J]. J Chem Soc, Perkin Trans 1, 1980: 1779. |
| [28] |
Kuo YH, Lin CH, Shih CC. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation[J]. J Agric Food Chem, 2015, 63(9): 2479-2489.
doi: 10.1021/acs.jafc.5b00073 URL |
| [29] |
Shao YY, Chen CC, Wang HY, et al. Chemical constituents of Antrodia camphorata submerged whole broth[J]. Nat Prod Res, 2008, 22(13): 1151-1157.
doi: 10.1080/14786410601132410 URL |
| [30] |
Nakamura N, Hirakawa A, Gao JJ, et al. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line[J]. J Nat Prod, 2004, 67(1): 46-48.
pmid: 14738384 |
| [31] |
Chen CC, Shiao YJ, Lin RD, et al. Neuroprotective diterpenes from the fruiting body of Antrodia camphorata[J]. J Nat Prod, 2006, 69(4): 689-691.
doi: 10.1021/np0581263 URL |
| [32] |
Chiang HC, Wu DP, Cherng IW, et al. A sesquiterpene lactone, phenyl and biphenyl compounds from Antrodia cinnamomea[J]. Phytochemistry, 1995, 39(3): 613-616.
doi: 10.1016/0031-9422(95)00025-3 URL |
| [33] |
Chen PY, Wu JD, Tang KY, et al. Isolation and synjournal of a bioactive benzenoid derivative from the fruiting bodies of Antrodia camphorata[J]. Molecules, 2013, 18(7): 7600-7608.
doi: 10.3390/molecules18077600 URL |
| [34] |
Chien SC, Chen ML, Kuo HT, et al. Anti-inflammatory activities of new succinic and maleic derivatives from the fruiting body of Antrodia camphorata[J]. J Agric Food Chem, 2008, 56(16): 7017-7022.
doi: 10.1021/jf801171x URL |
| [35] | Wu MD, Cheng MJ, Wang BC, et al. Chemical constituents from the mycelia of Antrodia cinnamomea[J]. J Chil Chem Soc, 2007, 52(4): 1338-1340. |
| [36] |
Lee TH, Lee CK, Tsou WL, et al. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata[J]. Planta Med, 2007, 73(13): 1412-1415.
doi: 10.1055/s-2007-990232 URL |
| [37] |
Chen YC, Chiu HL, Chao CY, et al. New anti-inflammatory aromatic components from Antrodia camphorata[J]. Int J Mol Sci, 2013, 14(3): 4629-4639.
doi: 10.3390/ijms14034629 URL |
| [38] |
Lin CC, Chen CC, Kuo YH, et al. 2, 3, 5-Trimethoxy-4-cresol, an anti-metastatic constituent from the solid-state cultured mycelium of Antrodia cinnamomea and its mechanism[J]. J Nat Med, 2015, 69(4): 513-521.
doi: 10.1007/s11418-015-0916-6 URL |
| [39] | Huang KF, Huang WM, Jiang HZ. Phenyl Compounds from Antrodia Cinnamomea[J]. The Chinese Pharmaceutical Journal, 2001, 53(6): 327-331. |
| [40] |
Yang CM, Zhou YJ, Wang RJ, et al. Anti-angiogenic effects and mechanisms of polysaccharides from Antrodia cinnamomea with different molecular weights[J]. Journal of Ethnopharmacology, 2009, 123(3): 407-412.
doi: 10.1016/j.jep.2009.03.034 URL |
| [41] |
Yang SS, Wang GJ, Wang SY, et al. New constituents with iNOS inhibitory activity from mycelium of Antrodia camphorata[J]. Planta Med, 2009, 75(5): 512-516.
doi: 10.1055/s-0029-1185305 URL |
| [42] |
Villaume MT, Sella E, Saul G, et al. Antroquinonol A:scalable synjournal and preclinical biology of a phase 2 drug candidate[J]. ACS Cent Sci, 2016, 2(1): 27-31.
doi: 10.1021/acscentsci.5b00345 URL |
| [43] |
Lin TJ, Lai KC, Lee AS, et al. Novel Antrodia cinnamomea extract reduced cancer stem-like phenotype changes and resensitized KRAS-mutant colorectal cancer via a MicroRNA-27a pathway[J]. Cancers, 2019, 11(11): 1657.
doi: 10.3390/cancers11111657 URL |
| [44] |
Yen IC, Yao CW, Kuo MT, et al. Anti-cancer agents derived from solid-state fermented Antrodia camphorata mycelium[J]. Fitoterapia, 2015, 102: 115-119.
doi: 10.1016/j.fitote.2015.02.010 pmid: 25721423 |
| [45] |
Wang SC, Lee TH, Hsu CH, et al. Antroquinonol D, isolated from Antrodia camphorata, with DNA demethylation and anticancer potential[J]. J Agric Food Chem, 2014, 62(24): 5625-5635.
doi: 10.1021/jf4056924 URL |
| [46] |
Chen YL, Yen IC, Lin KT, et al. 4-acetylantrocamol LT3, a new ubiquinone from Antrodia cinnamomea, inhibits hepatocellular carcinoma HepG2 cell growth by targeting YAP/TAZ, mTOR, and WNT/β-catenin signaling[J]. Am J Chin Med, 2020, 48(5): 1243-1261.
doi: 10.1142/S0192415X20500615 URL |
| [47] |
Lin YW, Chiang BH. 4-acetylantroquinonol B isolated from Antrodia cinnamomea arrests proliferation of human hepatocellular carcinoma HepG2 cell by affecting p53, p21 and p27 levels[J]. J Agric Food Chem, 2011, 59(16): 8625-8631.
doi: 10.1021/jf2011326 URL |
| [48] |
Boukouvalas J, Albert V, Loach RP, et al. Unified route to asymmetrically substituted butenolide, maleic anhydride, and maleimide constituents of Antrodia camphorata[J]. Tetrahedron, 2012, 68(47): 9592-9597.
doi: 10.1016/j.tet.2012.09.064 URL |
| [49] |
Wu MD, Cheng MJ, Wang BC, et al. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages[J]. J Nat Prod, 2008, 71(7): 1258-1261.
doi: 10.1021/np070634k URL |
| [50] |
Wu MD, Cheng MJ, Yech YJ, et al. Inhibitory effects of maleimide derivatives from the mycelia of the Fungus Antrodia cinnamomea BCRC 36799 on nitric oxide production in lipopolysaccharide(LPS)-activated RAW264. 7 macrophages[J]. Chem Biodivers, 2013, 10(3): 434-441.
doi: 10.1002/cbdv.v10.3 URL |
| [51] |
Lu MK, Lin TY, Chang CC. Chemical identification of a sulfated glucan from Antrodia cinnamomea and its anti-cancer functions via inhibition of EGFR and mTOR activity[J]. Carbohydr Polym, 2018, 202: 536-544.
doi: 10.1016/j.carbpol.2018.09.009 URL |
| [52] | 朱会霞, 孙金旭, 王灿. 樟芝多糖流变特性研究[J]. 食品研究与开发, 2011, 32(1): 10-13. |
| Zhu HX, Sun JX, Wang C. The rheology study for Antrodia camphorate[J]. Food Res Dev, 2011, 32(1): 10-13. | |
| [53] |
Lee MH, Chao CH, Hsu YC, et al. Production, characterization, and functions of sulfated polysaccharides from zinc sulfate enriched cultivation of Antrodia cinnamomea[J]. Int J Biol Macromol, 2020, 159: 1013-1021.
doi: 10.1016/j.ijbiomac.2020.05.068 URL |
| [54] |
Han HF, Nakamura N, Zuo F, et al. Protective effects of a neutral polysaccharide isolated from the mycelium of Antrodia cinnamomea on Propionibacterium acnes and lipopolysaccharide induced hepatic injury in mice[J]. Chem Pharm Bull, 2006, 54(4): 496-500.
doi: 10.1248/cpb.54.496 URL |
| [55] |
Chiu CH, Peng CC, Ker YB, et al. Physicochemical characteristics and anti-inflammatory activities of antrodan, a novel glycoprotein isolated from Antrodia cinnamomea mycelia[J]. Molecules, 2013, 19(1): 22-40.
doi: 10.3390/molecules19010022 URL |
| [56] |
Ker YB, Peng CC, Chang WL, et al. Hepatoprotective bioactivity of the glycoprotein, antrodan, isolated from Antrodia cinnamomea mycelia[J]. PLoS One, 2014, 9(4): e93191.
doi: 10.1371/journal.pone.0093191 URL |
| [57] |
Zhang Y, Wang Z, Li D, et al. A polysaccharide from Antrodia cinnamomea mycelia exerts antitumor activity through blocking of TOP1/TDP1-mediated DNA repair pathway[J]. Int J Biol Macromol, 2018, 120(pt b): 1551-1560.
doi: 10.1016/j.ijbiomac.2018.09.162 URL |
| [58] |
Cheng JJ, Huang NK, et al. Characterization and biological functions of sulfated polysaccharides from sulfated-salt treatment of Antrodia cinnamomea[J]. Process Biochem, 2009, 44(4): 453-459.
doi: 10.1016/j.procbio.2008.12.012 URL |
| [59] |
Tsai MC, Song TY, Shih PH, et al. Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture[J]. Food Chem, 2007, 104(3): 1115-1122.
doi: 10.1016/j.foodchem.2007.01.018 URL |
| [60] |
Lin TY, Lu MK, Tseng AJ, et al. Effects of sterol-type elicitors on biochemical characterization of polysaccharides from Antrodia cinnamomea[J]. Int J Biol Macromol, 2020, 162: 1476-1483.
doi: 10.1016/j.ijbiomac.2020.07.201 URL |
| [61] |
Lu MY, Fan WL, Wang WF, et al. Genomic and transcriptomic analyses of the medicinal fungus Antrodia cinnamomea for its metabolite biosynjournal and sexual development[J]. PNAS, 2014, 111(44): E4743-E4752.
doi: 10.1073/pnas.1417570111 URL |
| [62] | 李晶. 牛樟芝MVA途径中基因克隆及转录组、代谢组研究[D]. 福州:福建农林大学, 2016. |
| Li J. Molecular cloning of genes involved in MVA of Antrodia cinnamomea and its research on transcriptomics and metabolismics[D]. Fuzhou:Fujian Agriculture and Forestry University, 2016. | |
| [63] |
Hu PF, Huang J, Chen L, et al. Oxidative stress induction is a rational strategy to enhance the productivity of Antrodia cinnamomea fermentations for the antioxidant secondary metabolite antrodin C[J]. J Agric Food Chem, 2020, 68(13): 3995-4004.
doi: 10.1021/acs.jafc.9b07965 URL |
| [64] |
Yang SH, Lin YW, Chiang BH. Biosynjournal of 4-acetylantroquinonol B in Antrodia cinnamomea via a pathway related to coenzyme Q synjournal[J]. Biochem Eng J, 2017, 117: 23-29.
doi: 10.1016/j.bej.2016.09.019 URL |
| [65] |
Wei YY, Chou KCC, Yang SH, et al. Oxygen vector accelerates farnesylation and redox reaction to promote the biosynjournal of 4-acetylantroquinonol B and antroquinonol during submerged fermentation of Antrodia cinnamomea[J]. Food Bioprod Process, 2020, 120: 80-90.
doi: 10.1016/j.fbp.2019.12.012 URL |
| [66] |
Hsu CS, Chou HH, Fang JM. A short synjournal of(±)-antroqui-nonol in an unusual scaffold of 4-hydroxy-2-cyclohexenone[J]. Org Biomol Chem, 2015, 13(19): 5510-5519.
doi: 10.1039/c5ob00411j pmid: 25875221 |
| [67] |
Sulake RS, Chen C. Total synjournal of(+)-antroquinonol and(+)-antroquinonol D[J]. Org Lett, 2015, 17(5): 1138-1141.
doi: 10.1021/acs.orglett.5b00046 URL |
| [68] | 卢叶枫, 陈冠敏, 钟礼云, 等. 牛樟芝醇溶性提取物安全性评价[J]. 毒理学杂志, 2015, 29(5): 388-390. |
| Lu YF, Chen GM, Zhong LY, et al. Safety evaluation of alcohol soluble extract from Antrodia cinnamomea[J]. J Toxicol, 2015, 29(5): 388-390. | |
| [69] | 徐蔚, 王瑾, 王宫. 牛樟芝胶囊的毒性实验研究[J]. 海峡药学, 2011, 23(5): 41-43. |
| Xu W, Wang J, Wang G. Experimental study on toxicity of Antrodia cinnamomea capsule[J]. Strait Pharm J, 2011, 23(5): 41-43. | |
| [70] |
Senthil Kumar KJ, Gokila Vani M, Hsieh HW, et al. MicroRNA-708 activation by glucocorticoid receptor agonists regulate breast cancer tumorigenesis and metastasis via downregulation of NF-κB signaling[J]. Carcinogenesis, 2019, 40(2): 335-348.
doi: 10.1093/carcin/bgz011 pmid: 30726934 |
| [71] | 任毅. MicroRNA-200c对三阴性乳腺癌细胞增殖和凋亡的影响及其分子机制的研究[D]. 南京:南京医科大学, 2015. |
| Ren Y. The regulating of microRNA-200c on proliferation and apoptosis in triple negative breast cancer and it’s molecular mechanism[D]. Nanjing:Nanjing Medical University, 2015. | |
| [72] |
Kumar VB, Yuan TC, Liou JW, et al. Antroquinonol inhibits NSCLC proliferation by altering PI3K/mTOR proteins and miRNA expression profiles[J]. Mutat Res, 2011, 707(1/2): 42-52.
doi: 10.1016/j.mrfmmm.2010.12.009 URL |
| [73] |
Shang KM, Su TH, Lee WL, et al. Novel effect and the mechanistic insights of fruiting body extract of medicinal fungus Antrodia cinnamomea against T47D breast cancer[J]. Phytomedicine, 2017, 24: 39-48.
doi: 10.1016/j.phymed.2016.11.006 URL |
| [74] |
Lu MK, Lin TY, Chao CH, et al. Molecular mechanism of Antrodia cinnamomea sulfated polysaccharide on the suppression of lung cancer cell growth and migration via induction of transforming growth factor β receptor degradation[J]. Int J Biol Macromol, 2017, 95: 1144-1152.
doi: 10.1016/j.ijbiomac.2016.11.004 URL |
| [75] |
Lin TY, Tseng AJ, Qiu WL, et al. A sulfated glucan from Antrodia cinnamomea reduces Slug expression through regulation of TGFβ/AKT/GSK3β axis in lung cancer[J]. Carbohydr Polym, 2019, 210: 175-184.
doi: 10.1016/j.carbpol.2019.01.078 URL |
| [76] |
Yang HL, Lin KY, Juan YC, et al. The anti-cancer activity of Antrodia camphorata against human ovarian carcinoma(SKOV-3)cells via modulation of HER-2/neu signaling pathway[J]. J Ethnopharmacol, 2013, 148(1): 254-265.
doi: 10.1016/j.jep.2013.04.023 URL |
| [77] |
Liu YM, Liu YK, Huang PI, et al. Antrodia cinnamomea mycelial fermentation broth inhibits the epithelial-mesenchymal transition of human esophageal adenocarcinoma cancer cells[J]. Food Chem Toxicol, 2018, 119: 380-386.
doi: 10.1016/j.fct.2018.01.028 URL |
| [78] |
Long huei, Hu CT, Weng CF. Antrodia cinnamomea prolongs survival in a patient with small cell lung cancer[J]. Medicina, 2019, 55(10): 640.
doi: 10.3390/medicina55100640 URL |
| [79] | 单丽珠, 宋腾, 王华庆. 牛樟芝联合化疗对恶性肿瘤患者免疫功能的影响[J]. 中国中西医结合外科杂志, 2015, 21(6): 552-555. |
| Shan LZ, Song T, Wang HQ. Effect of Antrodia camphorata combined with chemotherapy on immunologic function in patients with malignant tumors[J]. Chin J Surg Integr Tradit West Med, 2015, 21(6): 552-555. | |
| [80] | Yue PK, Wong YY, Wong KK, et al. Current evidence for the hepatoprotective activities of the medicinal mushroom Antrodia cinnamomea[J]. Chin Med, 2013, 8(1): 1-7. |
| [81] |
Ho YC, Lin MT, et al. The hepatoprotective activity against ethanol-induced cytotoxicity by aqueous extract of Antrodia cinnamo-mea[J]. J Chin Inst Chem Eng, 2008, 39(5): 441-447.
doi: 10.1016/j.jcice.2008.03.008 URL |
| [82] |
Chang JS, Kuo HP, Chang KL, et al. Apoptosis of hepatocellular carcinoma cells induced by nanoencapsulated polysaccharides extracted from Antrodia camphorata[J]. PLoS One, 2015, 10(9): e0136782.
doi: 10.1371/journal.pone.0136782 URL |
| [83] |
Liu Y, Wang Z, Kong F, et al. Triterpenoids extracted from Antrodia cinnamomea mycelia attenuate acute alcohol-induced liver injury in C57BL/6 mice via suppression inflammatory response[J]. Front Microbiol, 2020, 11: 1113.
doi: 10.3389/fmicb.2020.01113 URL |
| [84] | Gokila Vani M, Kumar KJ, Liao JW, et al. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism[J]. Evid Based Complement Alternat Med, 2013, 2013: 296082. |
| [85] | Chen YF, Wang SH, Chang SJ, et al. Zhankuic acid A as a novel JAK2 inhibitor for the treatment of concanavalin A-induced hepatitis[J]. Biochem Pharmacol, 2014, 91(2): 217-230. |
| [86] |
Lu KH, Pan YC, Sheen LY. Combination of cut-log cultivated fruiting body and solid-state cultured mycelia of Taiwanofungus camphoratus ameliorates CCl4-induced liver injury in rats[J]. J Tradit Complement Med, 2020, 10(2): 166-174.
doi: 10.1016/j.jtcme.2019.04.008 URL |
| [87] |
Phuong do T, Ma CM, et al. Inhibitory effects of antrodins A-E from Antrodia cinnamomea and their metabolites on hepatitis C virus protease[J]. Phytother Res, 2009, 23(4): 582-584.
doi: 10.1002/ptr.2657 pmid: 19003946 |
| [88] |
Li TY, Chiang BH. 4-Acetylantroquinonol B from Antrodia cinnamomea enhances immune function of dendritic cells against liver cancer stem cells[J]. Biomed Pharmacother, 2019, 109: 2262-2269.
doi: 10.1016/j.biopha.2018.11.101 URL |
| [89] |
Wang HC, Chu FH, Chien SC, et al. Establishment of the metabolite profile for an Antrodia cinnamomea health food product and investigation of its chemoprevention activity[J]. J Agric Food Chem, 2013, 61(36): 8556-8564.
doi: 10.1021/jf402849b URL |
| [90] |
Chen JF, Tsai YT, Lai YH, et al. Proteomic analysis of Antrodia Cinnamomea-induced ER stress in liver cancer cells[J]. J Pharm Biomed Anal, 2020, 187: 113142.
doi: 10.1016/j.jpba.2020.113142 URL |
| [91] |
Chiou YL, Chyau CC, Li TJ, et al. Hepatoprotective effect of Antrodia cinnamomea mycelium in patients with nonalcoholic steatohepatitis:a randomized, double-blind, placebo-controlled trial[J]. J Am Coll Nutr, 2021, 40(4): 349-357.
doi: 10.1080/07315724.2020.1779850 URL |
| [92] | 张毅红, 赵宗杰, 谢海涛, 等. 樟芝三萜类化合物的抗炎作用[J]. 中国病理生理杂志, 2015, 31(2): 369-373. |
| Zhang YH, Zhao ZJ, Xie HT, et al. Anti-inflammatory effect of triterpenoids from Antrodia camphorat A[J]. Chin J Pathophysiol, 2015, 31(2): 369-373. | |
| [93] |
Chen YT, Shen YC, et al. Precursor-feeding strategy on the triterpenoid production and anti-inflammatory activity of Antrodia cinnamomea[J]. Process Biochem, 2016, 51(8): 941-949.
doi: 10.1016/j.procbio.2016.05.001 URL |
| [94] |
Kuo YH, Lin TY, You YJ, et al. Antiinflammatory and antiphotodamaging effects of ergostatrien-3β-ol, isolated from Antrodia camphorata, on hairless mouse skin[J]. Molecules, 2016, 21(9): 1213.
doi: 10.3390/molecules21091213 URL |
| [95] |
Lin CC, Pan IH, Li YR, et al. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines[J]. PLoS One, 2015, 10(2): e0116191.
doi: 10.1371/journal.pone.0116191 URL |
| [96] |
Lu MK, Lee MH, Chao CH, et al. Physiochemical changes and mechanisms of anti-inflammation effect of sulfated polysaccharides from ammonium sulfate feeding of Antrodia cinnamomea[J]. Int J Biol Macromol, 2020, 148: 715-721.
doi: 10.1016/j.ijbiomac.2020.01.110 URL |
| [97] |
Hsieh YH, Chu FH, Wang YS, et al. Antrocamphin A, an anti-inflammatory principal from the fruiting body of Taiwanofungus camphoratus, and its mechanisms[J]. J Agric Food Chem, 2010, 58(5): 3153-3158.
doi: 10.1021/jf903638p URL |
| [98] |
Shi Y, Yang SY, Lee DY, et al. Increasing anti-Aβ -induced neurotoxicity ability of Antrodia camphorata -fermented product with deep ocean water supplementary[J]. J Sci Food Agric, 2016, 96(14): 4690-4701.
doi: 10.1002/jsfa.7687 URL |
| [99] |
Chang CH, Wang HE, et al. Antrodia cinnamomea exhibits a potent neuroprotective effect in the PC12 Cell-Aβ25-35 model - pharmacologically through adenosine receptors and mitochondrial pathway[J]. Planta Med, 2012, 78(17): 1813-1823.
doi: 10.1055/s-00000058 URL |
| [100] |
Chang YY, Liu YC, et al. Effects of antrosterol from Antrodia camphorata submerged whole broth on lipid homeostasis, antioxidation, alcohol clearance, and anti-inflammation in livers of chronic-alcohol fed mice[J]. J Ethnopharmacol, 2017, 202: 200-207.
doi: 10.1016/j.jep.2017.03.003 URL |
| [101] | Huang BY, Liu CC, Jian ZY, et al. Antrodia camphorata declines oxidative stress and enhances antioxidant enzyme activity in the brain cortex of rats[J]. American Journal of Laboratory Medicine, 2016, 1(3): 29-33. |
| [102] |
Yu YL, Chen IH, Shen KY, et al. A triterpenoid methyl antcinate K isolated from Antrodia cinnamomea promotes dendritic cell activation and Th2 differentiation[J]. Eur J Immunol, 2009, 39(9): 2482-2491.
doi: 10.1002/eji.200839039 URL |
| [103] |
You RI, Lee YP, Su TY, et al. A benzenoid 4, 7-dimethoxy-5-methyl-L, 3-benzodioxole from Antrodia cinnamomea attenuates dendritic cell-mediated Th2 allergic responses[J]. Am J Chin Med, 2019, 47(6): 1271-1287.
doi: 10.1142/S0192415X19500654 URL |
| [104] |
Lai MN, Ko HJ, Ng LT. Hypolipidemic effects of Antrodia cinnamomea extracts in high-fat diet-fed hamsters[J]. J Food Biochem, 2012, 36(2): 233-239.
doi: 10.1111/jfbc.2012.36.issue-2 URL |
| [105] |
Wang CL, Huang WC, Chou CJ, et al. Aqueous extract of Antrodia cinnamomea reduced high-fat diet-induced obesity in mice and suppressed adipogenesis in 3T3-L1 cells[J]. J Funct Foods, 2017, 35: 185-196.
doi: 10.1016/j.jff.2017.05.041 URL |
| [106] |
Tsai YT, Ruan JW, et al. Antrodia cinnamomea confers obesity resistance and restores intestinal barrier integrity in leptin-deficient obese mice[J]. Nutrients, 2020, 12(3): 726.
doi: 10.3390/nu12030726 URL |
| [107] |
Sheu MJ, Teng YN, Chen YY, et al. The functional influences of common ABCB1 genetic variants on the inhibition of P-glycoprotein by Antrodia cinnamomea extracts[J]. PLoS One, 2014, 9(2): e89622.
doi: 10.1371/journal.pone.0089622 URL |
| [108] | Liu Y, Li L, An S, et al. Antifatigue effects of Antrodia cinnamomea cultured mycelium via modulation of oxidative stress signaling in a mouse model[J]. Biomed Res Int, 2017, 2017: 9374026. |
| [109] |
Wang SC, Yang CH, Grumezescu AM, et al. Renoprotective effects of shout camphor medicinal mushroom(Taiwanofungus camphorates, basidiomycetes)mycelia on several media in mice with chronic kidney disease[J]. Int J Med Mushrooms, 2016, 18(12): 1105-1114.
doi: 10.1615/IntJMedMushrooms.v18.i12 URL |
| [110] |
Johnson A, Cheng SC, Tsou D, et al. Attenuation of reproductive dysfunction in diabetic male rats with timber cultured Antrodia cinnamomea ethanol extract[J]. Biomed Pharmacother, 2019, 112: 108684.
doi: S0753-3322(18)38656-6 pmid: 30798138 |
| [111] |
Kong ZL, He JL, Sudirman S, et al. Nanoparticles of antroquinonol-rich extract from solid-state-cultured Antrodia cinnamomea improve reproductive function in diabetic male rats[J]. Int J Nanomedicine, 2020, 15: 4191-4203.
doi: 10.2147/IJN.S252885 URL |
| [112] | 李娟, 王毅, 樊丽, 等. 牛樟芝发酵液提取物抗耐药性细菌活性研究[J]. 福建师范大学学报:自然科学版, 2019, 35(1): 96-101. |
| Li J, Wang Y, Fan L, et al. Antimicrobial studies on Antrodia cinnamomea against multi-drug resistant human pathogenic bacteria[J]. J Fujian Norm Univ:Nat Sci Ed, 2019, 35(1): 96-101. | |
| [113] |
Chen CC, Li IC, Lin TW, et al. Efficacy and safety of oral Antrodia cinnamomea mycelium in mildly hypertensive adults:a randomized controlled pilot clinical study[J]. Eur J Integr Med, 2016, 8(5): 654-660.
doi: 10.1016/j.eujim.2016.06.001 URL |
| [114] |
Chou WL, Lee TH, Huang TH, et al. Coenzyme Q0 from Antrodia cinnamomea exhibits drug-resistant bacteria eradication and keratinocyte inflammation mitigation to ameliorate infected atopic dermatitis in mouse[J]. Front Pharmacol, 2019, 10: 1445.
doi: 10.3389/fphar.2019.01445 URL |
| [1] | YANG Zhi-xiao, HOU Qian, LIU Guo-quan, LU Zhi-gang, CAO Yi, GOU Jian-yu, WANG Yi, LIN Ying-chao. Responses of Rubisco and Rubisco Activase in Different Resistant Tobacco Strains to Brown Spot Stress [J]. Biotechnology Bulletin, 2023, 39(9): 202-212. |
| [2] | CHENG Ya-nan, ZHANG Wen-cong, ZHOU Yuan, SUN Xue, LI Yu, LI Qing-gang. Synthetic Pathway Construction of Producing 2'-fucosyllactose by Lactococcus lactis and Optimization of Fermentation Medium [J]. Biotechnology Bulletin, 2023, 39(9): 84-96. |
| [3] | LIU Bao-cai, CHEN Jing-ying, ZHANG Wu-jun, HUANG Ying-zhen, ZHAO Yun-qing, LIU Jian-chao, WEI Zhi-cheng. Characteristics Analysis of Seed Microrhizome Gene Expression of Polygonatum cyrtonema [J]. Biotechnology Bulletin, 2023, 39(8): 220-233. |
| [4] | LI Bo, LIU He-xia, CHEN Yu-ling, ZHOU Xing-wen, ZHU Yu-lin. Cloning, Subcellular Localization and Expression Analysis of CnbHLH79 Transcription Factor from Camellia nitidissima [J]. Biotechnology Bulletin, 2023, 39(8): 241-250. |
| [5] | YE Yun-fang, TIAN Qing-yin, SHI Ting-ting, WANG Liang, YUE Yuan-zheng, YANG Xiu-lian, WANG Liang-gui. Research Progress in the Biosynthesis and Regulation of β-ionone in Plants [J]. Biotechnology Bulletin, 2023, 39(8): 91-105. |
| [6] | WANG Ling, ZHUO Shen, FU Xue-sen, LIU Zi-xuan, LIU Xiao-rong, WANG Zhi-hui, ZHOU Ri-bao, LIU Xiang-dan. Advances in the Biosynthetic Pathways and Related Genes of Lotus Alkaloids [J]. Biotechnology Bulletin, 2023, 39(7): 56-66. |
| [7] | LI Zhi-qi, YUAN Yue, MIAO Rong-qing, PANG Qiu-ying, ZHANG Ai-qin. Melatonin Contents in Eutrema salsugineum and Arabidopsis thaliana Under Salt Stress, and Expression Pattern Analysis of Synthesis Related Genes [J]. Biotechnology Bulletin, 2023, 39(5): 142-151. |
| [8] | JIANG Qing-chun, DU Jie, WANG Jia-cheng, YU Zhi-he, WANG Yun, LIU Zhong-yu. Expression and Function Analysis of Transcription Factor PcMYB2 from Polygonum cuspidatum [J]. Biotechnology Bulletin, 2023, 39(5): 217-223. |
| [9] | ZHOU Ding-ding, LI Hui-hu, TANG Xing-yong, YU Fa-xin, KONG Dan-yu, LIU Yi. Research Progress in the Biosynthesis and Regulation of Glycyrrhizic Acid and Liquiritin [J]. Biotechnology Bulletin, 2023, 39(5): 44-53. |
| [10] | MA Yu-qian, SUN Dong-hui, YUE Hao-feng, XIN Jia-yu, LIU Ning, CAO Zhi-yan. Identification, Heterologous Expression and Functional Analysis of a GH61 Family Glycoside Hydrolase from Setosphaeria turcica with the Assisting Function in Degrading Cellulose [J]. Biotechnology Bulletin, 2023, 39(4): 124-135. |
| [11] | YU Hui-li, LI Ai-tao. Application of Cytochrome P450 in the Biosynthesis of Flavors and Fragrances [J]. Biotechnology Bulletin, 2023, 39(4): 24-37. |
| [12] | WANG Qi, HU Zhe, FU Wei, LI Guang-zhe, HAO Lin. Regulation of Burkholderia sp. GD17 on the Drought Tolerance of Cucumber Seedlings [J]. Biotechnology Bulletin, 2023, 39(3): 163-175. |
| [13] | YAO Xiao-wen, LIANG Xiao, CHEN Qing, WU Chun-ling, LIU Ying, LIU Xiao-qiang, SHUI Jun, QIAO Yang, MAO Yi-ming, CHEN Yin-hua, ZHANG Yin-dong. Study on the Expression Pattern of Genes in Lignin Biosynthesis Pathway of Cassava Resisting to Tetranychus urticae [J]. Biotechnology Bulletin, 2023, 39(2): 161-171. |
| [14] | MIAO Shu-nan, GAO Yu, LI Xin-ru, CAI Gui-ping, ZHANG Fei, XUE Jin-ai, JI Chun-li, LI Run-zhi. Functional Analysis of Soybean GmPDAT1 Genes in the Oil Biosynthesis and Response to Abiotic Stresses [J]. Biotechnology Bulletin, 2023, 39(2): 96-106. |
| [15] | YAN Meng-yu, WEI Xiao-wei, CAO Jing, LAN Hai-yan. Cloning of Basic Helix-loop-helix(bHLH)Transcription Factor Gene SabHLH169 in Suaeda aralocaspica and Analysis of Its Resistances to Drought Stress [J]. Biotechnology Bulletin, 2023, 39(11): 328-339. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||