








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (3): 103-112.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0619
Previous Articles Next Articles
GAO Ya-hui( ), JIANG Ming-guo, FENG Jing(
), JIANG Ming-guo, FENG Jing( ), ZHOU Gui
), ZHOU Gui
Received:2021-05-12
Online:2022-03-26
Published:2022-04-06
Contact:
FENG Jing
E-mail:1393901557@qq.com;fengjing0414141@163.com
GAO Ya-hui, JIANG Ming-guo, FENG Jing, ZHOU Gui. Screening of Potential PGPR Strains Producting Growth-promoting Volatile Compounds and Study on Their Growth-promoting Characteristics[J]. Biotechnology Bulletin, 2022, 38(3): 103-112.
| 菌株编号Strain No. | 菌株名称Name of strain |
|---|---|
| GX13159 | Bacillus cereus |
| GX13529 | Staphylococcus edaphicus |
| GX13571 | Asticcacaulis excentricus |
| GX13580 | Micrococcus aloeverae |
| GX13585 | Microbacterium hydrothermale |
| GX13594 | Microbacterium laevaniformans |
| GX13656 | Bacillus acidiceler |
| GX13667 | Bacillus aryabhattai |
| GX13668 | Micrococcus aloeverae |
| GX13676 | Streptomyces diastaticus subsp. ardesiacus |
| GX13678 | Streptomyces sanglieri |
| GX13679 | Falsibacillus pallidus |
| GX13685 | Sinomonas humi |
| GX13699 | Cellulosimicrobium funkei |
| GX13717 | Kocuria indica |
| GX13747 | Paenibacillus silvae |
| GX13767 | Bacillus mangrovi |
| GX13774 | Bacillus marisflavi |
| GX13777 | Halobacillus marinus |
| GX13785 | Kosakonia oryziphila |
| GX13790 | Serratia oryzae |
| GX14001 | Microbacterium aurantiacum |
| GX14008 | Microbacterium maritypicum |
| GX14011 | Exiguobacterium aestuarii |
| GX14016 | Staphylococcus haemolyticus |
| GX14017 | Staphylococcus hominis subsp. novobiosepticus |
| GX14022 | Staphylococcus nepalensis |
| GX14028 | Kytococcus sedentarius |
| GX14031 | Sporosarcina koreensis |
| GX14032 | Macrococcus canis |
| GX14046 | Glutamicibacter creatinolyticus |
| GX14059 | Pontibacter chinhatensis |
| GX14066 | Kocuria tytonicola |
| GX14070 | Micrococcus luteus |
| GX14080 | Macrococcus caseolyticus subsp. Caseolyticus |
| GX14105 | Bacillus altitudinis |
| GX14108 | Brachybacterium squillarum |
| GX14201 | Staphylococcus sciuri |
| GX14203 | Bacillus haikouensis |
| GX14062 | Agromyces indicus |
| GX14082 | Psychrobacter pulmonis |
| GX14096 | Pseudomonas deceptionensis |
| GX13112 | Klebsiella Trevisan |
| GX13213 | Klebsiella variicola subsp. Variicola |
| GX14104 | Streptomyces griseus subsp. griseus |
| GX14111 | Streptomyces koyangensis |
| GX14119 | Streptomyces gramineus |
| GX14125 | Streptomyces flavovirens |
Table 1 Strain information
| 菌株编号Strain No. | 菌株名称Name of strain |
|---|---|
| GX13159 | Bacillus cereus |
| GX13529 | Staphylococcus edaphicus |
| GX13571 | Asticcacaulis excentricus |
| GX13580 | Micrococcus aloeverae |
| GX13585 | Microbacterium hydrothermale |
| GX13594 | Microbacterium laevaniformans |
| GX13656 | Bacillus acidiceler |
| GX13667 | Bacillus aryabhattai |
| GX13668 | Micrococcus aloeverae |
| GX13676 | Streptomyces diastaticus subsp. ardesiacus |
| GX13678 | Streptomyces sanglieri |
| GX13679 | Falsibacillus pallidus |
| GX13685 | Sinomonas humi |
| GX13699 | Cellulosimicrobium funkei |
| GX13717 | Kocuria indica |
| GX13747 | Paenibacillus silvae |
| GX13767 | Bacillus mangrovi |
| GX13774 | Bacillus marisflavi |
| GX13777 | Halobacillus marinus |
| GX13785 | Kosakonia oryziphila |
| GX13790 | Serratia oryzae |
| GX14001 | Microbacterium aurantiacum |
| GX14008 | Microbacterium maritypicum |
| GX14011 | Exiguobacterium aestuarii |
| GX14016 | Staphylococcus haemolyticus |
| GX14017 | Staphylococcus hominis subsp. novobiosepticus |
| GX14022 | Staphylococcus nepalensis |
| GX14028 | Kytococcus sedentarius |
| GX14031 | Sporosarcina koreensis |
| GX14032 | Macrococcus canis |
| GX14046 | Glutamicibacter creatinolyticus |
| GX14059 | Pontibacter chinhatensis |
| GX14066 | Kocuria tytonicola |
| GX14070 | Micrococcus luteus |
| GX14080 | Macrococcus caseolyticus subsp. Caseolyticus |
| GX14105 | Bacillus altitudinis |
| GX14108 | Brachybacterium squillarum |
| GX14201 | Staphylococcus sciuri |
| GX14203 | Bacillus haikouensis |
| GX14062 | Agromyces indicus |
| GX14082 | Psychrobacter pulmonis |
| GX14096 | Pseudomonas deceptionensis |
| GX13112 | Klebsiella Trevisan |
| GX13213 | Klebsiella variicola subsp. Variicola |
| GX14104 | Streptomyces griseus subsp. griseus |
| GX14111 | Streptomyces koyangensis |
| GX14119 | Streptomyces gramineus |
| GX14125 | Streptomyces flavovirens |
| 组别 Group | 鲜重 Fresh weight/g | 根长 Root lengt/cm | 侧根数 Number of lateral root/roots | 叶片长 Leaf length/cm | 叶片宽 Leaf width/cm |
|---|---|---|---|---|---|
| 对照组Control | 0.081±0.015 | 2.855±0.656 | 1.887±0.726 | 0.551±0.093 | 0.443±0.089 |
| GX13594 | 0.503±0.214** | 4.627±1.481** | 4.400±1.183** | 0.990±0.217** | 0.730±0.158** |
| GX13747 | 0.387±0.078** | 5.187±1.351** | 4.267±0.799** | 1.023±0.145** | 0.772±0.157** |
| GX14001 | 0.703±0.300** | 4.530±1.129** | 4.267±1.870** | 1.250±0.260** | 0.880±0.190** |
| GX14016 | 0.487±0.106** | 4.853±1.255** | 5.333±1.589** | 1.133±0.247** | 0.775±0.191** |
| GX14066 | 0.620±0.100** | 4.243±0.918** | 4.533±1.552** | 1.285±0.244** | 0.958±0.260** |
| GX14070 | 0.300±0.183** | 4.600±0.858** | 3.867±1.125** | 0.950±0.163** | 0.742±0.129** |
| GX14201 | 0.457±0.093** | 4.486±1.143** | 4.643±1.082** | 1.107±0.289** | 0.764±0.209** |
Table 2 Effect of VOCs produced by some strains on tobacco
| 组别 Group | 鲜重 Fresh weight/g | 根长 Root lengt/cm | 侧根数 Number of lateral root/roots | 叶片长 Leaf length/cm | 叶片宽 Leaf width/cm |
|---|---|---|---|---|---|
| 对照组Control | 0.081±0.015 | 2.855±0.656 | 1.887±0.726 | 0.551±0.093 | 0.443±0.089 |
| GX13594 | 0.503±0.214** | 4.627±1.481** | 4.400±1.183** | 0.990±0.217** | 0.730±0.158** |
| GX13747 | 0.387±0.078** | 5.187±1.351** | 4.267±0.799** | 1.023±0.145** | 0.772±0.157** |
| GX14001 | 0.703±0.300** | 4.530±1.129** | 4.267±1.870** | 1.250±0.260** | 0.880±0.190** |
| GX14016 | 0.487±0.106** | 4.853±1.255** | 5.333±1.589** | 1.133±0.247** | 0.775±0.191** |
| GX14066 | 0.620±0.100** | 4.243±0.918** | 4.533±1.552** | 1.285±0.244** | 0.958±0.260** |
| GX14070 | 0.300±0.183** | 4.600±0.858** | 3.867±1.125** | 0.950±0.163** | 0.742±0.129** |
| GX14201 | 0.457±0.093** | 4.486±1.143** | 4.643±1.082** | 1.107±0.289** | 0.764±0.209** |
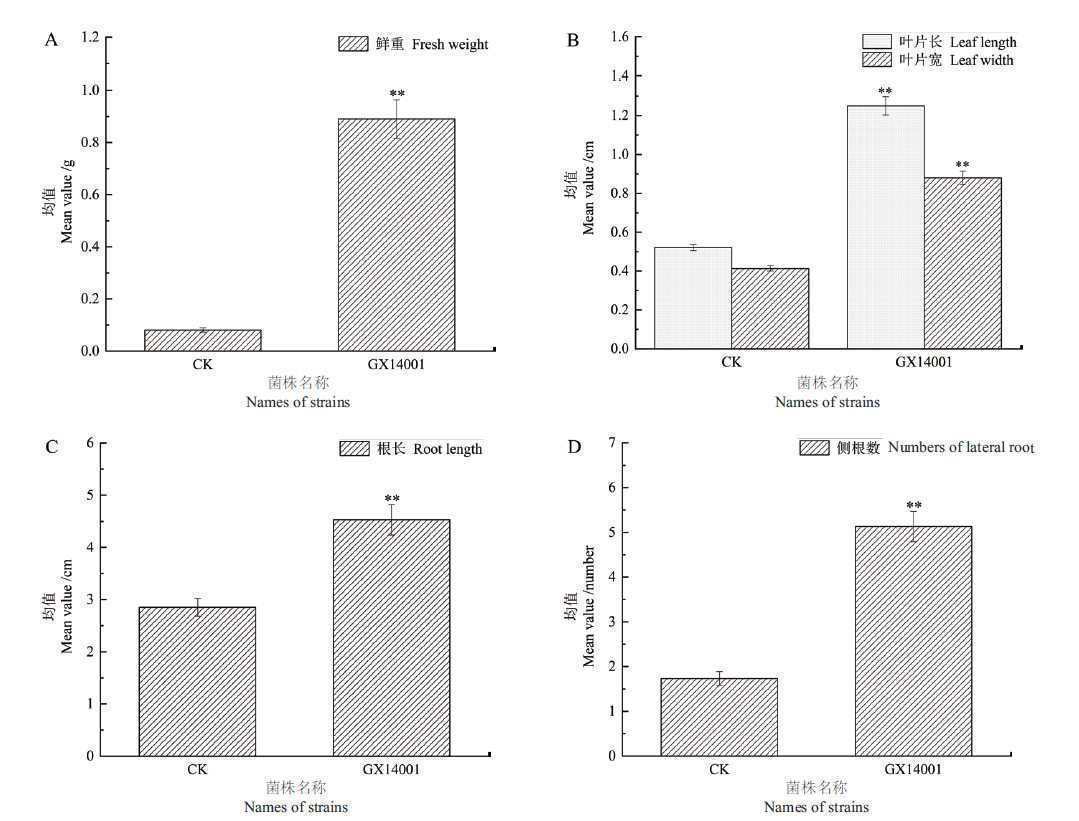
Fig. 2 Growth-promoting effects of VOCs produced by strain GX14001 on tobacco A:Effect of strain GX14001 on the fresh weight of tobacco. B:Effects of strain GX14001 on the tobacco leaf length and width. C:Effect of strain GX14001 on the root length of tobacco. D:Effect of strain GX14001 on the lateral root number of tobacco.“*” refers to significant difference between treatments,P < 0.05,“**” refers to P < 0.01,The same below
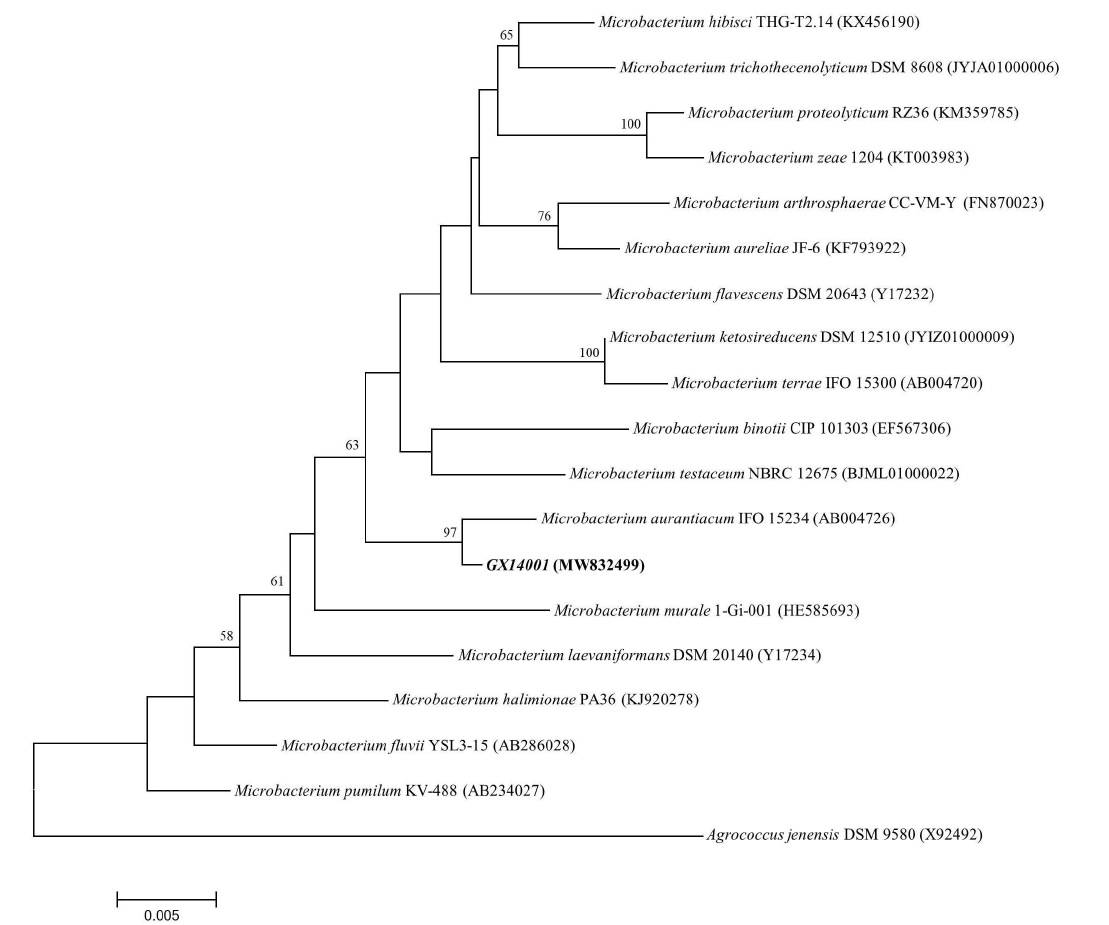
Fig. 3 Constructed phylogenetic tree of strain GX14001 based on 16S rRNA gene sequence The value on the branch represents the percentage of the node formed in 1 000 calculations when the Neighbor-Joining method is used to construct the system tree. The registration number of 16S rRNA gene sequence in GenBank is shown in brackets. The ruler represents the base substitution frequency

Fig. 4 Growth promoting effect of VOCs produced by strain GX14001 on rape A:Results of GX14001-VOCs pot culture. B:Results of GX14001-VOCs potted roots. C:The effects of GX14001-VOCs treatment on the leaf length and width of rape. D:Effect of GX14001-VOCs treatment on root length of rape. E:Effect of GX14001-VOCs on the fresh weight of rape. F:Effect of GX14001-VOCs on the dry weight of rape
| 编号No. | 保留时间Retaining time/ min | 化学名称Chemical name | 峰面积Peak area |
|---|---|---|---|
| 1 | 9.3353 | 邻乙基甲苯(1-ethyl-2-methyl- Benzene) | 1335701 |
| 2 | 9.9132 | 1,2,3-三甲苯(1,2,3-trimethyl- Benzene) | 2357671 |
| 3 | 11.1091 | 1,2,4-三甲苯(1,2,4-trimethyl- Benzene) | 294469 |
| 4 | 11.298 | 4-甲基-癸烷(4-methyl-Decane) | 917363 |
| 5 | 12.8658 | 十二烷(Dodecane) | 3497194 |
| 6 | 32.2232 | 二十五烷(Pentacosane) | 671059 |
| 7 | 34.1057 | 9-辛基-十七烷(9-octyl-Heptadecane) | 387135 |
Table 3 VOCs produced by strain GX14001
| 编号No. | 保留时间Retaining time/ min | 化学名称Chemical name | 峰面积Peak area |
|---|---|---|---|
| 1 | 9.3353 | 邻乙基甲苯(1-ethyl-2-methyl- Benzene) | 1335701 |
| 2 | 9.9132 | 1,2,3-三甲苯(1,2,3-trimethyl- Benzene) | 2357671 |
| 3 | 11.1091 | 1,2,4-三甲苯(1,2,4-trimethyl- Benzene) | 294469 |
| 4 | 11.298 | 4-甲基-癸烷(4-methyl-Decane) | 917363 |
| 5 | 12.8658 | 十二烷(Dodecane) | 3497194 |
| 6 | 32.2232 | 二十五烷(Pentacosane) | 671059 |
| 7 | 34.1057 | 9-辛基-十七烷(9-octyl-Heptadecane) | 387135 |
| 菌株编号 Strain No. | 菌株名称 Name of a strain | 溶无机磷 Inorganic phosphorus solubilization | 溶有机磷 Organic phosphorus solubilization | 固氮 Nitrogen fixation | IAA/(μg·mL-1) |
|---|---|---|---|---|---|
| GX14001 | Microbacterium aurantiacum | ++ | ++ | + | 1.737 |
Table 4 Summary of determining strain's IAA and ability of fixing nitrogen and solubilizing phosphorus
| 菌株编号 Strain No. | 菌株名称 Name of a strain | 溶无机磷 Inorganic phosphorus solubilization | 溶有机磷 Organic phosphorus solubilization | 固氮 Nitrogen fixation | IAA/(μg·mL-1) |
|---|---|---|---|---|---|
| GX14001 | Microbacterium aurantiacum | ++ | ++ | + | 1.737 |
| [1] |
Adesemoye AO, Kloepper JW. Plant-microbes interactions in enhanced fertilizer-use efficiency[J]. Appl Microbiol Biotechnol, 2009, 85(1):1-12.
doi: 10.1007/s00253-009-2196-0 pmid: 19707753 |
| [2] | 李俊, 姜昕, 马鸣超, 等. 我国微生物肥料产业需求与技术创新[J]. 中国土壤与肥料, 2019(2):1-5. |
| Li J, Jiang X, Ma MC, et al. Development demand and technical innovation for bio-fertilizer industry in China[J]. Soil Fertil Sci China, 2019(2):1-5. | |
| [3] |
Ryu CM, Farag MA, Hu CH, et al. Bacterial volatiles promote growth in Arabidopsis[J]. PNAS, 2003, 100(8):4927-4932.
doi: 10.1073/pnas.0730845100 URL |
| [4] |
Zhang H, Kim MS, Krishnamachari V, et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis[J]. Planta, 2007, 226(4):839-851.
doi: 10.1007/s00425-007-0530-2 URL |
| [5] |
Rudrappa T, Biedrzycki ML, Kunjeti SG, et al. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana[J]. Commun Integr Biol, 2010, 3(2):130-138.
doi: 10.4161/cib.3.2.10584 URL |
| [6] |
Zhang H, Sun Y, Xie X, et al. A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms[J]. Plant J, 2009, 58(4):568-577.
doi: 10.1111/tpj.2009.58.issue-4 URL |
| [7] |
Cappellari LDR, Banchio E. Microbial volatile organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission[J]. J Plant Growth Regul, 2020, 39(2):764-775.
doi: 10.1007/s00344-019-10020-3 URL |
| [8] |
Yasmin H, Rashid U, Hassan MN, et al. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize(Zea mays L.)[J]. Physiol Plant, 2021, 172(2):896-911.
doi: 10.1111/ppl.v172.2 URL |
| [9] | Choudoir M, Rossabi S, Gebert M, et al. A phylogenetic and functional perspective on volatile organic compound production by actinobacteria[J]. mSystems, 2019, 4(2):e00295-18. |
| [10] |
Lee S, Behringer G, Hung R, et al. Effects of fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression[J]. Fungal Ecol, 2019, 37:1-9.
doi: 10.1016/j.funeco.2018.08.004 URL |
| [11] |
Paul D, Park KS. Identification of volatiles produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes plant growth[J]. Sensors:Basel, 2013, 13(10):13969-13977.
doi: 10.3390/s131013969 URL |
| [12] | Tahir HA, Gu Q, Wu H, et al. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2[J]. Front Microbiol, 2017, 8:171. |
| [13] |
Zhang HM, Murzello C, Sun Y, et al. Choline and osmotic-stress tolerance induced in Arabidopsis by the soil microbe Bacillus subtilis(GB03)[J]. Mol Plant Microbe Interact, 2010, 23(8):1097-1104.
doi: 10.1094/MPMI-23-8-1097 URL |
| [14] |
Lee B, Farag MA, Park HB, et al. Induced resistance by a long-chain bacterial volatile:elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa[J]. PLoS One, 2012, 7(11):e48744.
doi: 10.1371/journal.pone.0048744 URL |
| [15] | 白变霞, 陈艳彬, 任嘉红. 蜡状芽孢杆菌CLY07菌株的解有机磷特性研究[J]. 西南林业大学学报, 2016, 36(4):75-81. |
| Bai BX, Chen YB, Ren JH. Study on Organophosphate-solubilizing characteristics of Bacillus cereus CLY07[J]. J Southwest For Univ, 2016, 36(4):75-81. | |
| [16] |
Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms[J]. FEMS Microbiol Lett, 1999, 170(1):265-270.
pmid: 9919677 |
| [17] | 徐晓芬. 类芽孢杆菌BD3526在无氮固体培养基上产胞外多糖的研究[J]. 食品工业科技, 2017, 38(16):95-100. |
| Xu XF. Study on the exopolysaccharides produced by Paenibacillus bovis sp. nov BD3526 in nitrogen-free solid culture medium[J]. Sci Technol Food Ind, 2017, 38(16):95-100. | |
| [18] | 胡江春, 薛德林, 马成新, 等. 植物根际促生菌(PGPR)的研究与应用前景[J]. 应用生态学报, 2004, 15(10):1963-1966. |
| Hu JC, Xue DL, Ma CX, et al. Research advances in plant growth-promoting rhizobacteria and its application prospects[J]. Chin J Appl Ecol, 2004, 15(10):1963-1966. | |
| [19] |
Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria:Current perspective[J]. J King Saud Univ Sci, 2014, 26(1):1-20.
doi: 10.1016/j.jksus.2013.05.001 URL |
| [20] |
Xie SS, Wu HJ, Zang HY, et al. Plant growth promotion by spermidine-producing Bacillus subtilis OKB105[J]. Mol Plant Microbe Interact, 2014, 27(7):655-663.
doi: 10.1094/MPMI-01-14-0010-R URL |
| [21] | Cordovez V, Schop S, Hordijk K, et al. Priming of plant growth promotion by volatiles of root-associated Microbacterium spp[J]. Appl Environ Microbiol, 2018, 84(22):e01865-18. |
| [22] |
Mills GA, Walker V. Headspace solid-phase microextraction procedures for gas chromatographic analysis of biological fluids and materials[J]. J Chromatogr A, 2000, 902(1):267-287.
pmid: 11192159 |
| [23] |
Blom D, Fabbri C, Connor EC, et al. Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions[J]. Environ Microbiol, 2011, 13(11):3047-3058.
doi: 10.1111/j.1462-2920.2011.02582.x pmid: 21933319 |
| [24] |
Velázquez-Becerra C, Macías-Rodríguez LI, López-Bucio J, et al. A volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro[J]. Plant Soil, 2011, 339(1/2):329-340.
doi: 10.1007/s11104-010-0583-z URL |
| [25] |
Yamagiwa Y, Inagaki Y, Ichinose Y, et al. Talaromyces wortmannii FS2 emits β-caryphyllene, which promotes plant growth and induces resistance[J]. J Gen Plant Pathol, 2011, 77(6):336-341.
doi: 10.1007/s10327-011-0340-z URL |
| [26] |
Jishma P, Hussain N, Chellappan R, et al. Strain-specific variation in plant growth promoting volatile organic compounds production by five different Pseudomonas spp. as confirmed by response of Vigna radiata seedlings[J]. J Appl Microbiol, 2017, 123(1):204-216.
doi: 10.1111/jam.13474 pmid: 28423218 |
| [27] |
Xu Z, Mulchandani A, Chen W. Detection of benzene, toluene, ethyl benzene, and xylenes(BTEX)using toluene dioxygenase-peroxidase coupling reactions[J]. Biotechnol Prog, 2003, 19(6):1812-1815.
doi: 10.1021/bp0341794 URL |
| [28] |
Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria(PGPR):emergence in agriculture[J]. World J Microbiol Biotechnol, 2012, 28(4):1327-1350.
doi: 10.1007/s11274-011-0979-9 URL |
| [29] |
Dastager SG, Damare S. Marine actinobacteria showing phosphate-solubilizing efficiency in chorao island, Goa, India[J]. Curr Microbiol, 2013, 66(5):421-427.
doi: 10.1007/s00284-012-0288-z pmid: 23288302 |
| [30] | Kishore N, Pindi PK, Ram Reddy S. Phosphate-solubilizing microorganisms:A critical review[M]//Plant Biology and Biotechnology. New Delhi:Springer India, 2015:307-333. |
| [31] |
Karlidag H, Esitken A, Turan M, et al. Effects of root inoculation of plant growth promoting rhizobacteria(PGPR)on yield, growth and nutrient element contents of leaves of apple[J]. Sci Hortic, 2007, 114(1):16-20.
doi: 10.1016/j.scienta.2007.04.013 URL |
| [32] |
Sheng XF, Xia JJ, Jiang CY, et al. Characterization of heavy metal-resistant endophytic bacteria from rape(Brassica napus)roots and their potential in promoting the growth and lead accumulation of rape[J]. Environ Pollut, 2008, 156(3):1164-1170.
doi: 10.1016/j.envpol.2008.04.007 pmid: 18490091 |
| [1] | WANG Qi-yuan, WANG Jia-chen, YE Lei, JIANG Fan. Research Advances on Enhancement of Plant Resistance to Salinity Stress by Rhizobacteria Containing ACC Deaminase [J]. Biotechnology Bulletin, 2021, 37(2): 174-186. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||