








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (1): 214-223.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0388
Previous Articles Next Articles
ZHANG Jun1,2,3( ), ZHANG Hong1,2,3, ZHANG Rui1,2,3, LU Guo-dong1,2,3, YONG Jing-jiao1,2,3, LANG Si-rui1,2,3, CHEN Ren1,2,3(
), ZHANG Hong1,2,3, ZHANG Rui1,2,3, LU Guo-dong1,2,3, YONG Jing-jiao1,2,3, LANG Si-rui1,2,3, CHEN Ren1,2,3( )
)
Received:2022-04-01
Online:2023-01-26
Published:2023-02-02
Contact:
CHEN Ren
E-mail:zj1340131221@qq.com;chenren@nxu.edu.cn
ZHANG Jun, ZHANG Hong, ZHANG Rui, LU Guo-dong, YONG Jing-jiao, LANG Si-rui, CHEN Ren. Transformation and Functional Identification of the Key Genes Associated with Steviol Glycosides Biosynthesis in Stevia rebaudiana[J]. Biotechnology Bulletin, 2023, 39(1): 214-223.
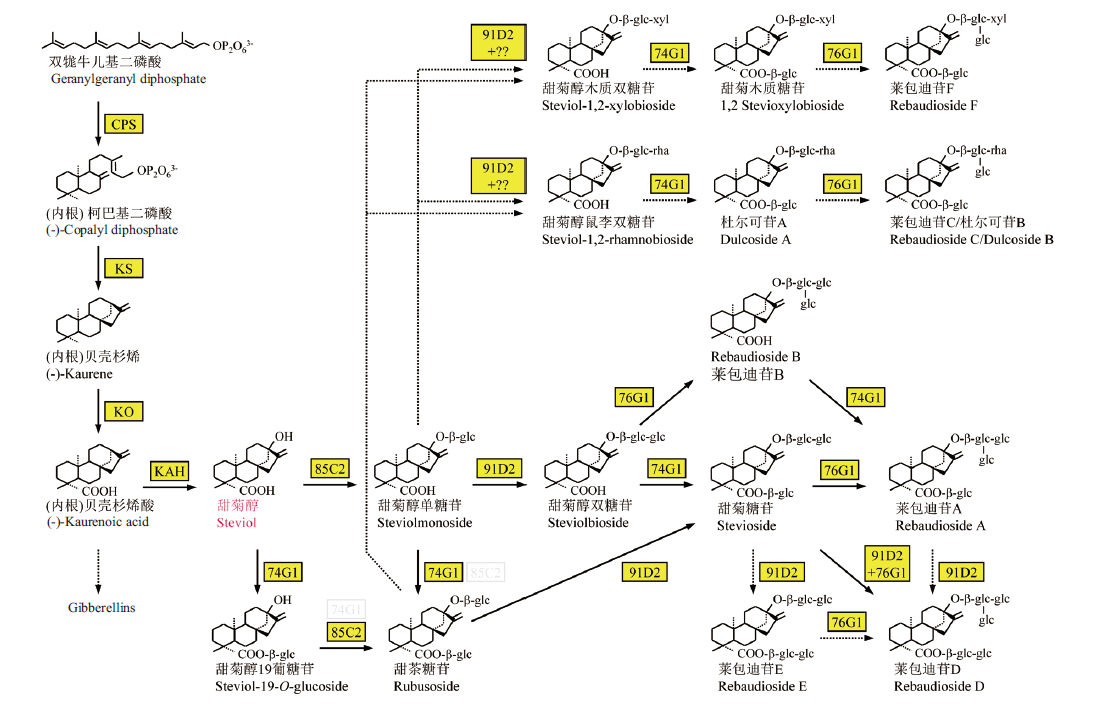
Fig. 1 Biosynthetic pathways of steviol glycosides CPS, copalylpyrophosphate synthase; KS, kaurene synthase; KO, kaurene oxidized; KAH, kaurenoicacid hydroxylase; 86C2, 91D2, 74G1, 76G1, UDP-glucosyltransferases(UGT86C2, UGT91D2, UGT74G1, UGT76G1)
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 引入酶切位点Restriction site |
|---|---|---|
| Sr76G1-S | TATGGATCCTTGCGTGTAAACGTCAGT | BamH I |
| Sr76G1-A | ATGCTCGAGTTATTTACAACGATGAAATGT | Xho I |
| Sr85C2-S | TATGGTACCATGGATGCAATGGCTACAAC | Kpn I |
| Sr85C2-A | GCCGAGCTCCAAAGTTACATCTTAAATAG | Sac I |
| Sr91D2m-S | TTACGGTACCATGGCTACCAGTGACTC | Kpn I |
| Sr91D2m-A | GTGGAGCTCTTAACTCTCATGATCGA | Sac I |
| CR80 MCS insert 35S-S | CACAATCCCACTATCCTTCGCA | |
| CR80 MCS insert NOS-A | TCCACTCTAATCATAAAAACCCATCTC | |
| CR80 MCS insert NOS-S | GAGATGGGTTTTTATGATTAGAGTCC | |
| CR100 Cla-S | CGCTACTGATTACGGTGCTGCTAT | |
| CR100 omega-A | TTGTTTGTTGTTTGTTGTTGTTGGTAA |
Table 1 Primers for PCR amplification
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 引入酶切位点Restriction site |
|---|---|---|
| Sr76G1-S | TATGGATCCTTGCGTGTAAACGTCAGT | BamH I |
| Sr76G1-A | ATGCTCGAGTTATTTACAACGATGAAATGT | Xho I |
| Sr85C2-S | TATGGTACCATGGATGCAATGGCTACAAC | Kpn I |
| Sr85C2-A | GCCGAGCTCCAAAGTTACATCTTAAATAG | Sac I |
| Sr91D2m-S | TTACGGTACCATGGCTACCAGTGACTC | Kpn I |
| Sr91D2m-A | GTGGAGCTCTTAACTCTCATGATCGA | Sac I |
| CR80 MCS insert 35S-S | CACAATCCCACTATCCTTCGCA | |
| CR80 MCS insert NOS-A | TCCACTCTAATCATAAAAACCCATCTC | |
| CR80 MCS insert NOS-S | GAGATGGGTTTTTATGATTAGAGTCC | |
| CR100 Cla-S | CGCTACTGATTACGGTGCTGCTAT | |
| CR100 omega-A | TTGTTTGTTGTTTGTTGTTGTTGGTAA |
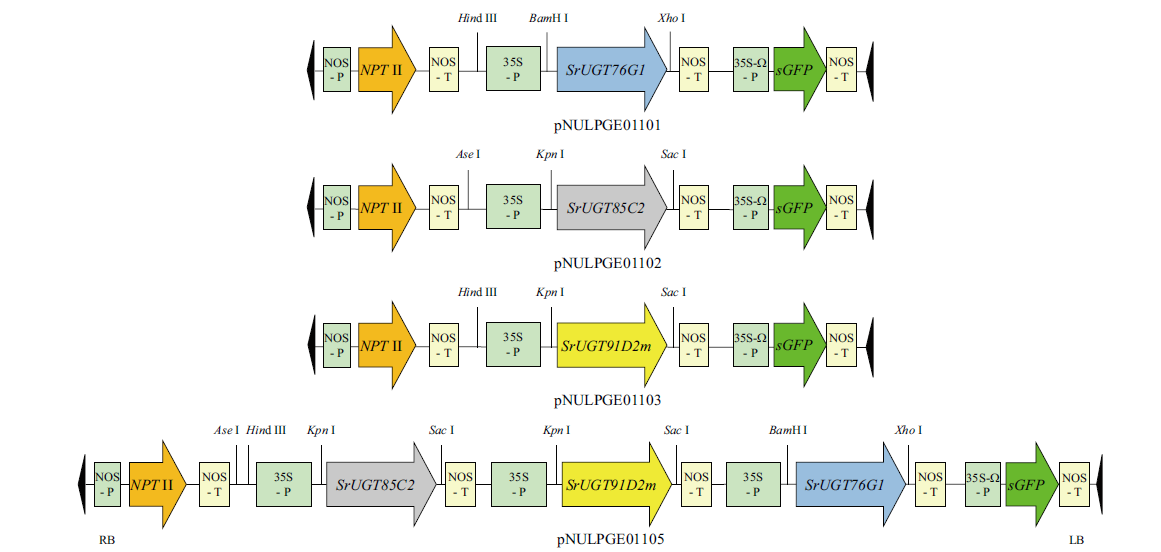
Fig. 2 Schematic structure of co-expression vectors of single genes or three genes NOS-P, nopaline synthase promoter; NOS-T, nopaline synthase terminator; 35S-P, cauliflower mosaic virus 35S promoter; 35S-W-P, 35S promoter with an additional omega element translational enhancer; NPT II, neomycin phosphotransferase II; sGFP, synthetic green-fluorescent protein with S65T mutation
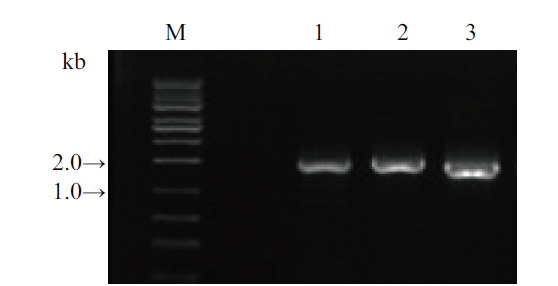
Fig. 3 PCR amplification of 3 target genes 1, SrUGT76G1(primer pair: Sr76G1-S, A; 1 425 bp); 2, SrUGT85C2(primer pair: Sr85C2-S, A; 1 511 bp); 3, SrUGT91D2m(primer pair: Sr91D2m-S, A; 1 441 bp); M, DNA marker
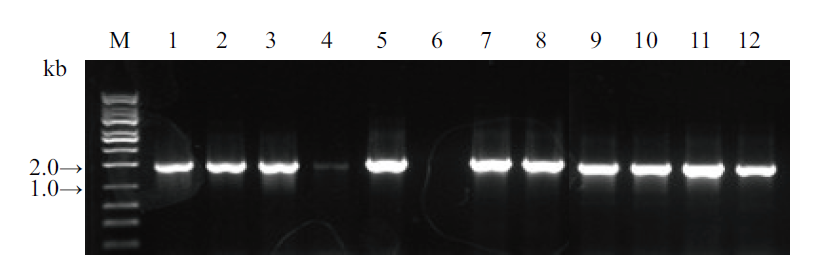
Fig. 4 Colony PCR identification of the 3 genes individually constructed into pKAFCR80 intermediate vector 1-4, SrUGT76G1(1 675 bp); 5-8, SrUGT85C2(1 764 bp); 9-12, SrUGT91D2m(1 693 bp)(All primer pair: CR80 MCS insert 35S-S, A); M, DNA marker
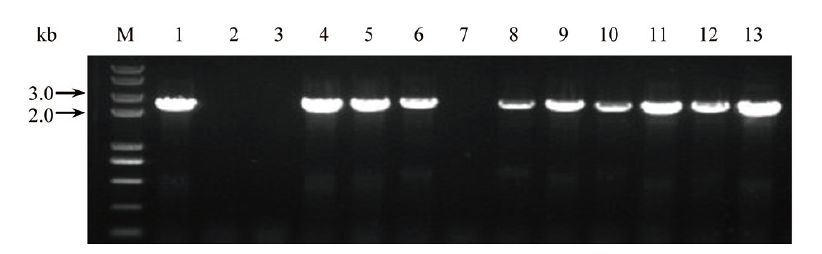
Fig. 5 Colony PCR identification of the 3 genes individu-ally constructed into pKAFCR100 binary vector 1-5, SrUGT76G1(2 901 bp); 6-9, SrUGT85C2(2 808 bp); 10-13, SrUGT91D2m(2 919 bp)(All primers pair: CR100 Cla-S, CR80 MCS insert NOS-A); M, DNA maker
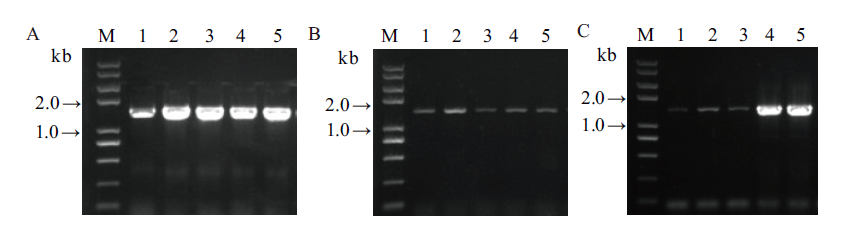
Fig. 6 Colony PCR identification of 3 genes in combination constructed into pKAFCR100 binary vector A: 1-5, SrUGT76G1(primer pair: Sr76G1-S, A; 1 425 bp); B: 1-5, SrUGT85C2(primer pair: Sr85C2-S, A; 1 511 bp); C: 1-5, SrUGT91D2m(primer pair: Sr91D2m-S, A; 1 441 bp); M, DNA marker
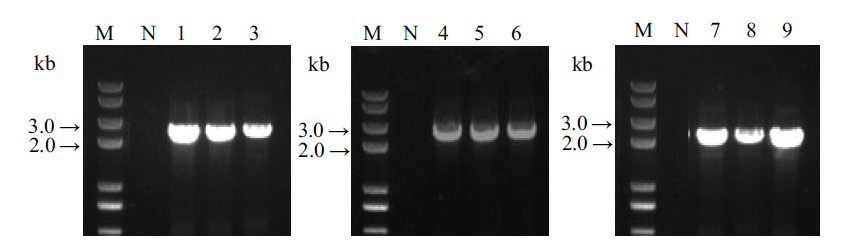
Fig. 7 PCR identification of the transgenic plant transferr-ed 3 genes individually N, wild-type negative control, 1-3, SrUGT76G1 gene transferred individually(primer pair: Sr76G1-S, CR100 omega-A; 2 151 bp); 4-6, SrUGT85C2 gene transferred individually(primer pair: CR100 Cla-S, Sr85C2-A; 2 643 bp); 7-9, SrUGT91D2m gene transferred individually(primer pair: CR80 MCS insert NOS-S, Sr91D2m-A; 2 434 bp); M, DNA marker
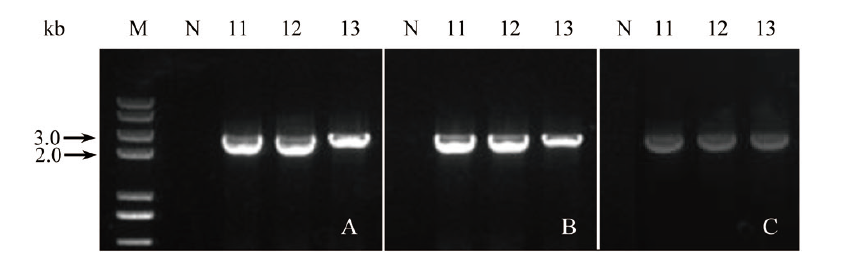
Fig. 8 PCR identification of the transgenic plant transferr-ed 3 genes in combination A: N, wild-type negative control, 11-13, three genes were transferred in combination(primer pair: Sr76G1-S, pKAFCR100 omega-A; 2 151 bp). B: N, wild-type negative control, 11-13, three genes were transferred in combination(primer pair: pKAFCR100 Cla-S, Sr85C2-A; 2 643 bp). C: N, wild-type negative control, 11-13, three genes were transferred in combination(primer pair: CR80 MCS insert NOS-S, Sr91D2m-A; 2 434 bp). M, DNA marker
| 转基因植株Transgenic plant | SGs/% | SMono/% | SBio/% | ST/% | RA/% | RA/SGs/% |
|---|---|---|---|---|---|---|
| 野生型对照Wild-type control | 12.74 ± 1.17c | 1.26 ± 0.13b | 0.66 ± 0.07c | 5.47 ± 0.65b | 5.34 ± 0.52c | 40.56 ± 2.36c |
| Sr01102(SrUGT85C2) | 13.22 ± 1.46bc | 1.57 ± 0.17a | 0.74 ± 0.06b | 5.55 ± 0.84b | 5.36 ± 0.64c | 40.79 ± 2.20c |
| Sr01103(SrUGT91D2m) | 13.65 ± 1.16b | 0.63 ± 0.07c | 1.10 ± 0.15a | 5.98 ± 0.56a | 5.95 ± 0.64c | 42.79 ± 2.73c |
| Sr01101(SrUGT76G1) | 15.22 ± 1.25ab | 1.22 ± 0.12b | 0.74 ± 0.06b | 2.49 ± 0.24d | 10.77 ± 1.02a | 71.70 ± 2.43a |
| Sr01105(3个基因组合/3 genes Combined) | 16.12 ± 1.59a | 1.23 ± 0.11b | 0.83 ± 0.09b | 4.60 ± 0.36c | 9.45 ± 1.15b | 58.27 ± 1.96b |
Table 2 Variety of steviol glycosides contents in different transgenic plant
| 转基因植株Transgenic plant | SGs/% | SMono/% | SBio/% | ST/% | RA/% | RA/SGs/% |
|---|---|---|---|---|---|---|
| 野生型对照Wild-type control | 12.74 ± 1.17c | 1.26 ± 0.13b | 0.66 ± 0.07c | 5.47 ± 0.65b | 5.34 ± 0.52c | 40.56 ± 2.36c |
| Sr01102(SrUGT85C2) | 13.22 ± 1.46bc | 1.57 ± 0.17a | 0.74 ± 0.06b | 5.55 ± 0.84b | 5.36 ± 0.64c | 40.79 ± 2.20c |
| Sr01103(SrUGT91D2m) | 13.65 ± 1.16b | 0.63 ± 0.07c | 1.10 ± 0.15a | 5.98 ± 0.56a | 5.95 ± 0.64c | 42.79 ± 2.73c |
| Sr01101(SrUGT76G1) | 15.22 ± 1.25ab | 1.22 ± 0.12b | 0.74 ± 0.06b | 2.49 ± 0.24d | 10.77 ± 1.02a | 71.70 ± 2.43a |
| Sr01105(3个基因组合/3 genes Combined) | 16.12 ± 1.59a | 1.23 ± 0.11b | 0.83 ± 0.09b | 4.60 ± 0.36c | 9.45 ± 1.15b | 58.27 ± 1.96b |
| [1] |
Soejarto DD, Kinghorn AD, Farnsworth NR. Potential sweetening agents of plant origin. III. Organoleptic evaluation of Stevia leaf herbarium samples for sweetness[J]. J Nat Prod, 1982, 45(5): 590-599.
pmid: 7153776 |
| [2] |
Kinghorn AD, Soejarto DD, Nanayakkara NPD, et al. A phytochemical screening procedure for sweet ent-kaurene glycosides in the genus Stevia[J]. J Nat Prod, 1984, 47(3): 439-444.
pmid: 6481357 |
| [3] |
Goyal SK, Samsher, Goyal RK. Stevia(Stevia rebaudiana)a bio-sweetener: a review[J]. Int J Food Sci Nutr, 2010, 61(1): 1-10.
doi: 10.3109/09637480903193049 pmid: 19961353 |
| [4] |
Yadav AK. A review on the improvement of Stevia[Stevia rebaudiana(Bertoni)[J]. Can J Plant Sci, 2011, 91(1): 1-27.
doi: 10.4141/cjps10086 URL |
| [5] | 倪军明, 李军平. 甜菊糖工业发展现状与前景[J]. 广州食品工业科技, 2004, 20(3): 156-158. |
| Ni JM, Li JP. Current development and prospect of stevioside industry[J]. Guangzhou Food Sci Technol, 2004, 20(3): 156-158. | |
| [6] | 徐鸿. 甜菊糖的特点及功能[J]. 现代农业科技, 2013(2): 286-287. |
| Xu H. Characteristic and functions of stevioside[J]. Mod Agric Sci Technol, 2013(2): 286-287. | |
| [7] |
Ohta M, Sasa S, Inoue A, et al. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana morita[J]. J Appl Glycosci, 2010, 57(3): 199-209.
doi: 10.5458/jag.57.199 URL |
| [8] |
Prakash I, Campbell M, Chaturvedula VSP. Catalytic hydrogenation of the sweet principles of Stevia rebaudiana, Rebaudioside B, Rebaudioside C, and Rebaudioside D and sensory evaluation of their reduced derivatives[J]. Int J Mol Sci, 2012, 13(11): 15126-15136.
doi: 10.3390/ijms131115126 pmid: 23203115 |
| [9] |
Wölwer-Rieck U. The leaves of Stevia rebaudiana(Bertoni), their constituents and the analyses thereof: a review[J]. J Agric Food Chem, 2012, 60(4): 886-895.
doi: 10.1021/jf2044907 URL |
| [10] |
Tada A, Takahashi K, Ishizuki K, et al. Absolute quantitation of stevioside and rebaudioside A in commercial standards by quantitative NMR[J]. Chem Pharm Bull(Tokyo), 2013, 61(1): 33-38.
pmid: 23124594 |
| [11] | Crammer B, Ikan R. Sweet glycosides from the stevia plant[J]. Chemistry in Britain, 1986, 22(10): 915-916. |
| [12] | 孙传范, 李进伟. 甜菊糖苷研究进展[J]. 食品科学, 2010, 31(9): 338-340. |
| Sun CF, Li JW. Research progress on steviosides[J]. Food Sci, 2010, 31(9): 338-340. | |
| [13] | Persinos G. Method of producing stevioside: US3723410[P]. 1973-03-27. |
| [14] |
Humphrey TV, Richman AS, Menassa R, et al. Spatial organisation of four enzymes from Stevia rebaudiana that are involved in steviol glycoside synthesis[J]. Plant Mol Biol, 2006, 61(1/2): 47-62.
doi: 10.1007/s11103-005-5966-9 URL |
| [15] |
Brandle JE, Telmer PG. Steviol glycoside biosynthesis[J]. Phytochemistry, 2007, 68(14): 1855-1863.
pmid: 17397883 |
| [16] |
Mohamed AAA, Ceunen S, Geuns JMC, et al. UDP-dependent glycosyltransferases involved in the biosynthesis of steviol glycosides[J]. J Plant Physiol, 2011, 168(10): 1136-1141.
doi: 10.1016/j.jplph.2011.01.030 URL |
| [17] |
Ceunen S, Geuns JMC. Steviol glycosides: chemical diversity, metabolism, and function[J]. J Nat Prod, 2013, 76(6): 1201-1228.
doi: 10.1021/np400203b pmid: 23713723 |
| [18] |
Yadav SK, Guleria P. Steviol glycosides from Stevia: biosynthesis pathway review and their application in foods and medicine[J]. Crit Rev Food Sci Nutr, 2012, 52(11): 988-998.
doi: 10.1080/10408398.2010.519447 URL |
| [19] |
Guleria P, Yadav SK. Agrobacterium mediated transient gene silencing(AMTS)in Stevia rebaudiana: insights into steviol glycoside biosynthesis pathway[J]. PLoS One, 2013, 8(9): e74731.
doi: 10.1371/journal.pone.0074731 URL |
| [20] |
Modi A, Litoriya N, Prajapati V, et al. Transcriptional profiling of genes involved in steviol glycoside biosynthesis in Stevia rebaudiana bertoni during plant hardening[J]. Dev Dyn, 2014, 243(9): 1067-1073.
doi: 10.1002/dvdy.24157 URL |
| [21] |
Madhav H, Bhasker S, Chinnamma M. Functional and structural variation of uridine diphosphate glycosyltransferase(UGT)gene of Stevia rebaudiana-UGTSr involved in the synthesis of rebaudioside A[J]. Plant Physiol Biochem, 2013, 63: 245-253.
doi: 10.1016/j.plaphy.2012.11.029 URL |
| [22] |
Guleria P, Masand S, Yadav SK. Overexpression of SrUGT85C2 from Stevia reduced growth and yield of transgenic Arabidopsis by influencing plastidial MEP pathway[J]. Gene, 2014, 539(2): 250-257.
doi: 10.1016/j.gene.2014.01.071 URL |
| [23] |
Yang YH, Huang SZ, Han YL, et al. Base substitution mutations in uridinediphosphate-dependent glycosyltransferase 76G1 gene of Stevia rebaudiana causes the low levels of rebaudioside A: mutations in UGT76G1, a key gene of steviol glycosides synthesis[J]. Plant Physiol Biochem, 2014, 80: 220-225.
doi: 10.1016/j.plaphy.2014.04.005 URL |
| [24] |
Kim MJ, Zheng JS, et al. Overexpression of SrUGT76G1 in Stevia alters major steviol glycosides composition towards improved quality[J]. Plant Biotechnol J, 2019, 17(6): 1037-1047.
doi: 10.1111/pbi.13035 URL |
| [25] | 陈任, 张虹, 张芮, 等. 用于植物多基因表达载体构建的质粒系统[J]. 分子植物育种, 2018, 16(4): 1138-1146. |
| Chen R, Zhang H, Zhang R, et al. A plasmid system for construction of plant multiple gene expression vectors[J]. Mol Plant Breed, 2018, 16(4): 1138-1146. | |
| [26] | 张芮. 甜菊醇糖苷生物合成途径关键基因的功能评价[D]. 银川: 宁夏大学, 2016. |
| Zhang R. The functional evaluation of key genes in steviol glycosides biosynthetic pathway[D]. Yinchuan: Ningxia University, 2016. | |
| [27] | 张虹, 张芮, 薄玉瑶, 等. 甜叶菊甜菊醇糖苷生物合成关键酶基因表达量与莱宝迪苷A含量的相关关系[J]. 分子植物育种, 2015, 13(8): 1802-1807. |
| Zhang H, Zhang R, Bo YY, et al. Correlation between the transcript levels of key enzyme-encoding genes and the content of rebaudioside a in steviol glycoside biosynthesis of Stevia rebaudiana[J]. Mol Plant Breed, 2015, 13(8): 1802-1807. | |
| [28] |
Jentzer JB, Alignan M, Vaca-Garcia C, et al. Response surface methodology to optimise accelerated solvent extraction of steviol glycosides from Stevia rebaudiana Bertoni leaves[J]. Food Chem, 2015, 166: 561-567.
doi: 10.1016/j.foodchem.2014.06.078 URL |
| [29] |
Periche A, Castelló ML, Heredia A, et al. Influence of drying method on steviol glycosides and antioxidants in Stevia rebaudiana leaves[J]. Food Chem, 2015, 172: 1-6.
doi: 10.1016/j.foodchem.2014.09.029 pmid: 25442516 |
| [30] |
Chranioti C, Chanioti S, Tzia C. Comparison of spray, freeze and oven drying as a means of reducing bitter aftertaste of steviol glycosides(derived from Stevia rebaudiana Bertoni plant)—Evaluation of the final products[J]. Food Chem, 2016, 190: 1151-1158.
doi: 10.1016/j.foodchem.2015.06.083 URL |
| [31] |
张虹, 路国栋, 袁春春, 等. 甜叶菊中9种甜菊醇糖苷积累与其生物合成关键基因表达量的相关性[J]. 核农学报, 2022, 36(1): 75-82.
doi: 10.11869/j.issn.100-8551.2022.01.0075 |
| Zhang H, Lu GD, Yuan CC, et al. Correlation between the accumulations of 9 steviol glycosides and the expressions of the key genes involved in their biosynthesis in Stevia rebaudiana[J]. J Nucl Agric Sci, 2022, 36(1): 75-82. | |
| [32] |
Zhang SS, Yang YS, Lyu CC, et al. Identification of the key residues of the uridine diphosphate glycosyltransferase 91D2 and its effect on the accumulation of steviol glycosides in Stevia rebaudiana[J]. J Agric Food Chem, 2021, 69(6): 1852-1863.
doi: 10.1021/acs.jafc.0c07066 URL |
| [33] | 刘欢, 李艳, 等. 甜叶菊糖基转移酶UGT76G1的克隆表达及其性质研究[J]. 食品工业科技, 2012, 33(20): 187-190. |
| Liu H, Li Y, et al. Cloning, expression and characteristic of glycosyl-transferase UGT76G1 from Stevia rebaudiana[J]. Sci Technol Food Ind, 2012, 33(20): 187-190. | |
| [34] |
Saifi M, Yogindran S, et al. Co-expression of anti-miR319g and miRStv_11 lead to enhanced steviol glycosides content in Stevia rebaudiana[J]. BMC Plant Biol, 2019, 19(1): 274.
doi: 10.1186/s12870-019-1871-2 URL |
| [35] |
Zhang T, Xu XY, Sun YM, et al. The SrWRKY71 transcription factor negatively regulates SrUGT76G1 expression in Stevia rebau-diana[J]. Plant Physiol Biochem, 2020, 148: 26-34.
doi: 10.1016/j.plaphy.2020.01.001 URL |
| [1] | FENG Cui-lian, ZHANG Shu-zhen. Breeding of Transgenic Insect-resistant Sugarcane and Strategies for Preventing the Its Resistance to Insects from Loss [J]. Biotechnology Bulletin, 2020, 36(7): 209-219. |
| [2] | ZHU Jing-wen GUO, Shu-qiao, SHU Hong-mei, GONG Yuan-yong, JIANG Lu, NI Wan-chao. Cloning and Expression Analysis of New KAH Genes from Stevia rebaudian [J]. Biotechnology Bulletin, 2017, 33(4): 104-107. |
| [3] | WANG Wen-zhi, YANG Ben-peng, CAI Wen-wei, XIONG Guo-ru, WANG Jun-gang, FENG Cui-lian, ZHANG Shu-zhen. Multiple Gene Transformation in Sugarcane and Multiple PCR Detection [J]. Biotechnology Bulletin, 2016, 32(1): 103-108. |
| [4] | Wang Qiong, Liu Bobin, Kong Qianqian, Chen Jienan. Advances in Molecular Breeding of Energy Plant Jatropha curcas [J]. Biotechnology Bulletin, 2014, 0(3): 1-8. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||