








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (3): 123-132.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0819
Previous Articles Next Articles
WANG Hai-long1( ), LI Yu-qian1,2, WANG Bo3, XING Guo-fang2(
), LI Yu-qian1,2, WANG Bo3, XING Guo-fang2( ), ZHANG Jie-wei1(
), ZHANG Jie-wei1( )
)
Received:2022-07-02
Online:2023-03-26
Published:2023-04-10
WANG Hai-long, LI Yu-qian, WANG Bo, XING Guo-fang, ZHANG Jie-wei. Isolation and Expression Analysis of SiMAPK3 in Setaria italica L.[J]. Biotechnology Bulletin, 2023, 39(3): 123-132.

Fig. 1 Sequence and structure analysis of SiMAPK3 in foxtail millet A:Hydrophobicity of SiMAPK3. B:Analysis of the SiMAPK3 secondary structure. C:Analysis of the SiMAPK3 domain. D:The tertiary structure model of SiMAPK3. E:Prediction of phosphorylation sites in SiMAPK3

Fig. 2 Multiple sequence alignment of the SiMAPK3 and MAPK proteins from other species Red box indicates the specific sequence of highly conservative TEY
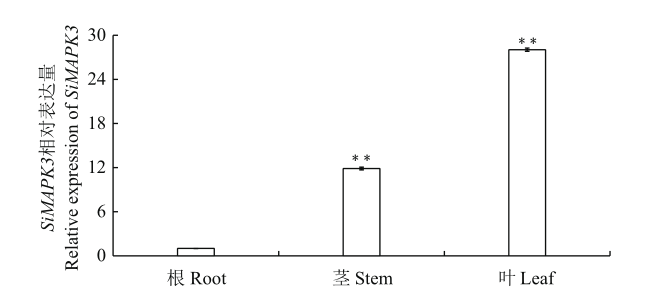
Fig. 4 Expression analysis of SiMAPK3 in different tissues of S. italica at the shooting stage of foxtail millet RT-qPCR analysis of SiMAPK3 gene expression in various organs, the SiActin in S. italica was used as an internal reference. n=3. Double asterisks(**)in each column indicate a significant difference at P<0.01 level. The same below
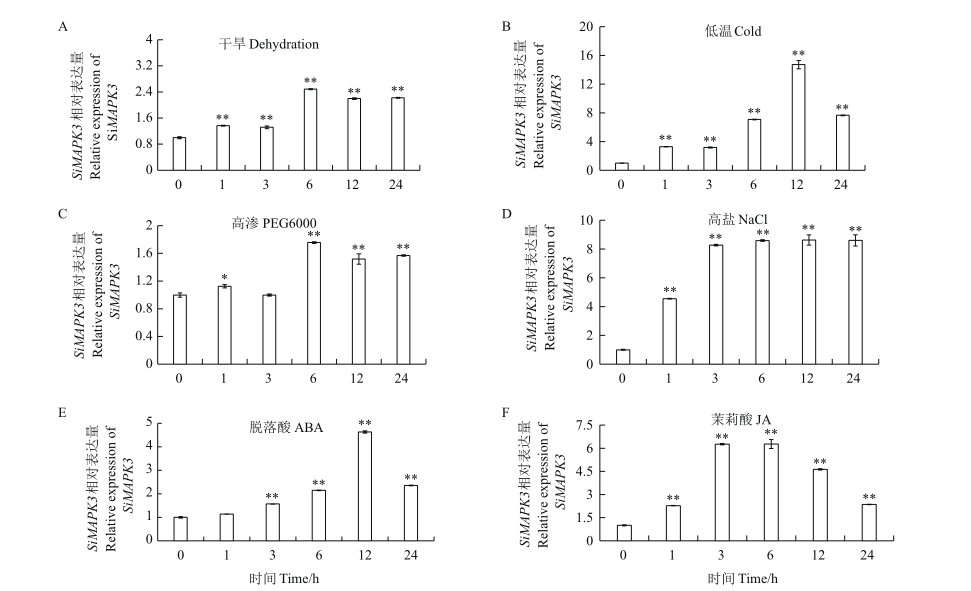
Fig. 5 SiMAPK3 expression patterns under different treatments A: SiMAPK3 gene expression under dehydration; B: SiMAPK3 gene expression under 4℃; C: SiMAPK3 gene expression under 20% PEG6000; D: SiMAPK3 gene expression under 300 mmol/L NaCl; E: SiMAPK3 gene expression under 200 μmol/L ABA; F: SiMAPK3 gene expression under 200 μmol/L JA. Asterisk(*)indicates a difference at P<0.05 level
| [1] |
Xu J, Zhang SQ. Mitogen-activated protein kinase cascades in signaling plant growth and development[J]. Trends Plant Sci, 2015, 20(1): 56-64.
doi: 10.1016/j.tplants.2014.10.001 pmid: 25457109 |
| [2] |
Zhang MM, Zhang SQ. Mitogen-activated protein kinase cascades in plant signaling[J]. J Integr Plant Biol, 2022, 64(2): 301-341.
doi: 10.1111/jipb.13215 |
| [3] |
Chen XX, Ding YL, Yang YQ, et al. Protein kinases in plant responses to drought, salt, and cold stress[J]. J Integr Plant Biol, 2021, 63(1): 53-78.
doi: 10.1111/jipb.13061 |
| [4] |
Rodriguez MCS, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants[J]. Annu Rev Plant Biol, 2010, 61: 621-649.
doi: 10.1146/annurev-arplant-042809-112252 pmid: 20441529 |
| [5] |
Zhang MM, Su JB, Zhang Y, et al. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense[J]. Curr Opin Plant Biol, 2018, 45(Pt A): 1-10.
doi: S1369-5266(17)30213-3 pmid: 29753266 |
| [6] |
Ichimura K, Shinozaki K, Tena G, et al. Mitogen-activated protein kinase cascades in plants: a new nomenclature[J]. Trends Plant Sci, 2002, 7(7): 301-308.
doi: 10.1016/s1360-1385(02)02302-6 pmid: 12119167 |
| [7] |
Rao KP, Richa T, Kumar K, et al. In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice[J]. DNA Res, 2010, 17(3): 139-153.
doi: 10.1093/dnares/dsq011 pmid: 20395279 |
| [8] |
Hamel LP, Nicole MC, Sritubtim S, et al. Ancient signals: comparative genomics of plant MAPK and MAPKK gene families[J]. Trends Plant Sci, 2006, 11(4): 192-198.
doi: 10.1016/j.tplants.2006.02.007 URL |
| [9] |
Jiang M, Chu ZQ. Comparative analysis of plant MKK gene family reveals novel expansion mechanism of the members and sheds new light on functional conservation[J]. BMC Genomics, 2018, 19(1): 407.
doi: 10.1186/s12864-018-4793-8 pmid: 29843611 |
| [10] |
Ichimura K, Mizoguchi T, Yoshida R, et al. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6[J]. Plant J, 2000, 24(5): 655-665.
doi: 10.1046/j.1365-313x.2000.00913.x pmid: 11123804 |
| [11] |
Guo T, Lu ZQ, Shan JX, et al. ERECTA1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice[J]. Plant Cell, 2020, 32(9): 2763-2779.
doi: 10.1105/tpc.20.00351 URL |
| [12] |
Lee H, Jun YS, Cha OK, et al. Mitogen-activated protein kinases MPK3 and MPK6 are required for stem cell maintenance in the Arabidopsis shoot apical meristem[J]. Plant Cell Rep, 2019, 38(3): 311-319.
doi: 10.1007/s00299-018-2367-5 |
| [13] |
Zuch DT, Doyle SM, Majda M, et al. Cell biology of the leaf epidermis: fate specification, morphogenesis, and coordination[J]. Plant Cell, 2022, 34(1): 209-227.
doi: 10.1093/plcell/koab250 URL |
| [14] |
Melvin P, Prabhu SA, Veena M, et al. The pearl millet mitogen-activated protein kinase PgMPK4 is involved in responses to downy mildew infection and in jasmonic- and salicylic acid-mediated defense[J]. Plant Mol Biol, 2015, 87(3): 287-302.
doi: 10.1007/s11103-014-0276-8 pmid: 25527312 |
| [15] |
Zhang T, Schneider JD, Lin CW, et al. MPK4 phosphorylation dynamics and interacting proteins in plant immunity[J]. J Proteome Res, 2019, 18(3): 826-840.
doi: 10.1021/acs.jproteome.8b00345 pmid: 30632760 |
| [16] |
Jammes F, Song C, Shin D, et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling[J]. Proc Natl Acad Sci USA, 2009, 106(48): 20520-20525.
doi: 10.1073/pnas.0907205106 pmid: 19910530 |
| [17] |
Yang XY, Wan ZW, Perry L, et al. Early millet use in Northern China[J]. Proc Natl Acad Sci USA, 2012, 109(10): 3726-3730.
doi: 10.1073/pnas.1115430109 pmid: 22355109 |
| [18] |
Diao XM, James S, Jeffrey LB, et al. Initiation of Setaria as a model plant[J]. Front Agr Sci Eng, 2014, 1(1): 16.
doi: 10.15302/J-FASE-2014011 URL |
| [19] |
Yang ZR, Zhang HS, Li XK, et al. A mini foxtail millet with an Arabidopsis-like life cycle as a C4 model system[J]. Nat Plants, 2020, 6(9): 1167-1178.
doi: 10.1038/s41477-020-0747-7 |
| [20] |
贾冠清, 刁现民. 中国谷子种业创新现状与未来展望[J]. 中国农业科学, 2022, 55(4): 653-665.
doi: 10.3864/j.issn.0578-1752.2022.04.003 |
|
Jia GQ, Diao XM. Current status and perspectives of innovation studies related to foxtail millet seed industry in China[J]. Sci Agric Sin, 2022, 55(4): 653-665.
doi: 10.3864/j.issn.0578-1752.2022.04.003 |
|
| [21] |
Zhao W, Zhang LL, Xu ZS, et al. Genome-wide analysis of MADS-box genes in foxtail millet(Setaria italica L.)and functional assessment of the role of SiMADS51 in the drought stress response[J]. Front Plant Sci, 2021, 12: 659474.
doi: 10.3389/fpls.2021.659474 URL |
| [22] |
Yang LY, Zhang Y, Guan RX, et al. Co-regulation of indole glucosinolates and camalexin biosynthesis by CPK5/CPK6 and MPK3/MPK6 signaling pathways[J]. J Integr Plant Biol, 2020, 62(11): 1780-1796.
doi: 10.1111/jipb.12973 |
| [23] |
Su JB, Yang LY, Zhu QK, et al. Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity[J]. PLoS Biol, 2018, 16(5): e2004122.
doi: 10.1371/journal.pbio.2004122 URL |
| [24] |
Shao YM, Yu XX, Xu XW, et al. The YDA-MKK4/MKK5-MPK3/MPK6 cascade functions downstream of the RGF1-RGI ligand-receptor pair in regulating mitotic activity in root apical meristem[J]. Mol Plant, 2020, 13(11): 1608-1623.
doi: 10.1016/j.molp.2020.09.004 pmid: 32916336 |
| [25] |
Rayapuram N, Bigeard J, Alhoraibi H, et al. Quantitative phosphoproteomic analysis reveals shared and specific targets of Arabidopsis mitogen-activated protein kinases(MAPKs)MPK3, MPK4, and MPK6[J]. Mol Cell Proteomics, 2018, 17(1): 61-80.
doi: 10.1074/mcp.RA117.000135 pmid: 29167316 |
| [26] | Yan ZW, Wang JX, Wang FX, et al. MPK3/6-induced degradation of ARR1/10/12 promotes salt tolerance in Arabidopsis[J]. EMBO Rep, 2021, 22(10): e52457. |
| [27] |
Liu YK, Liu LX, Qi JH, et al. Cadmium activates ZmMPK3-1 and ZmMPK6-1 via induction of reactive oxygen species in maize roots[J]. Biochem Biophys Res Commun, 2019, 516(3): 747-752.
doi: 10.1016/j.bbrc.2019.06.116 URL |
| [28] |
Wang NN, Li Y, Chen YH, et al. Phosphorylation of WRKY16 by MPK3-1 is essential for its transcriptional activity during fiber initiation and elongation in cotton(Gossypium hirsutum)[J]. Plant Cell, 2021, 33(8): 2736-2752.
doi: 10.1093/plcell/koab153 URL |
| [29] |
Long L, Xu FC, Zhao JR, et al. GbMPK3 overexpression increases cotton sensitivity to Verticillium dahliae by regulating salicylic acid signaling[J]. Plant Sci, 2020, 292: 110374.
doi: 10.1016/j.plantsci.2019.110374 URL |
| [30] |
Wu HJ, Si Q, Liu JM, et al. Regulation of Arabidopsis matrix metalloproteinases by mitogen-activated protein kinases and their function in leaf senescence[J]. Front Plant Sci, 2022, 13: 864986.
doi: 10.3389/fpls.2022.864986 URL |
| [31] |
Xin J, Li CL, Ning KX, et al. AtPFA-DSP3, an atypical dual-specificity protein tyrosine phosphatase, affects salt stress response by modulating MPK3 and MPK6 activity[J]. Plant Cell Environ, 2021, 44(5): 1534-1548.
doi: 10.1111/pce.v44.5 URL |
| [32] |
He CM, Liew LC, Yin LL, et al. The retrograde signaling regulator ANAC017 recruits the MKK9-MPK3/6, ethylene, and auxin signaling pathways to balance mitochondrial dysfunction with growth[J]. Plant Cell, 2022, 34(9): 3460-3481.
doi: 10.1093/plcell/koac177 URL |
| [1] | WANG Zi-ying, LONG Chen-jie, FAN Zhao-yu, ZHANG Lei. Screening of OsCRK5-interacted Proteins in Rice Using Yeast Two-hybrid System [J]. Biotechnology Bulletin, 2023, 39(9): 117-125. |
| [2] | LIU Wen-jin, MA Rui, LIU Sheng-yan, YANG Jiang-wei, ZHANG Ning, SI Huai-jun. Cloning of StCIPK11 Gene and Analysis of Its Response to Drought Stress in Solanum tuberosum [J]. Biotechnology Bulletin, 2023, 39(9): 147-155. |
| [3] | CHEN Xiao, YU Ming-lan, WU Long-kun, ZHENG Xiao-ming, PANG Hong-bo. Research Progress in lncRNA and Their Responses to Low Temperature Stress in Plant [J]. Biotechnology Bulletin, 2023, 39(7): 1-12. |
| [4] | WANG Shuai, FENG Yu-mei, BAI Miao, DU Wei-jun, YUE Ai-qin. Functional Analysis of Soybean Gene GmHMGR Responding to Exogenous Hormones and Abiotic Stresses [J]. Biotechnology Bulletin, 2023, 39(7): 131-142. |
| [5] | WEI Xi-ya, QIN Zhong-wei, LIANG La-mei, LIN Xin-qi, LI Ying-zhi. Mechanism of Melatonin Seed Priming in Improving Salt Tolerance of Capsicum annuum [J]. Biotechnology Bulletin, 2023, 39(7): 160-172. |
| [6] | ZHOU Wen-han, ZHENG Kang-ning, LI Yong-min. Stellera chamaejasme L. Inhibiting Cell Proliferation by Reducing YAP1 Expression in Hepatocellular Carcinoma [J]. Biotechnology Bulletin, 2023, 39(7): 316-324. |
| [7] | DING Kai-xin, WANG Li-chun, TIAN Guo-kui, WANG Hai-yan, LI Feng-yun, PAN Yang, PANG Ze, SHAN Ying. Research Progress in Uniconazole Alleviating Plant Drought Damage [J]. Biotechnology Bulletin, 2023, 39(6): 1-11. |
| [8] | WANG Chun-yu, LI Zheng-jun, WANG Ping, ZHANG Li-xia. Physiological and Biochemical Analysis of Drought Resistance in Sorghum Cuticular Wax-deficient Mutant sb1 [J]. Biotechnology Bulletin, 2023, 39(5): 160-167. |
| [9] | HOU Xiao-yuan, CHE Zheng-zheng, LI Heng-jing, DU Chong-yu, XU Qian, WANG Qun-qing. Construction of the Soybean Membrane System cDNA Library and Interaction Proteins Screening for Effector PsAvr3a [J]. Biotechnology Bulletin, 2023, 39(4): 268-276. |
| [10] | WANG Qi, HU Zhe, FU Wei, LI Guang-zhe, HAO Lin. Regulation of Burkholderia sp. GD17 on the Drought Tolerance of Cucumber Seedlings [J]. Biotechnology Bulletin, 2023, 39(3): 163-175. |
| [11] | LV Yu-jing, WU Dan-dan, KONG Chun-yan, YANG Yu, GONG Ming. Genome-wide Identification of XTH Gene Family and Their Interacting miRNAs and Possible Roles in Low Temperature Adaptation in Jatropha curcas L. [J]. Biotechnology Bulletin, 2023, 39(2): 147-160. |
| [12] | DU Qing-jie, ZHOU Lu-yao, YANG Si-zhen, ZHANG Jia-xin, CHEN Chun-lin, LI Juan-qi, LI Meng, ZHAO Shi-wen, XIAO Huai-juan, WANG Ji-qing. Overexpression of CaCP1 Enhances Salt Stress Sensibility in Transgenic Tobacco [J]. Biotechnology Bulletin, 2023, 39(2): 172-182. |
| [13] | WANG Ming-tao, LIU Jian-wei, ZHAO Chun-zhao. Molecular Mechanisms of Cell Wall Integrity in Plants Under Salt Stress [J]. Biotechnology Bulletin, 2023, 39(11): 18-27. |
| [14] | ZHANG Xiao-yan, YANG Shu-hua, DING Yang-lin. Molecular Mechanism of Cold Signal Perception and Transduction in Plants [J]. Biotechnology Bulletin, 2023, 39(11): 28-35. |
| [15] | YU Bo, QIN Xiao-hui, ZHAO Yang. Mechanisms of Plant Sensing Drought Signals [J]. Biotechnology Bulletin, 2023, 39(11): 6-17. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||