








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (4): 81-92.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0771
Previous Articles Next Articles
HAN Hui( ), ZHANG Jian, REN Yu-hong(
), ZHANG Jian, REN Yu-hong( )
)
Received:2022-06-25
Online:2023-04-26
Published:2023-05-16
HAN Hui, ZHANG Jian, REN Yu-hong. Molecular Modification of the Short-chain Dehydrogenase Lvchun and Its Application in the Synthesis of Chloromycetin[J]. Biotechnology Bulletin, 2023, 39(4): 81-92.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 大小 Size/bp |
|---|---|---|
| P1 | CGCGGATCCAAGGAGATATACATATGAAGATTGTCTTAGTTCTTTATGAT | 50 |
| P2 | CGGCTCGAGTTTCTTATCGTGTTTACCGTAAGCTTTAGTAACGTA | 45 |
| P3 | ACCACCACCACCACCACTGAAAGGAGATATACATATGGGCAGCA | 44 |
| P4 | GCTTTGTTAGCAGCCGGATCTCAGACCTGGCTGAAGCCG | 39 |
| P5 | GATCCGGCTGCTAACAAAGC | 20 |
| P6 | TCAGTGGTGGTGGTGGTGGT | 20 |
Table 1 Primer sequences of co-expression genes
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 大小 Size/bp |
|---|---|---|
| P1 | CGCGGATCCAAGGAGATATACATATGAAGATTGTCTTAGTTCTTTATGAT | 50 |
| P2 | CGGCTCGAGTTTCTTATCGTGTTTACCGTAAGCTTTAGTAACGTA | 45 |
| P3 | ACCACCACCACCACCACTGAAAGGAGATATACATATGGGCAGCA | 44 |
| P4 | GCTTTGTTAGCAGCCGGATCTCAGACCTGGCTGAAGCCG | 39 |
| P5 | GATCCGGCTGCTAACAAAGC | 20 |
| P6 | TCAGTGGTGGTGGTGGTGGT | 20 |
| 短肽名称 Short peptide name | 氨基酸序列 Amino acid sequence | 大小 Size/aa |
|---|---|---|
| L1 | GGGGSGGGGS | 10 |
| L2 | GGGGSGGGGSGGGGS | 15 |
| L3 | EEEEKKKKEEEEKKKK | 15 |
| L4 | KAKLKEEEERKQREEEERIKRLEELAKRKEEERK | 34 |
| L5 | EEEEKKKQQEEEAERLRRIQEEMEKERKRREEDEERRRKEEEERRMKLEMEAKRKQEEEERKKREDDEKRKKK | 51 |
Table 2 Amino acid sequences of linkers to short peptides
| 短肽名称 Short peptide name | 氨基酸序列 Amino acid sequence | 大小 Size/aa |
|---|---|---|
| L1 | GGGGSGGGGS | 10 |
| L2 | GGGGSGGGGSGGGGS | 15 |
| L3 | EEEEKKKKEEEEKKKK | 15 |
| L4 | KAKLKEEEERKQREEEERIKRLEELAKRKEEERK | 34 |
| L5 | EEEEKKKQQEEEAERLRRIQEEMEKERKRREEDEERRRKEEEERRMKLEMEAKRKQEEEERKKREDDEKRKKK | 51 |

Fig. 4 Molecular docking of Lvchun and substrate AHNA Yellow indicates coenzyme NADH, blue indicates amino acid residue, and green indicates substrate AHNA

Fig. 6 Expression and purification of mut-V112Y in E. coli BL21(DE3) M: Marker. 1: Crude enzyme solution. 2: Wear-off solution. 3: 50 mmol/L imidazole eluent. 4: 200 mmol/L imidazole eluent. 5: 500 mmol/L imidazole eluent
| 酶 Enzyme | 比活力Specific activity/(U·mg-1) | 米氏常数Km/(mmol·L-1) | 转换数kcat/(s-1) | 催化效率常数kcat/Km/(mmol·L-1·s-1) |
|---|---|---|---|---|
| Lvchun | 5.64 | 2.45 | 68.22 | 27.84 |
| mut-V112Y | 10.06 | 1.57 | 85.18 | 54.25 |
Table 3 Specific activity and kinetic parameters of Lvchun and mut-V112Y
| 酶 Enzyme | 比活力Specific activity/(U·mg-1) | 米氏常数Km/(mmol·L-1) | 转换数kcat/(s-1) | 催化效率常数kcat/Km/(mmol·L-1·s-1) |
|---|---|---|---|---|
| Lvchun | 5.64 | 2.45 | 68.22 | 27.84 |
| mut-V112Y | 10.06 | 1.57 | 85.18 | 54.25 |
| 重组菌株名称 Name of recombinant strain | 羰基还原酶 mut-V112Y | 甲酸脱氢酶 CbFDH | 融合蛋白 Fusion proteins | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | S | P | T | S | P | T | S | P | ||||
| mut-V112Y-CbFDH | + + + + | + + + + | - | + + + | + + + | - | - | - | - | |||
| CbFDH-mut-V112Y | + + + | + + + | - | + + | + + | - | - | - | - | |||
| F-L1-LY | - | - | - | - | - | - | + + + | + + | + + | |||
| F-L2-LY | - | - | - | - | - | - | + + + | + + | + + | |||
| F-L3-LY | - | - | - | - | - | - | + + | + + | - | |||
| F-L4-LY | - | - | - | - | - | - | + | + | - | |||
| F-L5-LY | - | - | - | - | - | - | + | + | - | |||
| LY-L1-F | - | - | - | - | - | - | + + + | + + | + + | |||
| LY-L2-F | - | - | - | - | - | - | + + + | + + | + + | |||
| LY-L3-F | - | - | - | - | - | - | + + + | + + + | + | |||
| LY-L4-F | - | - | - | - | - | - | + + + | + + + | + | |||
| LY-L5-F | - | - | - | - | - | - | + + + | + + + | + | |||
Table 4 Expressions of recombinant proteins in E. coli BL21(DE3)
| 重组菌株名称 Name of recombinant strain | 羰基还原酶 mut-V112Y | 甲酸脱氢酶 CbFDH | 融合蛋白 Fusion proteins | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | S | P | T | S | P | T | S | P | ||||
| mut-V112Y-CbFDH | + + + + | + + + + | - | + + + | + + + | - | - | - | - | |||
| CbFDH-mut-V112Y | + + + | + + + | - | + + | + + | - | - | - | - | |||
| F-L1-LY | - | - | - | - | - | - | + + + | + + | + + | |||
| F-L2-LY | - | - | - | - | - | - | + + + | + + | + + | |||
| F-L3-LY | - | - | - | - | - | - | + + | + + | - | |||
| F-L4-LY | - | - | - | - | - | - | + | + | - | |||
| F-L5-LY | - | - | - | - | - | - | + | + | - | |||
| LY-L1-F | - | - | - | - | - | - | + + + | + + | + + | |||
| LY-L2-F | - | - | - | - | - | - | + + + | + + | + + | |||
| LY-L3-F | - | - | - | - | - | - | + + + | + + + | + | |||
| LY-L4-F | - | - | - | - | - | - | + + + | + + + | + | |||
| LY-L5-F | - | - | - | - | - | - | + + + | + + + | + | |||
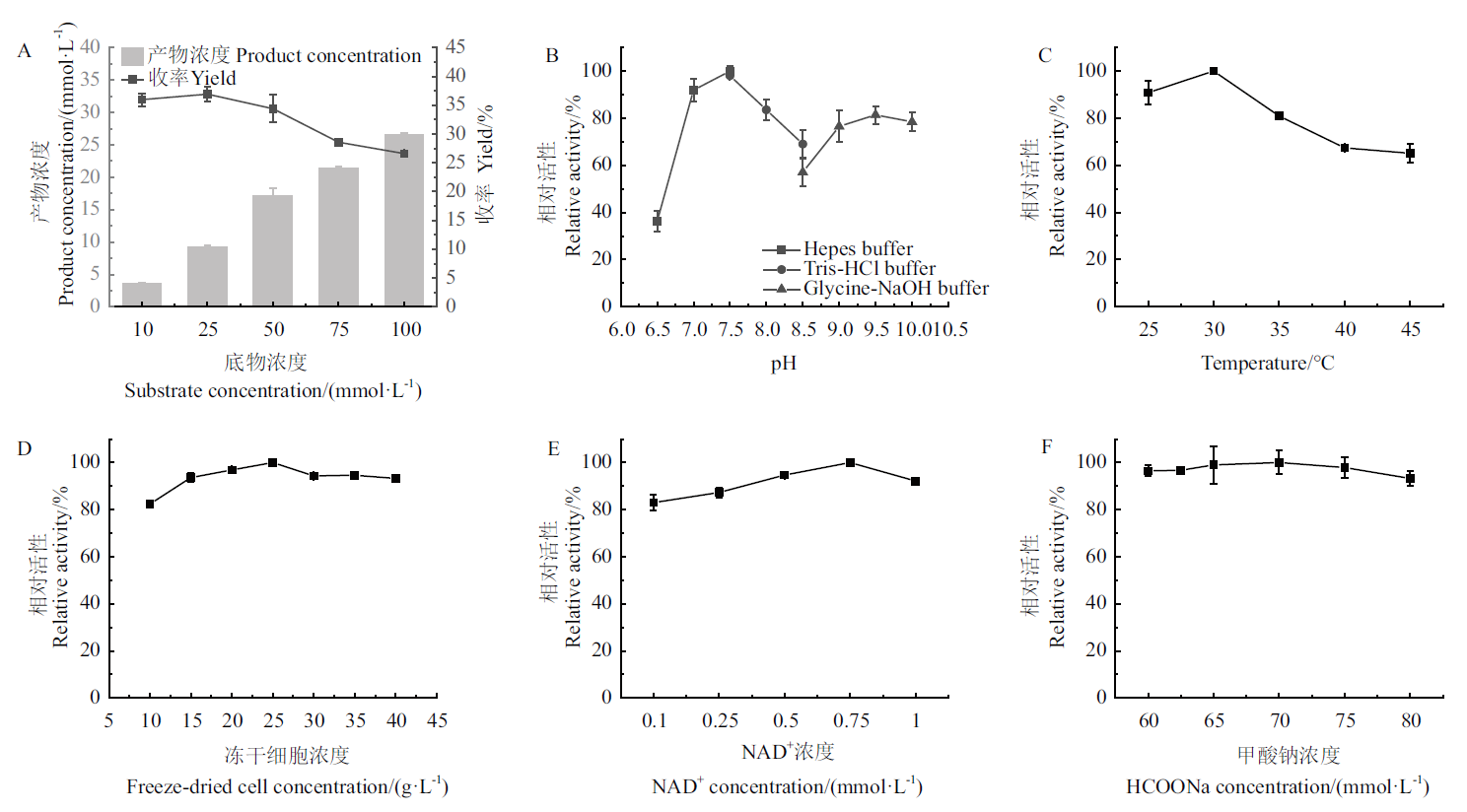
Fig. 9 Optimization of reaction conditions A: Substrate tolerance; B: pH; C: temperature; D: concentration of lyophilized cell; E: concentration of NAD+; F: concentration of HCOONa
| [1] | 贾美荣, 杨康辉, 江余祺, 等. 手性氯霉胺及其衍生物的应用进展[J]. 中国药物化学杂志, 2010, 20(6): 543-549, 551. |
| Jia MR, Yang KH, Jiang YQ, et al. The development of chiral chloramphenicol base and its derivatives[J]. Chin J Med Chem, 2010, 20(6): 543-549, 551. | |
| [2] |
Ha WZ, Shan ZX. An economic, practical access to enantiopure 1, 1'-bi-2-naphthols: enantioselective complexation of threo-(1S, 2S)-N-benzyl-N, N-dimethyl[1, 3-dihydroxy-1-(4'-nitrophenyl)]-2-propylammonium chloride[J]. Tetrahedron Asymmetry, 2006, 17(5): 854-859.
doi: 10.1016/j.tetasy.2006.02.015 URL |
| [3] |
Jiang B, Si YG. The first highly enantioselective alkynylation of chloral: a practical and efficient pathway to chiral trichloromethyl propargyl alcohols[J]. Adv Synth Catal, 2004, 346(6): 669-674.
doi: 10.1002/(ISSN)1615-4169 URL |
| [4] |
Tang HY, Zhao GF, Zhou ZH, et al. Chiral tertiary amine/L-proline cocatalyzed enantioselective morita-baylis-Hillman(MBH)reaction[J]. Eur J Org Chem, 2008, 2008(1): 126-135.
doi: 10.1002/(ISSN)1099-0690 URL |
| [5] |
Feng XC, Qiu GF, Liang SC, et al. Efficient synthesis of chiral β-and γ-N-tosylaminoalcohols from 1-aryl-2-aminopropane-1, 3-diols[J]. Russ J Org Chem, 2006, 42(4): 496-500.
doi: 10.1134/S107042800604004X URL |
| [6] |
Madesclaire M, Coudert P, Zaitsev VP, et al. Regioselectivity of the interaction of(1S, 2S)-2-amino- 1-(4-nitrophenyl)-1, 3-propanediol with some symmetrical ketones[J]. Chem Heterocycl Compd, 2004, 40(10): 1310-1314.
doi: 10.1007/s10593-005-0066-y URL |
| [7] |
Hazra B, Pore V, Dey S, et al. Bile acid amides derived from chiral amino alcohols: novel antimicrobials and antifungals[J]. Bioorg Med Chem Lett, 2004, 14(3): 773-777.
pmid: 14741287 |
| [8] | 杨尚金, 冯珂, 朱毅, 等. 一种氯霉素的合成方法: CN102399160A[P]. 2012-04-04. |
| Yang SJ, Feng K, Zhu Y, et al. Method for synthesizing chloramphenicol: CN102399160A[P]. 2012-04-04. | |
| [9] | 杨尚金, 冯珂, 朱毅, 等. 一种由硝基甲烷合成氯霉素的方法: CN102399164B[P]. 1970-01-17. |
| Yang SJ, Feng K, Zhu Y, et al. Method for synthesizing chloramphenicol from nitromethane: CN102399164B[P]. 1970-01-17. | |
| [10] | 谢新开, 黄晓飞, 杜好勉. 一种制备氯霉素的方法: CN111662937B[P]. 2021-09-03. |
| Xie XK, Huang XF, Du HM. Method for preparing chloramphenicol: CN111662937B[P]. 2021-09-03. | |
| [11] | 谢新开, 黄晓飞, 张金鑫, 等. 一种氯霉素类化合物的制备方法: CN106566851B[P]. 2020-11-10. |
| Xie XK, Huang XF, Zhang JX, et al. Preparation method of chloramphenicol compounds: CN106566851B[P]. 2020-11-10. | |
| [12] |
Sato R, Amao Y. Can formate dehydrogenase from Candida boidinii catalytically reduce carbon dioxide, bicarbonate, or carbonate to formate?[J]. New J Chem, 2020, 44(28): 11922-11926.
doi: 10.1039/D0NJ01183E URL |
| [13] |
Li J, Feng JH, Chen X, et al. Structure-guided directed evolution of a carbonyl reductase enables the stereoselective synthesis of(2S, 3S)-2, 2-disubstituted-3-hydroxycyclopentanones via desymmetric reduction[J]. Org Lett, 2020, 22(9): 3444-3448.
doi: 10.1021/acs.orglett.0c00892 URL |
| [14] |
Deng J, Yao ZQ, Chen KL, et al. Towards the computational design and engineering of enzyme enantioselectivity: a case study by a carbonyl reductase from Gluconobacter oxydans[J]. J Biotechnol, 2016, 217: 31-40.
doi: 10.1016/j.jbiotec.2015.11.003 URL |
| [15] |
Crowe J, Masone BS, Ribbe J. One-step purification of recombinant proteins with the 6xHis tag and Ni-NTA resin[J]. Mol Biotechnol, 1995, 4(3): 247-258.
pmid: 8680931 |
| [16] |
Tang W, Chen LL, Deng J, et al. Structure-guided evolution of carbonyl reductase for efficient biosynthesis of ethyl(R)-2-hydroxy-4-phenylbutyrate[J]. Catal Sci Technol, 2020, 10(22): 7512-7522.
doi: 10.1039/D0CY01411G URL |
| [17] |
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Anal Biochem, 1976, 72: 248-254.
pmid: 942051 |
| [18] |
Wang Y, Li LX, Ma CQ, et al. Engineering of cofactor regeneration enhances(2S, 3S)-2, 3-butanediol production from diacetyl[J]. Sci Rep, 2013, 3: 2643.
doi: 10.1038/srep02643 pmid: 24025762 |
| [19] |
Lineweaver H, Burk D. The determination of enzyme dissociation constants[J]. J Am Chem Soc, 1934, 56(3): 658-666.
doi: 10.1021/ja01318a036 URL |
| [20] |
Zhan JR, Shou C, Zheng YC, et al. Discovery and engineering of bacterial(-)-isopiperitenol dehydrogenases to enhance(-)-menthol precursor biosynthesis[J]. Adv Synth Catal, 2021, 363(16): 3973-3982.
doi: 10.1002/adsc.v363.16 URL |
| [21] |
Moreira C, Ramos MJ, Fernandes PA. Glutamine synthetase drugability beyond its active site: exploring oligomerization interfaces and pockets[J]. Molecules, 2016, 21(8): 1028.
doi: 10.3390/molecules21081028 URL |
| [22] |
Koumanov A, Benach J, Atrian S, et al. The catalytic mechanism of Drosophila alcohol dehydrogenase: evidence for a proton relay modulated by the coupled ionization of the active site Lysine/Tyrosine pair and a NAD+ ribose OH switch[J]. Proteins, 2003, 51(2): 289-298.
doi: 10.1002/prot.v51:2 URL |
| [23] |
Eixelsberger T, Woodley JM, Nidetzky B, et al. Scale-up and intensification of(S)-1-(2-chlorophenyl)ethanol bioproduction: economic evaluation of whole cell-catalyzed reduction of o-chloroacetophenone[J]. Biotechnol Bioeng, 2013, 110(8): 2311-2315.
doi: 10.1002/bit.24896 pmid: 23475609 |
| [24] |
He L, Ye WJ, Xie YY, et al. Efficient biocatalytic synthesis of(R)-2-chloro-1-(3, 4-difluorophenyl)ethanol by the short-chain dehydrogenase PpKR8 from Paraburkholderia phymatum STM815[J]. Org Process Res Dev, 2022, 26(2): 278-287.
doi: 10.1021/acs.oprd.1c00189 URL |
| [25] |
Kizaki N, Yasohara Y, Hasegawa J, et al. Synthesis of optically pure ethyl(S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehy-drogenase genes[J]. Appl Microbiol Biotechnol, 2001, 55(5): 590-595.
pmid: 11414326 |
| [26] |
Teng Y, Gu CL, Chen ZH, et al. Advances and applications of chiral resolution in pharmaceutical field[J]. Chirality, 2022, 34(8): 1094-1119.
doi: 10.1002/chir.23453 pmid: 35676772 |
| [1] | ZHONG Jian-feng, LI Xing-kui, XU Chong-xin, ZHANG Xiao, LIU Xian-jin. Biological Activity of Anti-idiotypic Single Chain Fragment Variable Antibody Against Cry1B by Site-directed Mutagenesis [J]. Biotechnology Bulletin, 2021, 37(10): 186-195. |
| [2] | HUA Chen, LI Xin-xin, TU Tao, YANG Hong, LUO Hui-ying, CHEN Jia-ming, YAO Bin, BAI Ying-guo, PENG Shu-chuan. Improving the Thermal Stability of Lactate Oxidase by ETSS [J]. Biotechnology Bulletin, 2018, 34(8): 144-150. |
| [3] | Liu Song, Lu Xinyao, Zhou Jingwen, Du Guocheng, Chen Jian. Research Advance on the Structure, Molecular Modification, and Fermentation of Lipoxygenases [J]. Biotechnology Bulletin, 2015, 31(12): 34-41. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||