








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (8): 275-287.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0110
Previous Articles Next Articles
MA Xiao-xiang1( ), MA Ze-yuan1, LIU Ya-yue1,2,3, ZHOU Long-jian1,2,3, HE Yi-fan4, ZHANG Yi1,2,3(
), MA Ze-yuan1, LIU Ya-yue1,2,3, ZHOU Long-jian1,2,3, HE Yi-fan4, ZHANG Yi1,2,3( )
)
Received:2024-01-31
Online:2024-08-26
Published:2024-07-02
Contact:
ZHANG Yi
E-mail:ma.xiao.xiang@163.com;hubeizhangyi@163.com
MA Xiao-xiang, MA Ze-yuan, LIU Ya-yue, ZHOU Long-jian, HE Yi-fan, ZHANG Yi. Effects of Simulated Mutational Biosynthetic Regulation on the Secondary Metabolites of Aspergillus terreus C23-3[J]. Biotechnology Bulletin, 2024, 40(8): 275-287.
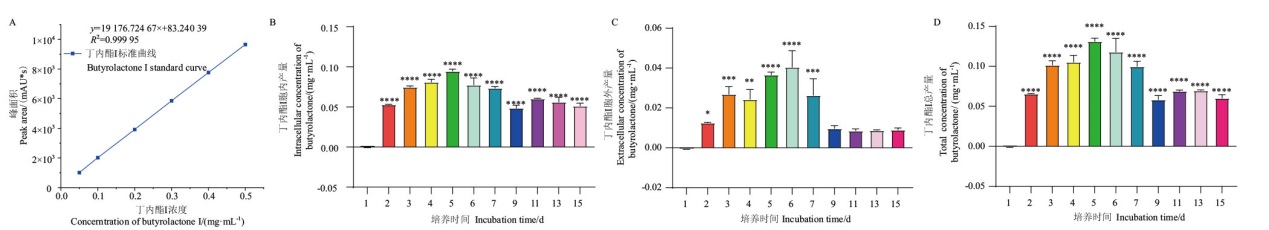
Fig. 2 Extracellular, intracellular, and total contents of butyrolactone I of strain C23-3 at different culture time A: Standard curve used to calculate the content of butyrolactone I in the extracts. B-D: Extracellular, intracellular, and total contents of butyrolactone I, the incubation time is calculated from the time after inoculation. *, **, ***, **** refers to the difference between the other days and the first day, which are P values < 0.05, 0.01, 0.001, and 0.000 1, respectively. Ordinary one-way ANOVA multiple comparisons were used for difference analysis between groups

Fig. 3 Butyrolactone I contents(A)and total extract mass(B)of strain C23-3 under the effect of different enzyme inhibitors or inducers ADA: Enzyme inhibitor 1,3-adamantane diacetic acid; NTSP: enzyme inhibitor N-(p-toluenesulfonyl)-L-phenylalanine; PS: enzyme inhibitor phenelzine sulfate; HT: inducer 3-hydroxy-L-tyrosine; DHPPA: inducer 3,4-dihydroxy-p-phenylpyruvate; Far: inducer farnesol; DMSO: the DMSO control group; blank: the blank group. The number after the abbreviations indicate the concentrations, and the unit is mmol/L. *, **, *** refer to the differences between the other groups and blank, which were P values < 0.05, 0.01, and 0.001, respectively. Ordinary one-way ANOVA multiple comparisons were used for difference analysis between groups
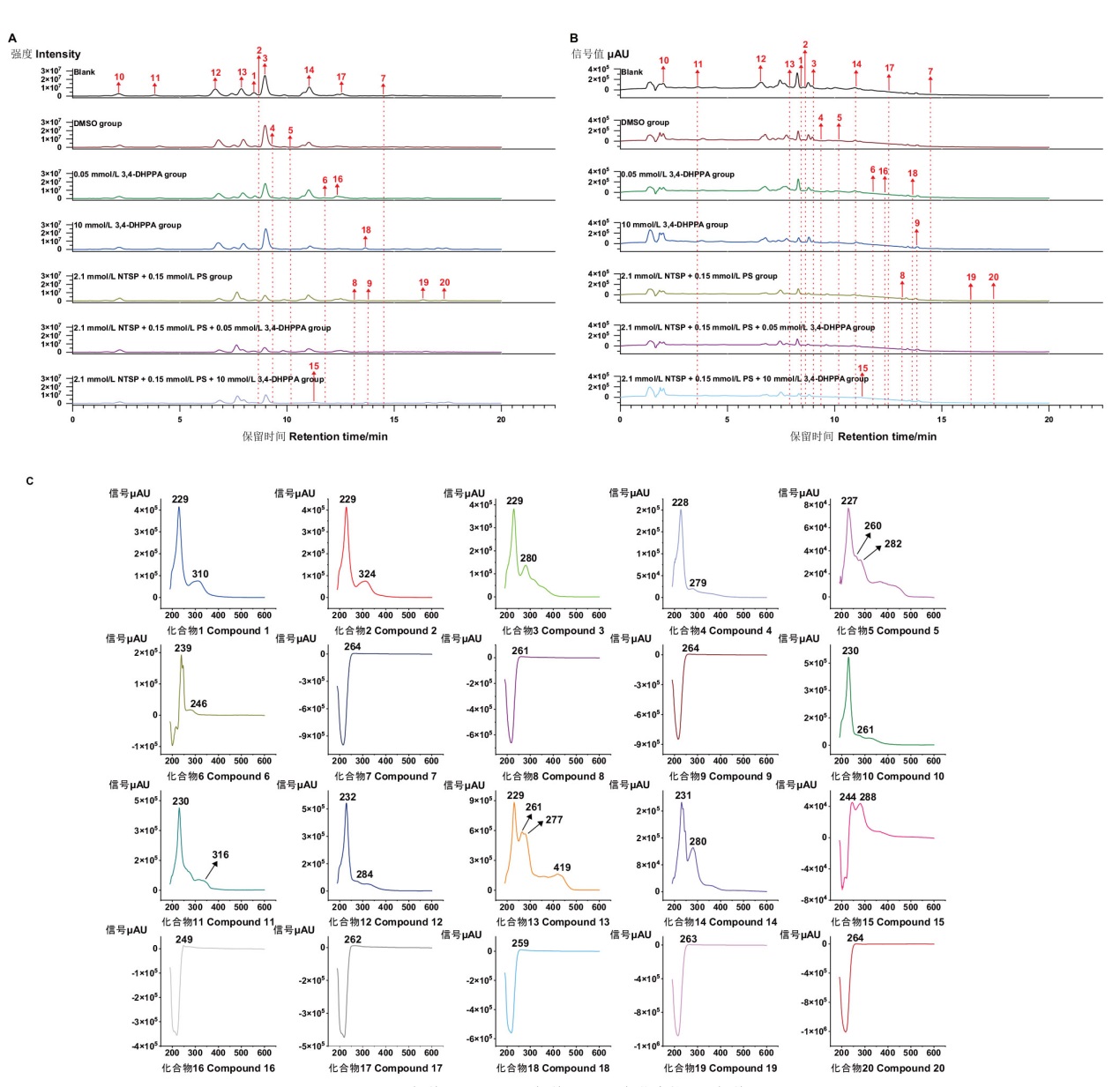
Fig. 4 LC-MS/MS-PDA chromatogram of Aspergillus terreus C23-3 extracts under different cultural conditions A: BPC chromatogram; B: PDA chromatogram; C: UV chromatogram of each compound
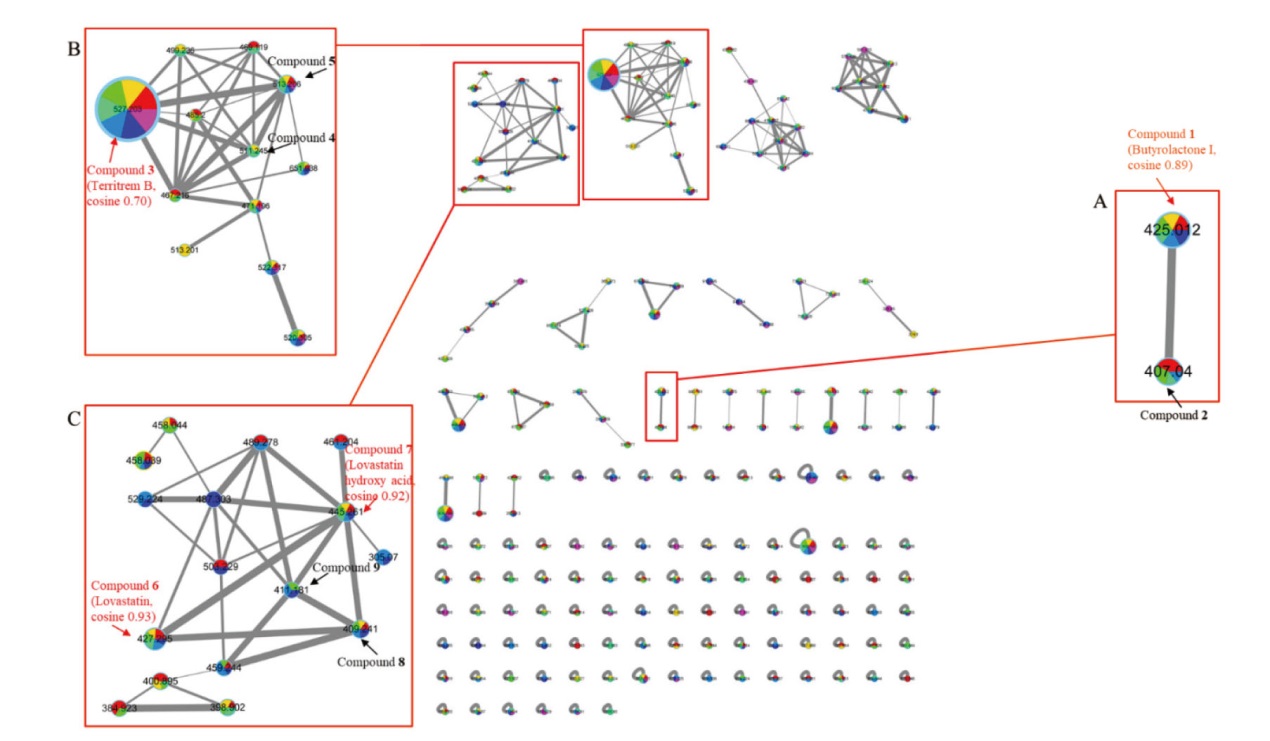
Fig. 5 Molecular network and its partial enlarged diagram of the metabolites of strain C23-3 under different cultural conditions based on MS2 relationships A-C:Enlarged images of metabolites clusters of butyrolactone I, territrem B and lovastatin congeners. Compounds 1, 3, 6, 7: Butyrolactone I,territrem B,lovastatin,and lovastatin hydroxy acid. Compounds 2, 4, 5, 8, 9:Possible derivatives of known compounds,i.e.,[M(butyrolactone I isomer)+ H]+,[M(butyrolactone I-2H)+ H]+,[M(butyrolactone I isomer + O)+ H]+,[M(butyrolactone I - H2O)+ H]+,[M(territrem B-O)+ H]+,[M(territrem B - CH2)+ H]+,[M(lovastatin - H2O)+ H]+,[M(lovastatin - O)+ H]+,and[M(lovastatin + 2H)+ H]+,respectively. The nodes in the molecular network are marked in 7 colors, , sequentially representing the 7 sample sources of compounds: blank group, control group with solvent DMSO; groups with the addition of 3,4-dihydroxyphenylpyruvate(0.05, 10 mmol/L), N-(p-toluenesulfonyl)-L-phenylalanine(2.1 mmol/L)+ phenelzine sulfate(0.15 mmol/L), 3,4-dihydroxyphenylpyruvate(0.05 mmol/L)+ N-(p-toluenesulfonyl)-L-phenylalanine(2.1 mmol/L)+ phenelzine sulfate(0.15 mmol/L), 3,4-dihydroxyphenylpyruvate(10 mmol/L)+N-(p-toluenesulfonyl)-L-phenylalanine(2.1 mmol/L)+ phenelzine sulfate(0.15 mmol/L)respectively
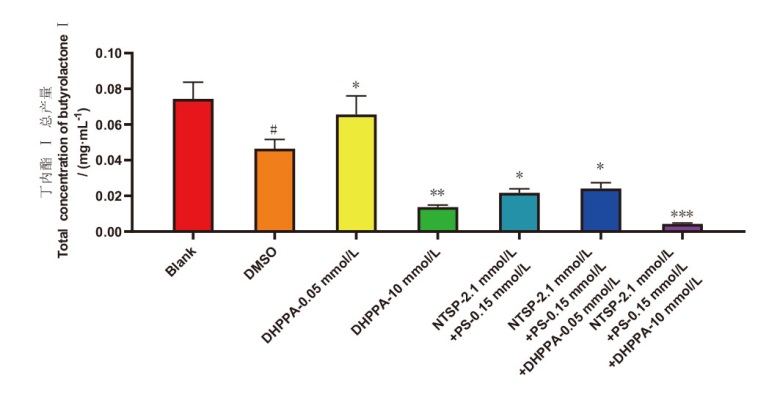
Fig. 7 Butyrolactone I content variation of strain C23-3 with the addition of enzyme inhibitors and inducers Blank: Blank group; DMSO: the solvent DMSO control group; NTSP: N-(p-toluenesulfonyl)-L-phenylalanine, PS: phenelzine sulfate. # P<0.05, DMSO control group vs blank group; * P<0.05, experimental group vs DMSO Control group; ** P<0.01, the sample group vs the control group, *** P<0.001, the sample group vs the control group
| 化合物序号Compound No. | 化合物注释 Compound note | 不同培养条件下的产物产量变化幅度 Changes in product yield under different cultivation conditions/% | |||||
|---|---|---|---|---|---|---|---|
| DMSO group | 0.05 mmol/L 3,4-DHPPA group | 10 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mmol/L PS group | 2.1 mmol/L NTSP + 0.15 mM PS + 0.05 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mM PS + 10 mmol/L 3,4-DHPPA group | ||
| 1 | 丁内酯I Butyrolactone I | ↓62.50 | ↓32.71 | ↓90.06 | ↓74.04 | ↓68.76 | ↓92.19 |
| 2 | 丁内酯I脱水产物 Butyrolactone I dehydrated product | ↓71.53 | ↓34.28 | ↓78.36 | ↓100.00 | ↓67.42 | ↓100.00 |
| 3 | 土震素B Territrem B | ↑4.40 | ↓26.45 | ↑9.58 | ↓73.15 | ↓78.94 | ↓57.47 |
| 4 | 土震素B脱氧产物 Territrem B deoxylation products | ↑65.52 | ↓100.00 | ↑38.07 | ↓80.67 | ↓84.58 | ↓100.00 |
| 5 | 土震素B降碳产物 Territrem B carbon reduction products | ↑50.57 | ↓56.52 | ↓33.36 | ↓100.00 | ↓100.00 | ↓86.79 |
| 土震素B类化合物总产量 Total production of territrem B compound | ↑5.12 | ↓27.10 | ↑9.36 | ↓73.42 | ↓79.14 | ↓57.95 | |
| 6 | 洛伐他汀Lovastatin | ↑6.60 | ↓28.74 | ↓29.12 | ↑86.14 | ↑46.16 | ↓100.00 |
| 7 | 洛伐他汀羟酸 Lovastatin hydroxy acid | ↓91.26 | ↓85.64 | ↓88.04 | ↓62.17 | ↓83.70 | ↓85.34 |
| 8 | 洛伐他汀脱水产物 Lovastatin dehydrated products | ↓34.19 | ↑10.05 | ↓57.14 | ↑50.70 | ↑15.97 | ↓98.61 |
| 9 | 洛伐他脱氧产物 Lovastatin deoxygenation products | ↓64.96 | ↓100.00 | ↓100.00 | ↑95.58 | ↑12.68 | ↓100.00 |
| 洛伐他汀类化合物总产量 Total production of lovastatin compounds | ↓43.48 | ↓48.02 | ↓60.20 | ↑16.07 | ↓15.59 | ↓93.41 | |
| 10 | N/A | ↓25.36 | ↓12.73 | ↓16.03 | ↓6.82 | ↓17.87 | ↓34.47 |
| 11 | Aspernolide C | ↑30.46 | ↓20.96 | ↑24.90 | ↓79.36 | ↓80.96 | ↓55.49 |
| 12 | Isochromophilone VI脱氢产物 Isochromophilone VI dehydrogenation product | ↓1.59 | ↓29.31 | ↓6.46 | ↓71.79 | ↓75.73 | ↓58.11 |
| 13 | Epi-aszonaleni A | ↑11.30 | ↓24.58 | ↓9.34 | ↓66.76 | ↓70.52 | ↓40.02 |
| 14 | N/A | ↓28.33 | ↑0.59 | ↓54.44 | ↓24.12 | ↓53.74 | ↓90.22 |
| 15 | Asterriquinone CT3 | ↓21.98 | ↓31.05 | ↑34.49 | ↓81.50 | ↓84.16 | ↓11.36 |
| 16 | Perinadine A | ↓43.49 | ↑38.06 | ↓66.44 | ↓15.69 | ↓46.11 | ↓88.34 |
| 17 | 12a-dehydroxyisoterreulactone A or Terreulactone C | ↓67.24 | ↓26.58 | ↓85.57 | ↑6.36 | ↓22.89 | ↓100.00 |
| 18 | Teraspiridole B | ↑8.11 | ↑14.79 | ↑148.27 | ↓13.45 | ↓38.63 | ↑94.77 |
| 19 | N/A | ↓26.37 | ↑1.29 | ↑13.45 | ↑50.55 | ↓3.21 | ↑19.44 |
| 20 | N/A | ↑111.26 | ↓81.63 | ↑1208.42 | ↑570.07 | ↑181.73 | ↑1399.99 |
Table 1 Variation of compounds 1-20's contents(integrated peak areas)under different cultural conditions compared to CK
| 化合物序号Compound No. | 化合物注释 Compound note | 不同培养条件下的产物产量变化幅度 Changes in product yield under different cultivation conditions/% | |||||
|---|---|---|---|---|---|---|---|
| DMSO group | 0.05 mmol/L 3,4-DHPPA group | 10 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mmol/L PS group | 2.1 mmol/L NTSP + 0.15 mM PS + 0.05 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mM PS + 10 mmol/L 3,4-DHPPA group | ||
| 1 | 丁内酯I Butyrolactone I | ↓62.50 | ↓32.71 | ↓90.06 | ↓74.04 | ↓68.76 | ↓92.19 |
| 2 | 丁内酯I脱水产物 Butyrolactone I dehydrated product | ↓71.53 | ↓34.28 | ↓78.36 | ↓100.00 | ↓67.42 | ↓100.00 |
| 3 | 土震素B Territrem B | ↑4.40 | ↓26.45 | ↑9.58 | ↓73.15 | ↓78.94 | ↓57.47 |
| 4 | 土震素B脱氧产物 Territrem B deoxylation products | ↑65.52 | ↓100.00 | ↑38.07 | ↓80.67 | ↓84.58 | ↓100.00 |
| 5 | 土震素B降碳产物 Territrem B carbon reduction products | ↑50.57 | ↓56.52 | ↓33.36 | ↓100.00 | ↓100.00 | ↓86.79 |
| 土震素B类化合物总产量 Total production of territrem B compound | ↑5.12 | ↓27.10 | ↑9.36 | ↓73.42 | ↓79.14 | ↓57.95 | |
| 6 | 洛伐他汀Lovastatin | ↑6.60 | ↓28.74 | ↓29.12 | ↑86.14 | ↑46.16 | ↓100.00 |
| 7 | 洛伐他汀羟酸 Lovastatin hydroxy acid | ↓91.26 | ↓85.64 | ↓88.04 | ↓62.17 | ↓83.70 | ↓85.34 |
| 8 | 洛伐他汀脱水产物 Lovastatin dehydrated products | ↓34.19 | ↑10.05 | ↓57.14 | ↑50.70 | ↑15.97 | ↓98.61 |
| 9 | 洛伐他脱氧产物 Lovastatin deoxygenation products | ↓64.96 | ↓100.00 | ↓100.00 | ↑95.58 | ↑12.68 | ↓100.00 |
| 洛伐他汀类化合物总产量 Total production of lovastatin compounds | ↓43.48 | ↓48.02 | ↓60.20 | ↑16.07 | ↓15.59 | ↓93.41 | |
| 10 | N/A | ↓25.36 | ↓12.73 | ↓16.03 | ↓6.82 | ↓17.87 | ↓34.47 |
| 11 | Aspernolide C | ↑30.46 | ↓20.96 | ↑24.90 | ↓79.36 | ↓80.96 | ↓55.49 |
| 12 | Isochromophilone VI脱氢产物 Isochromophilone VI dehydrogenation product | ↓1.59 | ↓29.31 | ↓6.46 | ↓71.79 | ↓75.73 | ↓58.11 |
| 13 | Epi-aszonaleni A | ↑11.30 | ↓24.58 | ↓9.34 | ↓66.76 | ↓70.52 | ↓40.02 |
| 14 | N/A | ↓28.33 | ↑0.59 | ↓54.44 | ↓24.12 | ↓53.74 | ↓90.22 |
| 15 | Asterriquinone CT3 | ↓21.98 | ↓31.05 | ↑34.49 | ↓81.50 | ↓84.16 | ↓11.36 |
| 16 | Perinadine A | ↓43.49 | ↑38.06 | ↓66.44 | ↓15.69 | ↓46.11 | ↓88.34 |
| 17 | 12a-dehydroxyisoterreulactone A or Terreulactone C | ↓67.24 | ↓26.58 | ↓85.57 | ↑6.36 | ↓22.89 | ↓100.00 |
| 18 | Teraspiridole B | ↑8.11 | ↑14.79 | ↑148.27 | ↓13.45 | ↓38.63 | ↑94.77 |
| 19 | N/A | ↓26.37 | ↑1.29 | ↑13.45 | ↑50.55 | ↓3.21 | ↑19.44 |
| 20 | N/A | ↑111.26 | ↓81.63 | ↑1208.42 | ↑570.07 | ↑181.73 | ↑1399.99 |
| [1] | 张偲, 张长生, 田新朋, 等. 中国海洋微生物多样性研究[J]. 中国科学院院刊, 2010, 25(6): 651-658. |
| Zhang S, Zhang CS, Tian XP, et al. The study of diversities of marine microbes in China[J]. Bull Chin Acad Sci, 2010, 25(6): 651-658. | |
| [2] |
马丽丽, 田新朋, 李桂菊, 等. 海洋微生物来源天然产物研究现状与态势[J]. 热带海洋学报, 2021, 40(5): 134-146.
doi: 10.11978/2020104 |
| Ma LL, Tian XP, Li GJ, et al. Research status and development trends of natural products from marine microorganisms[J]. J Trop Oceanogr, 2021, 40(5): 134-146. | |
| [3] |
Carroll AR, Copp BR, Davis RA, et al. Marine natural products[J]. Nat Prod Rep, 2020, 37(2): 175-223.
doi: 10.1039/c9np00069k pmid: 32025684 |
| [4] |
Weist S, Süssmuth RD. Mutational biosynthesis—a tool for the generation of structural diversity in the biosynthesis of antibiotics[J]. Appl Microbiol Biotechnol, 2005, 68(2): 141-150.
pmid: 15702315 |
| [5] | Rinehart KL Jr. Biosynthesis and mutasynthesis of aminocyclitol antibiotics[J]. Jpn J Antibiot, 1979, 32 Suppl: S32-S46. |
| [6] |
An X, Feng BM, Chen G, et al. Isolation and identification of phase I metabolites of butyrolactone I in rats[J]. Xenobiotica, 2017, 47(3): 236-244.
doi: 10.3109/00498254.2016.1172280 pmid: 27604497 |
| [7] |
Ibrahim SRM, Mohamed GA, Khedr AIM. γ-butyrolactones from Aspergillus species: structures, biosynthesis, and biological activities[J]. Nat Prod Commun, 2017, 12(5): 791-800.
pmid: 30496667 |
| [8] | Jiang M, Zhang HR. Engineering the shikimate pathway for biosynthesis of molecules with pharmaceutical activities in E. coli[J]. Curr Opin Biotechnol, 2016, 42: 1-6. |
| [9] | 江晶洁, 刘涛, 林双君. 基于莽草酸途径微生物合成芳香族化合物及其衍生物的研究进展[J]. 生命科学, 2019, 31(5): 430-448. |
| Jiang JJ, Liu T, Lin SJ. Research progress on the biosynthesis of aromatic compounds by microorganisms[J]. Chin Bull Life Sci, 2019, 31(5): 430-448. | |
| [10] | Pittard J, Yang J. Biosynthesis of the aromatic amino acids[J]. EcoSal Plus, 2008, 3(1): 10.1128/ecosalplus.3.6.1.8. |
| [11] |
Chen RD, Gao BQ, Liu X, et al. Molecular insights into the enzyme promiscuity of an aromatic prenyltransferase[J]. Nat Chem Biol, 2017, 13(2): 226-234.
doi: 10.1038/nchembio.2263 pmid: 27992881 |
| [12] | Hühner E, Öqvist K, Li SM. Design of α-keto carboxylic acid dimers by domain recombination of nonribosomal peptide synthetase(NRPS)-like enzymes[J]. Org Lett, 2019, 21(2): 498-502. |
| [13] |
van Dijk JWA, Wang CCC. Expanding the chemical space of nonribosomal peptide synthetase-like enzymes by domain and tailoring enzyme recombination[J]. Org Lett, 2018, 20(17): 5082-5085.
doi: 10.1021/acs.orglett.8b01581 pmid: 30106305 |
| [14] | Zheng YY, Ma ZL, Wu JS, et al. Induction of secondary metabolite biosynthesis by deleting the histone deacetylase HdaA in the marine-derived fungus Aspergillus terreus RA2905[J]. J Fungi, 2022, 8(10): 1024. |
| [15] | Fan H, Wei X, Si-Tu MX, et al. γ-aromatic butenolides of microbial source-A review of their structures, biological activities and biosynthesis[J]. Chem Biodivers, 2022, 19(6): e202200208. |
| [16] | 蔡彩虹, 郑浩, 盖翠娟, 等. 一株藤壶内生真菌的次生代谢产物[J]. 中国药科大学学报, 2023, 54(1): 62-67. |
| Cai CH, Zheng H, Gai CJ, et al. Secondary metabolites of the endophytic fungus Aspergillus sp. Dq-25 from barnacle[J]. J China Pharm Univ, 2023, 54(1): 62-67. | |
| [17] | 刘冰, 唐祉娟, 陈宁, 等. 海洋来源曲霉丁内酯类次级代谢产物研究进展[J]. 中国海洋药物, 2021, 40(5): 59-70. |
| Liu B, Tang ZJ, Chen N, et al. Research progress of butyrolactones isolated from marine-derived Aspergillus sp[J]. Chin J Mar Drugs, 2021, 40(5): 59-70. | |
| [18] | 杨静明, 杨文聪, 刘亚月, 等. 化学诱导对一株海洋来源土曲霉C23-3次生代谢产物及其生物活性的影响[J]. 微生物学通报, 2019, 46(3): 441-452. |
| Yang JM, Yang WC, Liu YY, et al. Influence of chemical induction on the secondary metabolites and biological activities of a marine-derived fungal strain Aspergillus terreus C23-3[J]. Microbiol China, 2019, 46(3): 441-452. | |
| [19] |
马小翔, 刘亚月, 聂影影, 等. 基于质谱的分子网络分析化学调控对土曲霉C23-3次生代谢产物及生物活性的影响[J]. 生物技术通报, 2021, 37(8): 95-110.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-1398 |
| Ma XX, Liu YY, Nie YY, et al. LC-MS/MS based molecular network analysis of the effects of chemical regulation on the secondary metabolites and biological activities of a fungal strain Aspergillus terreus C23-3[J]. Biotechnol Bull, 2021, 37(8): 95-110. | |
| [20] |
Andrews PR, Cain EN, Rizzardo E, et al. Rearrangement of chorismate to prephenate. Use of chorismate mutase inhibitors to define the transition state structure[J]. Biochemistry, 1977, 16(22): 4848-4852.
pmid: 911795 |
| [21] |
Smith GD, Roberts DV, Daday A. Affinity chromatography and inhibition of chorismate mutase-prephenate dehydrogenase by derivatives of phenylalanine and tyrosine[J]. Biochem J, 1977, 165(1): 121-126.
pmid: 889568 |
| [22] |
Dyck LE, Dewar KM. Inhibition of aromatic L-amino acid decarboxylase and tyrosine aminotransferase by the monoamine oxidase inhibitor phenelzine[J]. J Neurochem, 1986, 46(6): 1899-1903.
pmid: 2871132 |
| [23] | Guo CJ, Knox BP, Sanchez JF, et al. Application of an efficient gene targeting system linking secondary metabolites to their biosynthetic genes in Aspergillus terreus[J]. Org Lett, 2013, 15(14): 3562-3565. |
| [24] |
Palonen EK, Neffling MR, Raina S, et al. Butyrolactone I quantification from lovastatin producing Aspergillus terreus using tandem mass spectrometry-evidence of signalling functions[J]. Microorganisms, 2014, 2(2): 111-127.
doi: 10.3390/microorganisms2020111 pmid: 27682234 |
| [25] | Bok JW, Keller NP. LaeA, a regulator of secondary metabolism in Aspergillus spp[J]. Eukaryot Cell, 2004, 3(2): 527-535. |
| [26] | Palonen EK, Raina S, Brandt A, et al. Transcriptomic complexity of Aspergillus terreus velvet gene family under the influence of butyrolactone I[J]. Microorganisms, 2017, 5(1): 12. |
| [27] | Raina S, De Vizio D, Palonen EK, et al. Is quorum sensing involved in lovastatin production in the filamentous fungus Aspergillus terreus?[J]. Process Biochem, 2012, 47(5): 843-852. |
| [28] |
奚萌宇, 胡逸灵, 顾玉城, 等. 基因组挖掘指导天然药物分子的发现[J]. 合成生物学, 2024, 5(3): 447-473.
doi: 10.12211/2096-8280.2023-086 |
| Xi MY, Hu YL, Gu YC, et al. Genome mining-directed discovery for natural medicinal products[J]. Synth Biol J, 2024, 5(3): 447-473. | |
| [29] | Wang XH, Lin MY, Xu D, et al. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides[J]. Molecules, 2017, 22(12): 2069. |
| [30] | Wang XH, Li YY, Zhang XP, et al. Structural diversity and biological activities of the cyclodipeptides from fungi[J]. Molecules, 2017, 22(12): 2026. |
| [1] | SUN Shu-fang, LUO Yong-li, LI Chun-hui, JIN Min, XU Qian. Determination of Lignin Monomer Crosslinking Structures in Wheat Stems by UPLC-MS/MS [J]. Biotechnology Bulletin, 2022, 38(10): 66-72. |
| [2] | MA Xiao-xiang, LIU Ya-yue, NIE Ying-ying, LI Yan-mei, WANG Yuan, XUE Xin-yi, HONG Peng-zhi, ZHANG Yi. LC-MS/MS Based Molecular Network Analysis of the Effects of Chemical Regulation on the Secondary Metabolites and Biological Activities of a Fungal Strain Aspergillus terreus C23-3 [J]. Biotechnology Bulletin, 2021, 37(8): 95-110. |
| [3] | LIU Shan, YE Wei, ZHU Mu-zi, LI Sai-ni, DENG Zhang-shuang, ZHANG Wei-min. Cloning,Expression and Characterization of a Novel Acyltransferase GPAT [J]. Biotechnology Bulletin, 2021, 37(11): 257-266. |
| [4] | MENG Li-ná, PENG Chun-ying, LI Tie-dong, LI Bo-sheng. Proteomic ánálysis of Spiruliná plátensis in Response to ársenic Stress [J]. Biotechnology Bulletin, 2020, 36(4): 107-116. |
| [5] | GAN Chong-kun, ZHOU Hui-wen, CHEN Rong-fa, FAN Ye-geng, QIU Li-hang, HUANG Xing, LI Yang-rui, LU Xing-gao, WU Jian-ming. Application of Chemical Regulating Technology in Sugarcane Production [J]. Biotechnology Bulletin, 2019, 35(2): 163-170. |
| [6] | HUANG Zi-lei, ZHANG Wei-min, YE Wei, LI Sai-ni, LI Hao-hua, ZHU Mu-zi. Cloning and Identification of Gene Promoter for Gliotoxin Biosynthesis from Deep-Sea-Derived Fungus Dichotomomyces cejpii [J]. Biotechnology Bulletin, 2018, 34(4): 144-150. |
| [7] | Li Haohua, Chen Yuchan, Wang Lei, Zhang Weimin. Study on Antimicrobial and Antitumor Activities of Marine-derived Fungus Penicillium herquei FS83 [J]. Biotechnology Bulletin, 2013, 0(1): 151-155. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||