








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (7): 117-124.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1107
Previous Articles Next Articles
TIAN Xing-miao1( ), WANG Jian-lin1, GUO Lei2, SI Duo-duo1, GONG Zhen-xing1, LI Ji-dong1(
), WANG Jian-lin1, GUO Lei2, SI Duo-duo1, GONG Zhen-xing1, LI Ji-dong1( )
)
Received:2023-11-23
Online:2024-07-26
Published:2024-07-30
Contact:
LI Ji-dong
E-mail:1076049403@qq.com;lijidongi@foxmail.com
TIAN Xing-miao, WANG Jian-lin, GUO Lei, SI Duo-duo, GONG Zhen-xing, LI Ji-dong. Establishment of Dual LFD-RPA Rapid Detection Method for Mycoplasma gallisepticum and Mycoplasma synoviae[J]. Biotechnology Bulletin, 2024, 40(7): 117-124.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 片段大小 Product length/bp |
|---|---|---|
| PCR-MG-F | GTGTTCTCGGGTGCTATTGCTC | 819 |
| PCR-MG-R | TGGGGGCTGATTCATTCCTTGA | |
| PCR-MS-F | TAGACCCTTAATCGAATTTTG | 506 |
| PCR-MS-R | CTTTTGAGTATTCAGCATCTTC |
Table 1 PCR primers
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 片段大小 Product length/bp |
|---|---|---|
| PCR-MG-F | GTGTTCTCGGGTGCTATTGCTC | 819 |
| PCR-MG-R | TGGGGGCTGATTCATTCCTTGA | |
| PCR-MS-F | TAGACCCTTAATCGAATTTTG | 506 |
| PCR-MS-R | CTTTTGAGTATTCAGCATCTTC |
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 片段大小Product length/bp |
|---|---|---|
| RPA-MG-F1 | AGATTACCTCCGAACCCTGATTTTATCCAGT | 362 |
| RPA-MG-R1 | AATTCTTTGTTGGGGATTAGGACCAAATTGC | |
| RPA-MG-F2 | CCTCCGAACCCTGATTTTATCCAGTAGTG | 202 |
| RPA-MG-R2 | GTTGGCTCAATCGCTTCTGTTTTATTTTGT | |
| RPA-MG-F3 | ATTTGGTCCTAATCCCCAACAAAGAATTAACCC | 301 |
| RPA-MG-R3 | ATTAAACCCACCTCCAGCTTTATTTCCCATCG | |
| RPA-MS-F1 | AAAATTAAATTACTATTAGCAGCTAGTGCAGT | 130 |
| RPA-MS-R1 | GGTTTTGAGGATTATCAGTATTTGGGTTTCCAG | |
| RPA-MS-F2 | AATTAAATTACTATTAGCAGCTAGTGCAGT | 244 |
| RPA-MS-R2 | TTCTGTTTTAGCAGCCTCTACAGGGTCAAC | |
| RPA-MS-F3 | CCTGAACCAACACCTGGAAACCCAAATACTG | 167 |
| RPA-MS-R3 | TTAGCTTCTGTTTTAGCAGCCTCTACAGGGTC |
Table 2 Basic RPA primers
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 片段大小Product length/bp |
|---|---|---|
| RPA-MG-F1 | AGATTACCTCCGAACCCTGATTTTATCCAGT | 362 |
| RPA-MG-R1 | AATTCTTTGTTGGGGATTAGGACCAAATTGC | |
| RPA-MG-F2 | CCTCCGAACCCTGATTTTATCCAGTAGTG | 202 |
| RPA-MG-R2 | GTTGGCTCAATCGCTTCTGTTTTATTTTGT | |
| RPA-MG-F3 | ATTTGGTCCTAATCCCCAACAAAGAATTAACCC | 301 |
| RPA-MG-R3 | ATTAAACCCACCTCCAGCTTTATTTCCCATCG | |
| RPA-MS-F1 | AAAATTAAATTACTATTAGCAGCTAGTGCAGT | 130 |
| RPA-MS-R1 | GGTTTTGAGGATTATCAGTATTTGGGTTTCCAG | |
| RPA-MS-F2 | AATTAAATTACTATTAGCAGCTAGTGCAGT | 244 |
| RPA-MS-R2 | TTCTGTTTTAGCAGCCTCTACAGGGTCAAC | |
| RPA-MS-F3 | CCTGAACCAACACCTGGAAACCCAAATACTG | 167 |
| RPA-MS-R3 | TTAGCTTCTGTTTTAGCAGCCTCTACAGGGTC |
| RPA-MG-F2/R2/μL | RPA-MS-F1/R1/μL |
|---|---|
| 0.0 | 2.0 |
| 0.2 | 1.8 |
| 0.4 | 1.6 |
| 0.6 | 1.4 |
| 0.8 | 1.2 |
| 1.0 | 1.0 |
| 1.2 | 0.8 |
| 1.4 | 0.6 |
| 1.6 | 0.4 |
| 1.8 | 0.2 |
| 2.0 | 0.0 |
Table 3 Optimization of primer ratios for dual basic RPA
| RPA-MG-F2/R2/μL | RPA-MS-F1/R1/μL |
|---|---|
| 0.0 | 2.0 |
| 0.2 | 1.8 |
| 0.4 | 1.6 |
| 0.6 | 1.4 |
| 0.8 | 1.2 |
| 1.0 | 1.0 |
| 1.2 | 0.8 |
| 1.4 | 0.6 |
| 1.6 | 0.4 |
| 1.8 | 0.2 |
| 2.0 | 0.0 |
| 引物/探针名称 Primer/Probe name | 引物序列 Primer sequence(5'-3') |
|---|---|
| MG-LFD-RPA-F | CCTCCGAACCCTGATTTTATCCAGTAGTG |
| MG-LFD-RPA-R | TAMRA-GTTGGCTCAATCGCTTCTGTTTTATTTTGT |
| MG-LFD-RPA-Probe | Digoxin-AGAATGATGATCCAAGAACGTGAAGAACA CCA/idSp/AAGATGGTTGAATCC-C3-spacer |
| MS-LFD-RPA-F | AAAATTAAATTACTATTAGCAGCTAGTGCAGT |
| MS-LFD-RPA-R | Biotin-GGTTTTGAGGATTATCAGTATTTGGGTTTCCAG |
| MS-LFD-RPA-Probe | FAM-TGCTCCTGCTGTTATAGCAATTTCATGTGGTGA /idSp/CAAACTCCAGCACCT-C3-spacer |
Table 4 LFD-RPA primers and probes
| 引物/探针名称 Primer/Probe name | 引物序列 Primer sequence(5'-3') |
|---|---|
| MG-LFD-RPA-F | CCTCCGAACCCTGATTTTATCCAGTAGTG |
| MG-LFD-RPA-R | TAMRA-GTTGGCTCAATCGCTTCTGTTTTATTTTGT |
| MG-LFD-RPA-Probe | Digoxin-AGAATGATGATCCAAGAACGTGAAGAACA CCA/idSp/AAGATGGTTGAATCC-C3-spacer |
| MS-LFD-RPA-F | AAAATTAAATTACTATTAGCAGCTAGTGCAGT |
| MS-LFD-RPA-R | Biotin-GGTTTTGAGGATTATCAGTATTTGGGTTTCCAG |
| MS-LFD-RPA-Probe | FAM-TGCTCCTGCTGTTATAGCAATTTCATGTGGTGA /idSp/CAAACTCCAGCACCT-C3-spacer |
| 试剂Reagent | 体积Volume/μL |
|---|---|
| MG-LFD-RPA-F | 0.6 |
| MG-LFD-RPA-R | 0.6 |
| MG-LFD-RPA-Probe | 0.3 |
| MS-LFD-RPA-F | 1.4 |
| MS-LFD-RPA-R | 1.4 |
| MS-LFD-RPA-Probe | 0.3 |
| A buffer | 29.4 |
| Template | 3(MG)+3(MS) |
| ddH2O | 7.5 |
| B buffer | 2.5 |
Table 5 LFD-RPA reaction system(50 μL)
| 试剂Reagent | 体积Volume/μL |
|---|---|
| MG-LFD-RPA-F | 0.6 |
| MG-LFD-RPA-R | 0.6 |
| MG-LFD-RPA-Probe | 0.3 |
| MS-LFD-RPA-F | 1.4 |
| MS-LFD-RPA-R | 1.4 |
| MS-LFD-RPA-Probe | 0.3 |
| A buffer | 29.4 |
| Template | 3(MG)+3(MS) |
| ddH2O | 7.5 |
| B buffer | 2.5 |
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 片段大小 Product length/bp |
|---|---|---|
| MG 16S rRNA F | GAGCTAATCTGTAAAGTTGGTC | 183 |
| MG 16S rRNA R | GCTTCCTTGCGGTTAGCAAC | |
| MS 16S rRNA F | GAGAAGCAAAATAGTGATATCA | 211 |
| MS 16S rRNA R | CAGTCGTCTCCGAAGTTAACAA |
Table 6 OIE PCR primers
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 片段大小 Product length/bp |
|---|---|---|
| MG 16S rRNA F | GAGCTAATCTGTAAAGTTGGTC | 183 |
| MG 16S rRNA R | GCTTCCTTGCGGTTAGCAAC | |
| MS 16S rRNA F | GAGAAGCAAAATAGTGATATCA | 211 |
| MS 16S rRNA R | CAGTCGTCTCCGAAGTTAACAA |
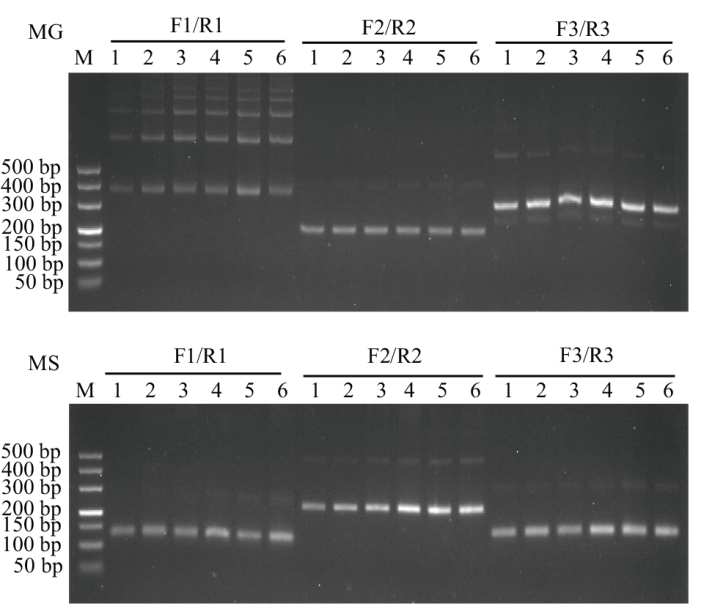
Fig. 3 Primer selection of MG and MS basic RPA F1/R1, F2/R2, F3/R3: The first, second, and third primer pair, respectively;M: DL500 DNA marker; 1: 5 min; 2: 10 min; 3: 15 min; 4: 20 min; 5: 25 min; 6: 30 min
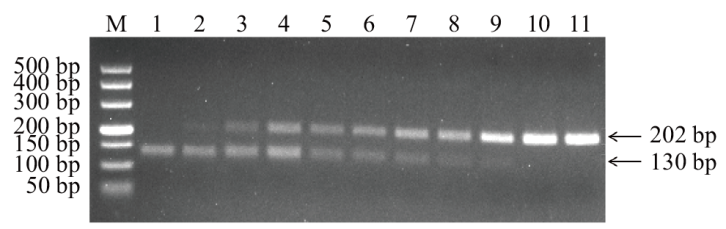
Fig. 4 Optimization of optimal primer ratios for MG and MS dual basic RPA M: DL500 DNA marker. 1-11: The ratios of RPA-MG-F2/R2 and RPA-MS-F1/R1 are 0∶2, 0.2∶1.8, 0.4∶1.6, 0.6∶1.4, 0.8∶1.2, 1∶1, 1.2∶0.8, 1.4∶0.6, 1.6∶0.4, 1.8∶0.2, 2∶0, respectively
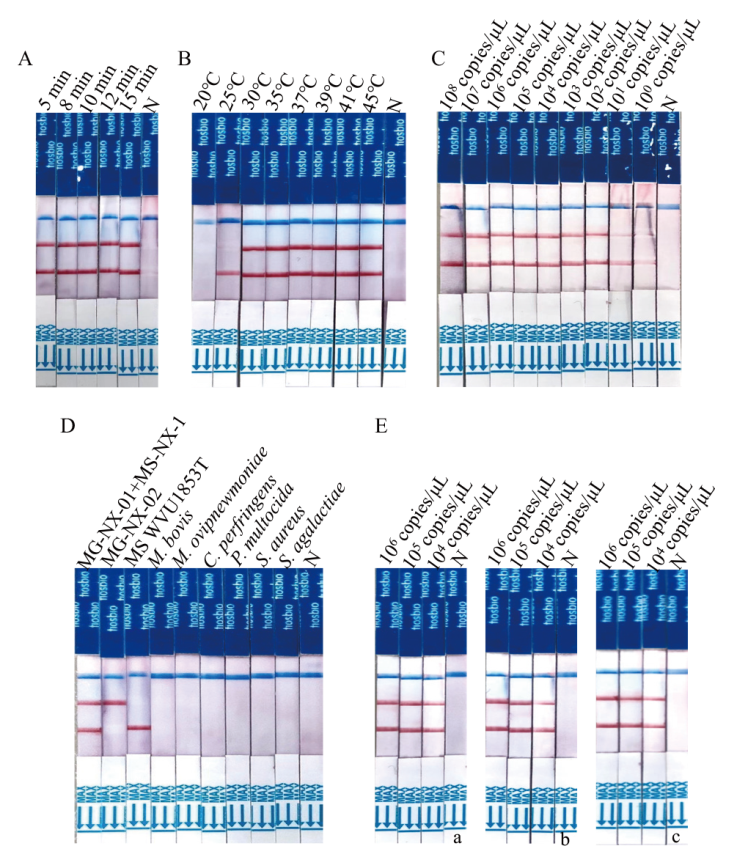
Fig. 5 Establishment of dual LFD-RPA method with MG and MS A: Reaction time optimization. B: Reaction temperature optimization. C: Sensitivity test. D: Specificity test. E: Repeatability test(a: The first repeat; b: the second repetition; c: the third repetition). N: Negative control
| 样品名称 Sample name | LFD-RPA检测结果 LFD-RPA test result | OIE PCR检测结果 OIE PCR test result |
|---|---|---|
| MG阳性 | 37 | 33 |
| MG阴性 | 83 | 87 |
| MS阳性 | 52 | 50 |
| MS阴性 | 68 | 70 |
Table 7 Comparative analysis of MG and MS dual LFD-RPA and OIE PCR detection methods
| 样品名称 Sample name | LFD-RPA检测结果 LFD-RPA test result | OIE PCR检测结果 OIE PCR test result |
|---|---|---|
| MG阳性 | 37 | 33 |
| MG阴性 | 83 | 87 |
| MS阳性 | 52 | 50 |
| MS阴性 | 68 | 70 |
| [1] | Saif YM. 禽病学[M]. 12版. 北京: 中国农业出版社, 2012. |
| Saif YM. Diseases of poultry[M]. 12th ed. Beijing: China Agriculture Press, 2012. | |
| [2] | 赵宇馨, 祁晶晶, 尚原冰, 等. 鸡毒支原体磷酸甘油酸变位酶的亚细胞定位及免疫原性分析[J/OL]. 中国动物传染病学报, 2023. DOI: 10.19958/j.cnki.cn31-2031/s.20230322.002. |
| Zhao YX, Qi JJ, Shang YB, et al. Subcellular localization and immunogenicity of phosphoglycerate mutase(PGM)in Mycoplasma gallisepticum[J/OL]. Chin J Anim Infect Dis, 2023. DOI: 10.19958/j.cnki.cn31-2031/s.20230322.002. | |
| [3] |
Winner F, Rosengarten R, Citti C. In vitro cell invasion of Mycoplasma gallisepticum[J]. Infect Immun, 2000, 68(7): 4238-4244.
doi: 10.1128/IAI.68.7.4238-4244.2000 pmid: 10858241 |
| [4] | Xu B, Chen X, Lu FY, et al. Comparative genomics of Mycoplasma synoviae and new targets for molecular diagnostics[J]. Front Vet Sci, 2021, 8: 640067. |
| [5] | Dufour-Gesbert F, Dheilly A, Marois C, et al. Epidemiological study on Mycoplasma synoviae infection in layers[J]. Vet Microbiol, 2006, 114(1/2): 148-154. |
| [6] | Wei XN, Zhong Q, Wang DA, et al. Epidemiological investigations and multilocus sequence typing of Mycoplasma gallisepticum collected in China[J]. Poult Sci, 2023, 102(11): 102930. |
| [7] | 向勇, 曹伟胜. 禽支原体检测技术进展[J]. 养禽与禽病防治, 2018(4): 2-9. |
| Xiang Y, Cao WS. Progress in detection technology of Mycoplasma avians[J]. Poult Husb Dis Contr, 2018(4): 2-9. | |
| [8] |
王艳丰, 张丁华, 朱金凤. 鸡滑液囊支原体病流行现状及防控技术研究进展[J]. 中国畜牧兽医, 2021, 48(8): 3038-3049.
doi: 10.16431/j.cnki.1671-7236.2021.08.037 |
| Wang YF, Zhang DH, Zhu JF. Research progress on epidemic status and prevention and control technology of Mycoplasma synoviae infection[J]. China Anim Husb Vet Med, 2021, 48(8): 3038-3049. | |
| [9] |
Bencina D. Haemagglutinins of pathogenic avian mycoplasmas[J]. Avian Pathol, 2002, 31(6): 535-547.
pmid: 12593736 |
| [10] | Piepenburg O, Williams CH, Stemple DL, et al. DNA detection using recombination proteins[J]. PLoS Biol, 2006, 4(7): e204. |
| [11] | 于灵芝, 陶凌云, 魏晓锋. 可视化核酸检测技术RPA-LFD的研究和应用进展[J]. 实验动物与比较医学, 2021, 41(6): 547-553. |
| Yu LZ, Tao LY, Wei XF. Research and application progress in visualized RPA-LFD nucleic acid detection technology[J]. Lab Anim Comp Med, 2021, 41(6): 547-553. | |
| [12] | 韩勇, 刘玉洁, 马珍驰, 等. 鸡毒支原体和鸡滑液囊支原体TaqMan-MGB双重荧光定量PCR检测方法的建立和应用[J]. 中国家禽, 2022, 44(12): 59-64. |
| Han Y, Liu YJ, Ma ZC, et al. Establishment and application on TaqMan-MGB dual fluorescent quantitative PCR assay for Mycoplasma gallisepticum and Mycoplasma synoviae[J]. China Poult, 2022, 44(12): 59-64. | |
| [13] | 胡欣月. 鸡毒支原体与鸡滑液囊支原体双重荧光定量PCR检测方法的建立与初步应用[D]. 武汉: 华中农业大学, 2022. |
| Hu XY. Establishment and application of real-time duplex fluorescence PCR assay for detection of Mycoplasma gallisepticum and Mycoplasma synoviae[D]. Wuhan: Huazhong Agricultural University, 2022. | |
| [14] | 朱小甫, 吴旭锦, 李怡婷, 等. 鸡毒支原体与滑液囊支原体双重PCR检测方法的建立与应用[J]. 动物医学进展, 2021, 42(6): 31-35. |
| Zhu XF, Wu XJ, Li YT, et al. Establishment and application of duplex PCR method for detection of Mycoplasma gallisepticum and Mycoplasma synovialum[J]. Prog Vet Med, 2021, 42(6): 31-35. | |
| [15] | Xia WL, Chen K, Liu WS, et al. Rapid and visual detection of Mycoplasma synoviae by recombinase-aided amplification assay combined with a lateral flow dipstick[J]. Poult Sci, 2022, 101(7): 101860. |
| [16] | 陆璐, 邱万伟, 丁巧玲, 等. 基于核酸等温扩增的侧流层析试纸条在病原微生物检测中的研究进展[J]. 食品安全质量检测学报, 2022, 13(5): 1462-1470. |
| Lu L, Qiu WW, Ding QL, et al. Research progress of side flow chromatography strip based on isothermal amplification of nucleic acid in the detection of pathogenic microorganisms[J]. J Food Saf Qual, 2022, 13(5): 1462-1470. | |
| [17] | 袁京磊, 李娜. 可视化检测方法在食品安全检测中的应用研究进展[J]. 食品安全导刊, 2021(32): 189-192. |
| Yuan JL, Li N. Progress on application of visual detection methods on food safety detection[J]. China Food Saf Mag, 2021(32): 189-192. | |
| [18] | Zhao GM, Wang HM, Hou PL, et al. Rapid visual detection of Mycobacterium avium subsp. paratuberculosis by recombinase polymerase amplification combined with a lateral flow dipstick[J]. J Vet Sci, 2018, 19(2): 242. |
| [19] | Onchan W, Ritbamrung O, Changtor P, et al. Sensitive and rapid detection of Babesia species in dogs by recombinase polymerase amplification with lateral flow dipstick(RPA-LFD)[J]. Sci Rep, 2022, 12(1): 20560. |
| [20] | Wang L, Wang Y, Wang F, et al. Development and application of rapid clinical visualization molecular diagnostic technology for Cryptococcus neoformans/C. gattii based on recombinase polymerase amplification combined with a lateral flow strip[J]. Front Cell Infect Microbiol, 2022, 11: 803798. |
| [21] |
张闪闪, 何斌, 李书光, 等. 可视化RPA-LFD技术快速检测猪链球菌[J]. 畜牧兽医学报, 2022, 53(2): 538-547.
doi: 10.11843/j.issn.0366-6964.2022.02.020 |
| Zhang SS, He B, Li SG, et al. Rapid detection of Streptococcus suis with visual RPA-LFD technology[J]. Acta Vet Zootechnica Sin, 2022, 53(2): 538-547. |
| [1] | LUO Xue-cong, AN Meng-nan, WU Yuan-hua, XIA Zi-hao. Applications of Recombinase Polymerase Amplification in Plant Virus Detection [J]. Biotechnology Bulletin, 2022, 38(2): 269-280. |
| [2] | FANG Yuan, WU Xun, LIN Yu, WANG Hai-yan, WU Hui-ping, JU Yu-liang. Duplex-RPA Detection for Bursaphelenchus xylophilus and Bursaphelenchus mucronatus [J]. Biotechnology Bulletin, 2021, 37(7): 183-190. |
| [3] | DOU Wen, LI You, LU Xin-yu, QIAN Xue-mei, SHEN Dan-yu. Construction of a RPA Detection Method for Transgenic Soybean and Its Application [J]. Biotechnology Bulletin, 2019, 35(5): 170-175. |
| [4] | JING Zhi-gang, DONG Hao, DI Dong-dong, TIAN Li-li, FAN Wei-xing. Research Progress on Recombinase Polymerase Amplification [J]. Biotechnology Bulletin, 2016, 32(6): 47-53. |
| [5] | CHENG Die, CHAI Fang-chao, CAI Yi, ZHOU Qian-jin, CHEN Jiong. Visual Detection of Vibrio harveyi Based on Loop-mediated Isothermal Amplification Combined with a Lateral Flow Dipstick [J]. Biotechnology Bulletin, 2016, 32(6): 60-68. |
| [6] | Wang Yaohuan, Wang Ruina, Zhou Qianjin, Chen Jiong. Rapid Detection of Vibrio vulnificus by Loop-mediated Isothermal Amplification Combined with Lateral Flow Dipstick Assay [J]. Biotechnology Bulletin, 2014, 30(6): 81-87. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||