








生物技术通报 ›› 2021, Vol. 37 ›› Issue (2): 138-148.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0529
吴蓉1( ), 曹佳睿1, 曹君1, 刘飞翔1, 杨猛1, 苏二正1,2(
), 曹佳睿1, 曹君1, 刘飞翔1, 杨猛1, 苏二正1,2( )
)
收稿日期:2020-05-25
出版日期:2021-02-26
发布日期:2021-02-26
作者简介:吴蓉,女,博士研究生,研究方向:酶与生物催化;E-mail: 基金资助:
WU Rong1( ), CAO Jia-rui1, CAO Jun1, LIU Fei-xiang1, YANG Meng1, SU Er-zheng1,2(
), CAO Jia-rui1, CAO Jun1, LIU Fei-xiang1, YANG Meng1, SU Er-zheng1,2( )
)
Received:2020-05-25
Published:2021-02-26
Online:2021-02-26
摘要:
旨在利用大肠杆菌实现南极假丝酵母脂肪酶B(CALB)基因的高效可溶性表达,并降低生产成本。构建带有不同信号肽的CALB基因表达质粒,转化至不同大肠杆菌宿主中,在摇瓶中进行基础培养基、诱导条件、培养基组成成分和进程曲线的优化。结果显示,带有PelB信号肽的重组菌pET25b-CALB-1/Rosetta(DE3)在20℃下使用0.5%(W/V)乳糖在TY培养基中诱导表达效果最好,优化后的合成培养基成分为1.75%(W/V)山梨醇、2.25%(W/V)鱼蛋白胨、1%(W/V)安琪酵母提取物、0.75%(W/V)Na2HPO4。在摇瓶中诱导60 h后,CALB酶活力最高达到35.67 U/mL,相比于初始发酵酶活力提高了17.77倍,是目前大肠杆菌生产CALB的最高水平。成功地构建了大肠杆菌表达系统,经过系统优化后,CALB的高水平可溶性表达得以实现。
吴蓉, 曹佳睿, 曹君, 刘飞翔, 杨猛, 苏二正. 南极假丝酵母脂肪酶B基因在大肠杆菌中的表达和发酵优化[J]. 生物技术通报, 2021, 37(2): 138-148.
WU Rong, CAO Jia-rui, CAO Jun, LIU Fei-xiang, YANG Meng, SU Er-zheng. Expression and Fermentation Optimization of Candida antarctica Lipase B in Escherichia coli[J]. Biotechnology Bulletin, 2021, 37(2): 138-148.
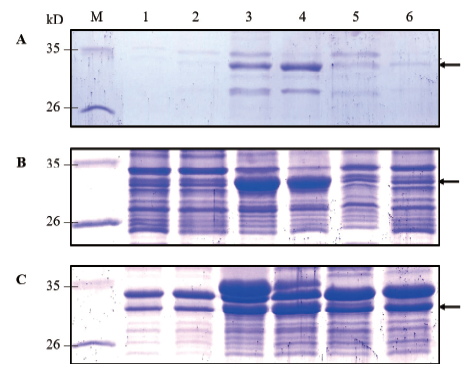
图2 重组菌的SDS-PAGE分析图 A:发酵液;B:菌体上清液;C:菌体沉淀;M:标准蛋白Marker;1:pET25b/BL21(DE3)空载体对照;2:pET25b/Rosetta(DE3)空载体对照;3:诱导后的重组菌pET25b-CALB-1/BL(DE3);4:诱导后的重组菌pET25b-CALB-1/Rosetta(DE3);5:诱导后的重组菌pET25b-CALB-2/BL(DE3);6:诱导后的重组菌pET25b-CALB-2/Rosetta(DE3)
| Carbon sources (1.0%,W/V) | OD600 | Enzyme activity(U/mL) | Specific activity(U/mg) |
|---|---|---|---|
| Sucrose | 4.72±0.12 | 1.59±0.02 | 2.83±0.05 |
| Glucose | 2.84±0.09 | 0.78±0.01 | 5.77±0.07 |
| Glycerol | 10.10±0.24 | 5.93±0.06 | 5.84±0.08 |
| Sorbitol | 13.20±0.21 | 12.12±0.11 | 7.61±0.08 |
| Starch | 8.82±0.17 | 2.45±0.02 | 2.58±0.04 |
| Dextrin | 12.70±0.43 | 7.43±0.07 | 6.07±0.05 |
表1 不同碳源对重组大肠杆菌的生长和CALB表达的影响
| Carbon sources (1.0%,W/V) | OD600 | Enzyme activity(U/mL) | Specific activity(U/mg) |
|---|---|---|---|
| Sucrose | 4.72±0.12 | 1.59±0.02 | 2.83±0.05 |
| Glucose | 2.84±0.09 | 0.78±0.01 | 5.77±0.07 |
| Glycerol | 10.10±0.24 | 5.93±0.06 | 5.84±0.08 |
| Sorbitol | 13.20±0.21 | 12.12±0.11 | 7.61±0.08 |
| Starch | 8.82±0.17 | 2.45±0.02 | 2.58±0.04 |
| Dextrin | 12.70±0.43 | 7.43±0.07 | 6.07±0.05 |
| Nitrogen sources (2.0%,W/V) | OD600 | Enzyme activity (U/mL) | Specific activity (U/mg) |
|---|---|---|---|
| Tryptone | 12.69±0.32 | 19.41±0.20 | 12.15±0.09 |
| SM | 15.16±0.37 | 9.50±0.07 | 4.22±0.06 |
| BBP | 12.88±0.25 | 20.99±0.19 | 6.66±0.07 |
| FP | 16.14±0.40 | 22.70±0.17 | 6.97±0.06 |
| PP | 12.55±0.11 | 1.32±0.03 | 0.66±00.05 |
| CSL | 11.59±0.31 | 5.32±0.06 | 2.48±0.02 |
表2 不同氮源对重组大肠杆菌的生长和CALB表达的影响
| Nitrogen sources (2.0%,W/V) | OD600 | Enzyme activity (U/mL) | Specific activity (U/mg) |
|---|---|---|---|
| Tryptone | 12.69±0.32 | 19.41±0.20 | 12.15±0.09 |
| SM | 15.16±0.37 | 9.50±0.07 | 4.22±0.06 |
| BBP | 12.88±0.25 | 20.99±0.19 | 6.66±0.07 |
| FP | 16.14±0.40 | 22.70±0.17 | 6.97±0.06 |
| PP | 12.55±0.11 | 1.32±0.03 | 0.66±00.05 |
| CSL | 11.59±0.31 | 5.32±0.06 | 2.48±0.02 |
| [1] |
Liu ZQ, Zheng XB, Zhang SP, et al. Cloning, expression and characterization of a lipase gene from the Candida antarctica ZJB09193 and its application in biosynjournal of vitamin A esters[J]. Microbiological Research, 2012,167(8):452-460.
doi: 10.1016/j.micres.2011.12.004 URL pmid: 22281522 |
| [2] |
Gonçalves FD, Gonçalves SA, Zanella GC. Lipases:sources, immobilization methods, and industrial applications[J]. Applied Microbiology and Biotechnology, 2019,103(18):7399-7423.
doi: 10.1007/s00253-019-10027-6 URL pmid: 31375880 |
| [3] | Anderson EM, Larsson KM, Kirk O. One biocatalyst-many applications:The use of Candida Antarctica B-lipase in organic synjournal[J]. Biocatalysis, 1998,16(3):181-204. |
| [4] |
Hama S, Noda H, Kondo A. How lipase technology contributes to evolution of biodiesel production using multiple feedstocks[J]. Current Opinion in Biotechnology, 2018,50:57-64.
doi: 10.1016/j.copbio.2017.11.001 URL pmid: 29172108 |
| [5] |
Aguieiras EC, Cavalcanti-Oliveira ED, Freire DM. Current status and new developments of biodiesel production using fungal lipases[J]. Fuel, 2015,159:52-67.
doi: 10.1016/j.fuel.2015.06.064 URL |
| [6] |
Debuissy T, Pollet E, Avérous L. Enzymatic synjournal of a bio-based copolyester from poly(butylene succinate)and poly((R)-3-hydroxybutyrate):study of reaction parameters on the transesterification rate[J]. Biomacromolecules, 2016,17(12):4054-4063.
doi: 10.1021/acs.biomac.6b01494 URL pmid: 27936726 |
| [7] |
Nguyen HD, Löf D, Hvilsted S, et al. Highly branched bio-based unsaturated polyesters by enzymatic polymerization[J]. Polymers, 2016,8(10):1-12.
doi: 10.3390/polym8010001 URL |
| [8] |
Cai C, Gao Y, Liu Y, et al. Immobilization of Candida antarctica lipase B onto SBA-15 and their application in glycerolysis for diacylglycerols synjournal[J]. Food Chemistry, 2016,212:205-212.
doi: 10.1016/j.foodchem.2016.05.167 URL pmid: 27374525 |
| [9] |
Du Y, Gao J, Kong W, et al. Enzymatic synjournal of glycerol carbonate using a lipase immobilized on magnetic organosilica nanoflowers as a catalyst[J]. Acs Omega, 2018,3(6):6642-6650.
doi: 10.1021/acsomega.8b00746 URL pmid: 30023956 |
| [10] |
Melo AD, Silva FF, Dos Santos J, et al. Synjournal of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads[J]. Molecules, 2017,22(12):2165.
doi: 10.3390/molecules22122165 URL |
| [11] |
Chen Y, Liu J, Geng S, et al. Lipase-catalyzed synjournal mechanism of tri-acetylated phloridzin and its antiproliferative activity against HepG2 cancer cells[J]. Food Chemistry, 2019,277:186-194.
doi: 10.1016/j.foodchem.2018.10.111 URL pmid: 30502134 |
| [12] |
Diaz-Vidal T, Armenta-Perez VP, Rosales-Rivera LC, et al. Cross-linked enzyme aggregates of recombinant Candida antarctica lipase B for the efficient synjournal of olvanil, a nonpungent capsaicin analogue[J]. Biotechnology Progress, 2019,35(4):e2807.
doi: 10.1002/btpr.2807 URL pmid: 30883025 |
| [13] |
Lund IT, Bøckmann PL, Jacobsen EE. Highly enantioselective CALB-catalyzed kinetic resolution of building blocks for β-blocker atenolol[J]. Tetrahedron, 2016,72(46):7288-7292.
doi: 10.1016/j.tet.2016.02.018 URL |
| [14] |
Vásquez-Garay F, Teixeira Mendona R, Peretti SW. Chemoenzymatic lignin valorization:Production of epoxidized pre-polymers using Candida antarctica lipase B[J]. Enzyme and Microbial Technology, 2018,112:6-13.
doi: 10.1016/j.enzmictec.2018.01.007 URL pmid: 29499782 |
| [15] |
Mouad AM, Taupin D, Lehr L, et al. Aminolysis of linoleic and salicylic acid derivatives with Candida antarctica lipase B:a solvent-free process to obtain amphiphilic amides for cosmetic application[J]. Journal of Molecular Catalysis B Enzymatic, 2016,126:64-68.
doi: 10.1016/j.molcatb.2016.01.002 URL |
| [16] | 李燕妮, 衣婷婷, 李龙森. 南极假丝酵母产脂肪酶在15L发酵罐中培养条件的研究[J]. 化学与生物工程, 2007,7:43-44, 48. |
| Li YN, Yi TT, Li LS. Studies on fermentation process for lipase production by Candida antarctica in 15 L fermentor[J]. Chemistry and Bioengineering, 2007,7:43-44, 48. | |
| [17] |
Larsen MW, Bornscheuer UT, Hult K. Expression of Candida antarctica lipase B in Pichia pastoris and various Escherichia coli systems[J]. Protein Expression and Purification, 2008,62(1):90-97.
doi: 10.1016/j.pep.2008.07.012 URL pmid: 18725303 |
| [18] |
Liu D, Schmid RD, Rusnak M. Functional expression of Candida antarcticalipase B in the Escherichia coli cytoplasm-a screening system for a frequently used biocatalyst[J]. Applied Microbiology and Biotechnology, 2006,72(5):1024-1032.
URL pmid: 16703321 |
| [19] |
Ujiie A, Nakano H, Iwasaki Y. Extracellular production of Pseudozyma(Candida)antarctica lipase B with genuine primary sequence in recombinant Escherichia coli[J]. Journal of Bioscience and Bioengineering, 2016,121(3):303.
doi: 10.1016/j.jbiosc.2015.07.001 URL pmid: 26272415 |
| [20] |
Kim SK, Park YC, Lee HH, et al. Simple amino acid tags improve both expression and secretion of Candida antarctica lipase B in recombinant Escherichia coli[J]. Biotechnology and Bioengineering, 2015,112(2):346-355.
doi: 10.1002/bit.25361 URL pmid: 25182473 |
| [21] |
Smith MT, Hawes AK, Shrestha P, et al. Alternative fermentation conditions for improved Escherichia coli-based cell-free protein synjournal for proteins requiring supplemental components for proper synjournal[J]. Process Biochemistry, 2014,49(2):217-222.
doi: 10.1016/j.procbio.2013.10.012 URL |
| [22] |
Johar SS, Talbert JN. Strep-tag II fusion technology for the modification and immobilization of lipase B from Candida antarctica(CALB)[J]. Journal of Genetic Engineering and Biotechnology, 2017,15(2):359-367.
doi: 10.1016/j.jgeb.2017.06.011 URL pmid: 30647674 |
| [23] |
Robert JM, Lattari FS, Machado AC, et al. Production of recombinant lipase B from Candida antarctica in Pichia pastoris under control of the promoter PGK using crude glycerol from biodiesel production as carbon source[J]. Biochemical Engineering Journal, 2017,118:123-131.
doi: 10.1016/j.bej.2016.11.018 URL |
| [24] | Jayachandran C, Athiyaman BP, Sankaranarayanan M. Formate co-feeding improved Candida antarctica lipase B activity in Pichia pastoris[J]. Research Journal of Biotechnology, 2017,12(12):29-36. |
| [25] |
Robert JM, Betancur MO, Machado ACO, et al. Increase of Candida antarctica lipase B production under PGK promoter in Pichia pastoris:effect of multicopies[J]. Brazilian Journal of Microbiology, 2019,50(2):405-413.
doi: 10.1007/s42770-019-00056-8 URL pmid: 30827000 |
| [26] |
Han SY, Pan ZY, Huang DF, et al. Highly efficient synjournal of ethyl hexanoate catalyzed by CALB-displaying Saccharomyces cerevisiae whole-cells in non-aqueous phase[J]. Journal of Molecular Catalysis B:Enzymatic, 2009,59(1-3):168-172.
doi: 10.1016/j.molcatb.2009.02.007 URL |
| [27] |
Haegh I, Patkar S, Halkier T, et al. Two lipases from Candida antarctica:cloning and expression in Aspergillus oryzae[J]. Canadian Journal of Botany, 1995,73(S1):869-875.
doi: 10.1139/b95-333 URL |
| [28] |
Hayat SMG, Farahani N, Golichenari B, et al. Recombinant protein expression in Escherichia coli(E. coli):what we need to know[J]. Current Pharmaceutical Design, 2018,24(6):718-725.
doi: 10.2174/1381612824666180131121940 URL pmid: 29384059 |
| [29] |
Rosano GL, Ceccarelli EA. Recombinant protein expression in Escherichia coli:advances and challenges[J]. Frontiers in Microbiology, 2014,5:172-189.
doi: 10.3389/fmicb.2014.00172 URL pmid: 24860555 |
| [30] |
Blank K, Morfill J, Gumpp H, et al. Functional expression of Candida antarctica lipase B in Eschericha coli[J]. Journal of Biotechnology, 2006,125(4):474-483.
URL pmid: 16713003 |
| [31] |
Ghahremanifard P, Rezaeinezhad N, Rigi G, et al. Designing a novel signal sequence for efficient secretion of Candida antarctica lipase B in E. coli:The molecular dynamic simulation, codon optimization and statistical analysis approach[J]. International Journal of Biological Macromolecules, 2018,119:291-305.
doi: 10.1016/j.ijbiomac.2018.07.150 URL pmid: 30055273 |
| [32] |
Zhou X, Han Y, Lv Z, et al. Simultaneously achieve soluble expression and biomimetic immobilization of Candida antarctica lipase B by introducing polyamine tags[J]. Journal of Biotechnology, 2017,249:1-9.
doi: 10.1016/j.jbiotec.2017.03.015 URL pmid: 28323015 |
| [33] | 苏二正, 吴向萍, 高蓓, 等. 短小芽孢杆菌脂肪酶基因的克隆、表达及酶学性质研究[J]. 生物技术通报, 2014,4:132-138. |
| Su EZ, Wu XP, Gao B, et al. Gene cloning, expression and characterization of the lipase from Bacillus pumilus S6[J]. Biotechnology Bulletin, 2014,4:132-138. | |
| [34] |
Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of potein-dye binding[J]. Analytical Biochemistry, 1976,72(S1-2):248-254.
doi: 10.1016/0003-2697(76)90527-3 URL |
| [35] |
张宇萌, 童梅, 陆小冬, 等. 提高大肠杆菌可溶性重组蛋白表达产率的研究进展[J]. 中国生物工程杂志, 2016,36(5):118-124.
doi: 10.13523/j.cb.20160517 URL |
| Zhang YM, Tong M, Lu XD, et al. Advances in promoting soluble expression of recombinant protein in Escherichia coli[J]. China Biotechnology, 2016,36(5):118-124. | |
| [36] | 罗曼, 关怡新, 姚善泾. 大肠杆菌二硫键形成蛋白A(DsbA)研究进展[J]. 生物工程学报, 2007,1:7-15. |
| Luo M, Guan YX, Yao SJ. Study on disulfide bond formation protein A in Escherichia coli[J]. Chinese Journal of Biotechnology, 2007,1:7-15. | |
| [37] | 刘开泉. 利用原核系统表达富含二硫键蛋白质的探索与改进[D]. 泰安:山东农业大学, 2011. |
| Liu KQ. Exploring and impraving the approach for the prokaryotic expression of multi-disulfide bonds protein[D]. Taian:Shandong Agricultural University, 2011. | |
| [38] | 王重喜, 姜雅杰, 陈小颖, 等. Atsttrin在大肠杆菌中的表达和条件优化研究[J]. 药物生物技术, 2019,26(4):289-295. |
| Wang CX, Jiang YJ, Chen XY, et al. Optimization of gene engineering expression of Atsttrin in Escherichia Coli[J]. Pharmaceutical Biotechnology, 2019,26(4):289-295. | |
| [39] |
Ma XQ, Su EZ, Zhu Y, et al. High-level expression of glutaryl-7-aminocephalosporanic acid acylase from Pseudomonas diminuta NK703 in Escherichia coli by combined optimization strategies[J]. Journal of Biotechnology, 2013,168(4):607-615.
doi: 10.1016/j.jbiotec.2013.08.024 URL pmid: 23994688 |
| [40] |
Su LQ, Huang Y, Wu J. Enhanced production of recombinant Escherichia coli glutamate decarboxylase through optimization of induction strategy and addition of pyridoxine[J]. Bioresource Technology, 2015,198:63-69.
doi: 10.1016/j.biortech.2015.08.153 URL pmid: 26364229 |
| [41] | Nielsen TB, Ishii M, Kirk O. Lipases A and B from the yeast Candida antarctica[M]// Biotechnological Applications of Cold-Adapted Organisms, 1999: 49-61. |
| [42] | 黎继烈, 崔培梧, 鲁耀邦, 等. Penicillium sp. 1523产柚苷酶摇瓶发酵培养基优化[J]. 食品科学, 2011,9:158-162. |
| Li JL, Cui PW, Lu YB, et al. Optimization of shaking-flask fermentation medium for naringinase production by Penicillium sp. 1523[J]. Food Science, 2011,9:158-162. |
| [1] | 程亚楠, 张文聪, 周圆, 孙雪, 李玉, 李庆刚. 乳酸乳球菌生产2'-岩藻糖基乳糖的途径构建及发酵培养基优化[J]. 生物技术通报, 2023, 39(9): 84-96. |
| [2] | 陈彩萍, 任昊, 龙腾飞, 何冰, 鲁兆祥, 孙坚. 大肠杆菌Nissle 1917对炎症性肠病治疗作用的研究进展[J]. 生物技术通报, 2023, 39(6): 109-118. |
| [3] | 唐瑞琪, 赵心清, 朱笃, 汪涯. 大肠杆菌对木质纤维素水解液抑制物的胁迫耐受性[J]. 生物技术通报, 2023, 39(11): 205-216. |
| [4] | 车永梅, 刘广超, 郭艳苹, 叶青, 赵方贵, 刘新. 一种耐盐复合菌剂的制备和促生作用研究[J]. 生物技术通报, 2023, 39(11): 217-225. |
| [5] | 李仁瀚, 张乐乐, 刘春立, 刘秀霞, 白仲虎, 杨艳坤, 李业. 基于紫色杆菌素生物合成途径的L-色氨酸生物传感器的构建[J]. 生物技术通报, 2023, 39(10): 80-92. |
| [6] | 高伟欣, 黄火清, 赵晶, 张鑫, 杨宁, 杨浩萌. 应用于基因编辑的核糖核蛋白复合体的构建与活性验证[J]. 生物技术通报, 2022, 38(8): 60-68. |
| [7] | 孙曼銮, 葛赛, 卜佳, 朱壮彦. 大肠杆菌核糖核酸酶调控机制研究[J]. 生物技术通报, 2022, 38(3): 234-245. |
| [8] | 李晓芳, 刘慧燕, 潘琳, 艾治宇, 李一鸣, 张恒, 方海田. 常温常压等离子体诱变选育高产L-异亮氨酸大肠杆菌[J]. 生物技术通报, 2022, 38(1): 150-156. |
| [9] | 张瑶心, 王亮节, 郑文, 徐汉琴, 郑恋, 钟静. 产几丁质酶的无色杆菌ZWW8的发酵产酶及酶学性质研究[J]. 生物技术通报, 2021, 37(4): 96-106. |
| [10] | 王凯凯, 王晓璐, 苏小运, 张杰. 大肠杆菌双质粒CRISPR-Cas9系统的优化及应用[J]. 生物技术通报, 2021, 37(12): 252-264. |
| [11] | 陈桥, 吴海英, 王宗寿, 谢雨康, 李宜青, 孙俊松. 聚羟基丁酸酯合成引发的高密度生长大肠杆菌的多位点突变分析[J]. 生物技术通报, 2020, 36(7): 112-118. |
| [12] | 李静舒, 赵佳. 生防细菌ML-3抗菌蛋白发酵条件的优化及其防治应用[J]. 生物技术通报, 2020, 36(6): 83-92. |
| [13] | 张春晨, 胡双艳, 阮海华. 人源溶菌酶在大肠杆菌中的表达与复性研究[J]. 生物技术通报, 2020, 36(3): 153-161. |
| [14] | 李鹏昊, 梁严予, 王彦伟, 关洋, 逄文强, 田克恭. 非洲猪瘟病毒K196R和A240L蛋白的可溶性表达及酶活力分析[J]. 生物技术通报, 2020, 36(11): 70-75. |
| [15] | 王琦, 颜春蕾, 高洪伟, 吴薇, 杨庆利. 基于核酸适配体传感器检测食品致病菌的研究进展[J]. 生物技术通报, 2020, 36(11): 245-258. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||