








生物技术通报 ›› 2021, Vol. 37 ›› Issue (10): 143-151.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1401
胡晓( ), 王宝宝, 窦少华, 姜南, 付常振, 金行, 高凤山(
), 王宝宝, 窦少华, 姜南, 付常振, 金行, 高凤山( )
)
收稿日期:2020-11-17
出版日期:2021-10-26
发布日期:2021-11-12
作者简介:胡晓,女,硕士,研究方向:动物分子免疫学;E-mail: 基金资助:
HU Xiao( ), WANG Bao-bao, DOU Shao-hua, JIANG Nan, FU Chang-zhen, JIN Hang, GAO Feng-shan(
), WANG Bao-bao, DOU Shao-hua, JIANG Nan, FU Chang-zhen, JIN Hang, GAO Feng-shan( )
)
Received:2020-11-17
Published:2021-10-26
Online:2021-11-12
摘要:
为构建烟台黑猪SLA-2基因(SLA-2-YT)的真核表达载体SLA-2-YT/pCDH并将其在真核细胞中表达,根据SLA-2-YT编码区基因序列设计1对引物,以SLA-2-YT/pMD18-T全基因克隆表达载体为模板进行PCR扩增获得SLA-2-YT编码区基因片段,在上下游引物的5'端分别添加限制性内切酶Xba I和Not I的酶切位点,将目的基因经pMD 19-T Simple Vector TA克隆后与pCDH-CMV-MCS-EF1-Puro真核表达载体连接,获得的重组质粒转化至大肠杆菌Stbl 3感受态细胞,对扩大培养的单克隆菌株抽提质粒,使用双酶切和测序验证插入序列。对真核表达载体构建正确的菌株抽提无内毒素质粒,通过慢病毒包装和感染将质粒转染至sT2细胞,通过Western Blotting检测sT2细胞中SLA-2-YT基因的表达情况。结果显示,成功构建了SLA-2-YT/pCDH重组真核表达载体。重组质粒进行慢病毒包装和感染sT2细胞,经嘌呤霉素筛选后,成功获得阳性细胞克隆。Western Blotting检测显示SLA-2-YT/pCDH在sT2细胞中得到了优势表达,其融合蛋白的分子量大小为45 kD,与理论设计值相符。该实验成功构建了SLA-2-YT编码区基因的真核表达载体,并证实了SLA-2-YT编码区基因能够在sT2细胞中优势表达,为下一步开展SLA-2-YT递呈CTL表位的研究提供了材料。
胡晓, 王宝宝, 窦少华, 姜南, 付常振, 金行, 高凤山. 烟台黑猪SLA-2基因真核表达载体的构建及表达[J]. 生物技术通报, 2021, 37(10): 143-151.
HU Xiao, WANG Bao-bao, DOU Shao-hua, JIANG Nan, FU Chang-zhen, JIN Hang, GAO Feng-shan. Construction of a Eukaryotic Expression Vector of SLA-2 Gene from Yantai Black Pigs and Its Expression[J]. Biotechnology Bulletin, 2021, 37(10): 143-151.
| 基因 Gene | 登录号 GenBank accession No. | 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 限制性内切酶 Cleavage sites of restriction enzyme |
|---|---|---|---|---|
| SLA-2-YT | AB672508.1T | pSLA-2-YT-F | GCTCTAGAATGCGGGTCAGGGGCCCTCAAGCCATCCTC | Xba I |
| pSLA-2-YT-R | GTTGCGGCCGCTCACACTCTAGGATCCTTGGTAAGGGACAC | Not I |
表1 PCR引物信息
Table 1 Information of the primers used in PCR
| 基因 Gene | 登录号 GenBank accession No. | 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 限制性内切酶 Cleavage sites of restriction enzyme |
|---|---|---|---|---|
| SLA-2-YT | AB672508.1T | pSLA-2-YT-F | GCTCTAGAATGCGGGTCAGGGGCCCTCAAGCCATCCTC | Xba I |
| pSLA-2-YT-R | GTTGCGGCCGCTCACACTCTAGGATCCTTGGTAAGGGACAC | Not I |
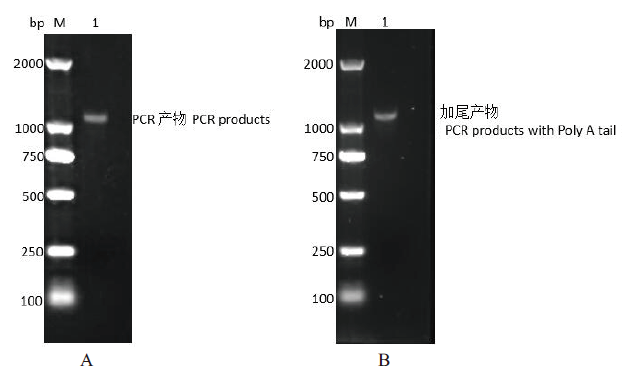
图2 烟台黑猪SLA-2-YT基因CDS区PCR扩增与加尾 A 为烟台黑猪SLA-2-YT基因CDS区PCR扩增产物的检测图,M:DNA marker 2000;1:SLA-2-YT 基因CDS区的PCR扩增产物;B为PCR 扩增产物加Poly A尾后的回收产物检测图,M:DNA marker 2000;1:PCR 扩增产物经加尾后的回收产物
Fig. 2 PCR amplification and tailing of SLA-2-YT gene CDS region in Yantai black pig A is the PCR amplified product of SLA-2-YT gene CDS region of Yantai black pig; M: DNA marker 2000; 1: The PCR productsed of the CDS region of SLA-2-YT gene. B is the detection map of PCR amplificated products plus poly A tail; M: DNA marker 2000, 1: Recovery of PCR products after tailing
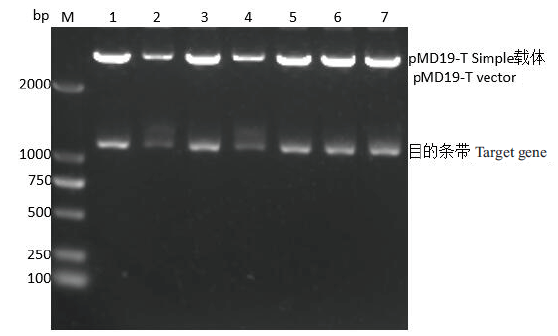
图3 重组质粒SLA-2-YT/pMD19-T Simple双酶切鉴定 M: DNA marker 2000, 1-7: 重组质粒SLA-2-YT/pMD19-T Simple双酶切产物
Fig. 3 Identification of recombinant plasmid SLA-2-YT/ pMD19-T Simple by double digestion M: DNA marker 2000, 1-7: double digestion products of recombinant plasmid SLA-2-YT/pMD19-T
图4 Vector NTI 11.5分析SLA-2-YT/pMD 19-T Simple重组克隆载体中插入序列
Fig. 4 Insertion sequences in SLA-2-YT/pMD 19-T Simple recombinant vector analyzed by Vector NTI 11.5
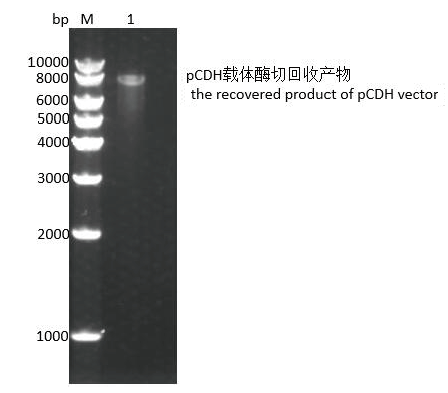
图5 pCDH-CMV-MCS-EF1-Puro 载体回收 M:DNA marker 10000, 1:pCDH-CMV-MCS-EF1-Puro载体经Xba I和Not I 双酶切后的回收产物
Fig. 5 pCDH-CMV-MCS-EF1-Puro vector recovery M:DNA marker 10000, and 1: the recovered product of pCDH-CMV-MCSEF1- Puro vector digested by Xba I and Not I
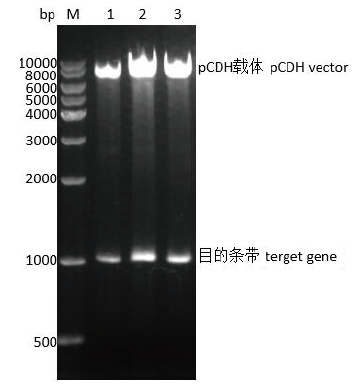
图6 重组质粒SLA-2-YT/pCDH双酶切鉴定 M:DNA marker 10000, 1-3:重组质粒SLA-2-YT/pCDH双酶切产物
Fig. 6 Identification of recombinant plasmid SLA-2-YT/pCDH by double digestion M:DNA marker 10000, and 1-3: double digestion products of recombinant plasmid SLA-2-YT/pCDH
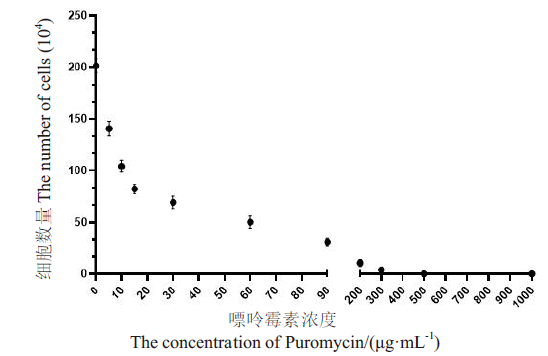
图7 嘌呤霉素作用于sT2细胞的致死浓度曲线 X轴为嘌呤霉素作用的浓度,Y轴不同浓度对应孔板中活细胞的数量
Fig. 7 Lethal concentration curve of puromycin on sT2 cells The x-axis is the concentration of puromycin, and the y-axis is the number of living cells in the plate
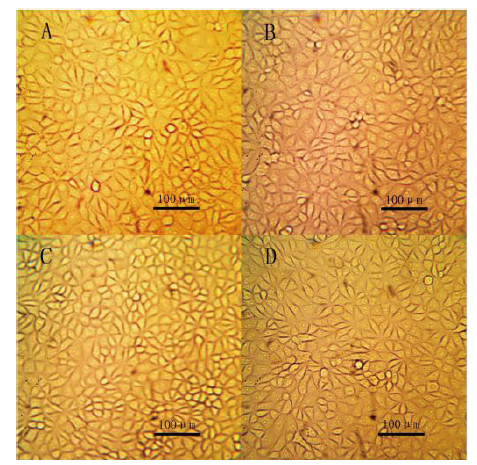
图8 光学显微镜下观察慢病毒感染后的细胞状态 A为PK15细胞,B为sT2细胞,C为转染空载体pCDH-CMV-MCS-EF1-Puro的sT2细胞,D为转染SLA-2-YT/pCDH的sT2细胞
Fig. 8 Observation of cell state after lentivirus infection under light microscope A is PK15 cell, B is sT2 cell, C is sT2 cell transfected with pCDH-CMVMCS-EF1-Puro, D is sT2 cell transfected with SLA-2-YT/pCDH
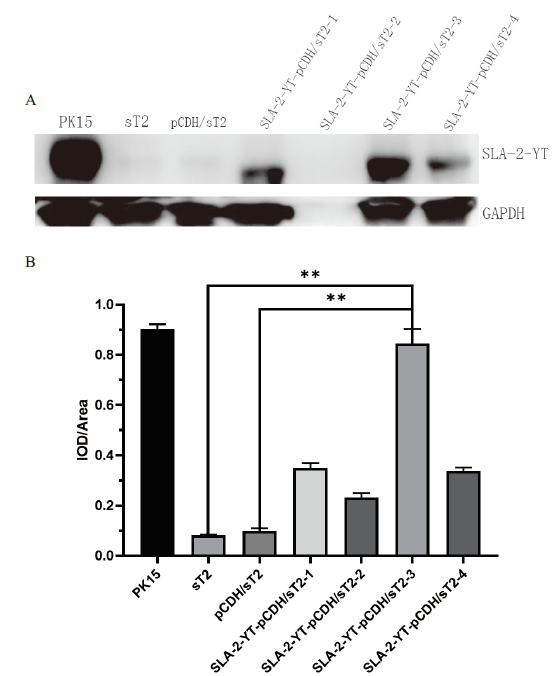
图9 Western Blotting 检测 SLA-2-YT/pCDH 在sT2细胞中的表达 A 为Western Blotting检测结果;B为相对IOD值统计分析结果,以GAPDH(36 kD)作为内参衡量SLA-2-YT/pCDH蛋白(45 kD)的表达水平(**表示 P<0.01)
Fig. 9 Western Blotting to detect the expression of SLA-2-YT/pCDH in sT2 cells A is the result of Western blotting. The expression of SLA-2-YT/pCDH protein (45 kD) is measured with GAPDH (36 kD) as internal parameter (**P<0.01)
| [1] |
Zhang N, Qi J, Feng S, et al. Crystal structure of swine major histocompatibility complex class I SLA-1 0401 and identification of 2009 pandemic swine-origin influenza A H1N1 virus cytotoxic T lymphocyte epitope peptides[J]. J Virol, 2011, 85(22):11709-11724.
doi: 10.1128/JVI.05040-11 URL |
| [2] |
Smith DM, Lunney JK, Martens GW, et al. Nomenclature for factors of the SLA class-I system, 2004[J]. Tissue Antigens, 2005, 65(2):136-149.
pmid: 15713212 |
| [3] |
Trowsdale J. Genomic structure and function in the MHC[J]. Trends Genet, 1993, 9(4):117-122.
pmid: 8516845 |
| [4] |
Osborne BA, Lunney JK, Pennington L, et al. Two-dimensional gel analysis of swine histocompatibility antigens[J]. J Immunol, 1983, 131(6):2939-2944.
pmid: 6580344 |
| [5] |
Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells[J]. Curr Opin Immunol, 2010, 22(1):109-117.
doi: 10.1016/j.coi.2010.01.022 URL |
| [6] |
Van De Weijer ML, Luteijn RD, Wiertz EJ. Viral immune evasion:Lessons in MHC class I antigen presentation[J]. Semin Immunol, 2015, 27(2):125-137.
doi: 10.1016/j.smim.2015.03.010 URL |
| [7] | 郭建凤, 蔺海朝, 王继英, 等. 不同屠宰体重烟台黑猪胴体性能、肉质性状及其相关关系分析[J]. 养猪, 2020(4):49-53. |
| Guo JF, Lin HC, Wang JY, et al. Carcass performance, meat quality traits and their correlation analysis of Yantai black pigs with different slaughter weights[J]. Swine Production, 2020, (4):49-53. | |
| [8] | 李润桦 . 披荆斩棘, 守得云开见月明——访山东威海市烟台黑猪原种猪场曲宏宇总经理[J]. 猪业科学, 2020, 37(6):74-75. |
| Li RY. Overcome the thorns and thorns, guard the clouds and see the moonlight-Interview with Qu Hongyu, general manager of Yantai black pig original breeding pig farm in Weihai, Shandong[J]. Swine Industry Science, 2020, 37(6):74-75 | |
| [9] | 姜巍, 董宋鹏, 李子彬, 等. 烟台黑猪SLA-2-YTH原核表达载体的构建及表达[J]. 中国畜牧兽医, 2014, 41(4):85-89. |
| Jiang W, Dong SP, Li ZB, et al. Construction and expression of prokaryotic expression vector of SLA-2-YTH derived from yantai black pig[J]. China Animal Husbandry &Veterinary Medicine, 2014, 41(4):85-89. | |
| [10] | 杨靖. 大鼠AQP4基因RNAi慢病毒载体的构建及病毒滴度测定方法的建立[D]. 郑州:郑州大学, 2007. |
| Yang J. Preparation of lentiviral vector expressing rat AQP4-shRNA and establishment of a titration method[D]. Zhengzhou:Zhengzhou University, 2007. | |
| [11] | 冶昡青, 郭寿清, 乔自林, 等. 慢病毒介导沉默CDH1基因的MDCK细胞株建立中最佳的MOI值及嘌呤霉素浓度的筛选[J]. 甘肃畜牧兽医, 2020, 50(1):43-45. |
| Ye XQ, Guo SQ, Qiao ZL, et al. Selection of the best MOI value and puromycin concentration in the establishment of a MDCK cell line with CDH1 gene silenced by lentivirus[J]. Gansu Animal Husbandry and Veterinary, 2020, 50(1):43-45. | |
| [12] | Dong Y, Zhang X, Wang F, et al. Construction of lentivirus plasmid pCDH-NLRX1 and stable expression of NLRX1 in A549 cells[J]. Chinese Journal of Cellular and Molecular Immunology, 2020, 36(2):152-156. |
| [13] | 刘爱玲. DF-1细胞中禽流感病毒免疫相关基因比较分析及免疫缺陷型细胞株重构[D]. 济南:山东师范大学, 2015. |
| Liu AL. Comparative analysis of selected innate immune-related genes following avian influenza viruses and reconstruction of immunodeficient cell strain in DF-1 cells[D]. Jinan:Shandong Normal University, 2015. | |
| [14] | 刘筏, 杨金刚, 翟晓鑫, 等. 烟台黑猪SLA-I重链基因末端生物素修饰及表达[J]生物技术通报, 2014(1):191-195. |
| Liu F, Yang JG, Zhai XX, et al. Modification and expression of Biotin at the end of SLA-I heavy chain gene in Yantai Black Pig[J]. Biotechnology Bulletin, 2014(1):191-195. |
| [1] | 陈中元, 王玉红, 代为俊, 张艳敏, 叶倩, 刘旭平, 谭文松, 赵亮. 柠檬酸铁铵对悬浮HEK293细胞转染的影响机制探究[J]. 生物技术通报, 2023, 39(9): 311-318. |
| [2] | 吴坤坤, 徐行, 季策, 任建峰, 李伟明, 张庆华. 斑马鱼notch3基因真核表达载体的构建及其表达分析[J]. 生物技术通报, 2022, 38(1): 179-186. |
| [3] | 周美琪, 肖欣怡, 杨卓一, 白思益, 陈会, 袁运生. 重组人骨桥蛋白在哺乳动物细胞中的表达、纯化和活性研究[J]. 生物技术通报, 2020, 36(8): 129-135. |
| [4] | 孟利, 杜彩萍. 大鼠His-Akt1重组蛋白的真核表达、蛋白纯化及活性鉴定[J]. 生物技术通报, 2020, 36(12): 98-103. |
| [5] | 杨雷, 叶洲杰, 李兆龙, 沈阳坤, 傅雅娟. 利用电转的方法对T细胞TET2基因敲除并探讨TET2对T细胞增殖的影响[J]. 生物技术通报, 2020, 36(1): 229-237. |
| [6] | 邓晓芬, 杨晓佳, 易天红, 冯英, 柯潇, 赖维莉. 融合蛋白基因与抗体基因电转染CHO-S细胞的条件摸索优化[J]. 生物技术通报, 2019, 35(4): 223-228. |
| [7] | 翟晓鑫, 高花, 姜平, 许崇波, 张宗辉, 李文哲, 高凤山. 荷包猪SLA-2-HB01真核表达载体的构建及在PK15细胞中的表达[J]. 生物技术通报, 2018, 34(12): 132-139. |
| [8] | 欧阳婧,孙园园,唐泽民,唐政山,李放军,杨寅柯. 慢病毒介导沉默PD-L1基因在乳腺癌细胞中的表达[J]. 生物技术通报, 2017, 33(9): 259-266. |
| [9] | 徐文, 杨华瑜, 郑永昌. 外源DNA在哺乳动物细胞中不依赖于启动子的转录和剪接现象[J]. 生物技术通报, 2017, 33(8): 139-145. |
| [10] | 王佳雯, 高云鹏, 孙天霞, 刘美辰, 赵雨, 王思明. 梅花鹿胸腺素beta10基因真核表达载体的构建及鉴定[J]. 生物技术通报, 2017, 33(6): 149-154. |
| [11] | 张晶晶, 金小宝, 李小波, 汪洁, 马艳. 过表达Glypican-3对高转移性肝癌细胞HCCLM3生物学行为的影响[J]. 生物技术通报, 2017, 33(12): 176-184. |
| [12] | 程华, 方向东, 崔硕, 吴萌, 张昭军, 李泽夏. 磷脂酰肌醇蛋白聚糖3真核表达载体的构建及在细胞中的表达[J]. 生物技术通报, 2016, 32(5): 146-150. |
| [13] | 阮征, 李杰, 刘开武 ,王莲芳 ,华娟 ,胡小明3 ,刘杰 ,黄海军. PRRS 病毒主要受体蛋白CD151和CD163真核表达载体的构建[J]. 生物技术通报, 2014, 0(12): 190-194. |
| [14] | 曹腾威,谷凌云,黄和,高振,郦明芳. 哺乳动物中RNA干扰沉默基因表达的研究进展[J]. 生物技术通报, 2014, 0(11): 24-31. |
| [15] | 刘筏,杨金刚,翟晓鑫,董宋鹏,高凤山. 烟台黑猪SLA-I 重链基因末端生物素修饰及表达[J]. 生物技术通报, 2014, 0(1): 191-195. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||