








生物技术通报 ›› 2021, Vol. 37 ›› Issue (10): 152-152.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0064
姚琼( ), 全林发, 徐淑, 董易之, 李文景, 池艳艳, 陈炳旭(
), 全林发, 徐淑, 董易之, 李文景, 池艳艳, 陈炳旭( )
)
收稿日期:2021-01-15
出版日期:2021-10-26
发布日期:2021-11-12
作者简介:姚琼,女,博士,副研究员,研究方向:农业昆虫与害虫防治;E-mail: 基金资助:
YAO Qiong( ), QUAN Lin-fa, XU Shu, DONG Yi-zhi, LI Wen-jing, CHI Yan-yan, CHEN Bing-xu(
), QUAN Lin-fa, XU Shu, DONG Yi-zhi, LI Wen-jing, CHI Yan-yan, CHEN Bing-xu( )
)
Received:2021-01-15
Published:2021-10-26
Online:2021-11-12
摘要:
章鱼胺(OA)是无脊椎动物中一种非常重要的生物单体胺,作为神经递质与其受体结合共同参与调节昆虫的嗅觉、产卵、运动、学习、记忆和免疫反应等多种生理功能。为了探究叉角厉蝽章鱼胺受体功能,为捕食性天敌昆虫叉角厉蝽应对逆境胁迫响应机制的研究提供参考,利用转录组测序结果和RACE技术获得2个OctβRs基因,在对其序列特征进行生物信息学分析之后,通过RT-qPCR 技术分析2个OctβRs基因的发育模式以及在亚致死剂量的高效氯氟氢菊酯和毒死蜱处理后的表达变化。结果表明扩增得到EfOctβ1R和EfOctβ2R的基因开放阅读框长度为1 245和1 230 bp,分别编码414和409个氨基酸,均属于疏水性蛋白,蛋白序列包含有7个跨膜结构域,序列中存在半胱氨酸残基位点、蛋白激酶C、配体结合位点和糖基化位点等高度保守结构域,属于典型的G蛋白偶联受体超家族成员。同源性分析结果显示,2个EfOctβRs基因分属于 Octβ1R和Octβ2R两个明显分支。在叉角厉蝽整个发育周期的不同发育阶段中,均可检测到2个EfOctβRs基因,但二者在不同发育阶段的表达水平有所差异,在叉角厉蝽成虫期均有较高量表达,其中EfOctβ2R在卵期表达要高于若虫期,而EfOctβ1R则相反。亚致死剂量高效氯氟氢菊酯和毒死蜱处理后,2个EfOctβRs基因响应强烈,均呈现不同程度的表达上升变化,分别在2种药剂处理后24 h和36 h时,EfOctβ1R和EfOctβ2R表达上升高达12倍以上。本研究结果表明,EfOctβRs可能参与叉角厉蝽在杀虫剂肋迫下的应激过程。
姚琼, 全林发, 徐淑, 董易之, 李文景, 池艳艳, 陈炳旭. 叉角厉蝽2个章鱼胺受体的基因克隆及化学农药对其表达的影响[J]. 生物技术通报, 2021, 37(10): 152-152.
YAO Qiong, QUAN Lin-fa, XU Shu, DONG Yi-zhi, LI Wen-jing, CHI Yan-yan, CHEN Bing-xu. Gene Cloning of 2 Octopamine Receptors from Eocanthecona furcellata and Effects of Chemical Pesticide on Its Expression[J]. Biotechnology Bulletin, 2021, 37(10): 152-152.
| 用途Purpose | 基因Gene | 5'扩增引物 primers for 5' RACE | 3'扩增引物 primers for 3' RACE |
|---|---|---|---|
| 巢式PCR Nested gene-specific primers | EfOctβ1R | CAATATGGTCGTACTTGGTAG | AAAGAGTGGAAACAGCTTCT |
| AGACTACTGCAATTCGTGA | AACAGCTTCTTGACAAATGG | ||
| EfOctβ2R | CGCTCCATGTAGTACAAGT | ACTTGTACTACATGGAGCG | |
| CACAGCGATACAGGACAG | AGTGGGCCAAGATTGTACG | ||
| 基因Gene | 5'扩增引物 5' primers | 3'扩增引物3' primers | |
| 实时荧光定量PCR RT-qPCR | EfOctβ1R | CCTGTGGGTATTGTCGTTCGTC | CGTAGCCTGCGTCGTTGGTTA |
| EfOctβ2R | TATCGTTCTGGGTGCCTGGAG | CGGACATAGATGCTGCGTAAC | |
| EfEF1a | CCGCCAACATCACTACTGAAGT | GCAACATAACCACGACGCAAT |
表1 本研究中EfOctβ1R和EfOctβ2R克隆及实时荧光定量PCR所需引物列表
Table 1 Primers for EfOctβ1R and EfOctβ2R cloning and qRT-PCR used in this study
| 用途Purpose | 基因Gene | 5'扩增引物 primers for 5' RACE | 3'扩增引物 primers for 3' RACE |
|---|---|---|---|
| 巢式PCR Nested gene-specific primers | EfOctβ1R | CAATATGGTCGTACTTGGTAG | AAAGAGTGGAAACAGCTTCT |
| AGACTACTGCAATTCGTGA | AACAGCTTCTTGACAAATGG | ||
| EfOctβ2R | CGCTCCATGTAGTACAAGT | ACTTGTACTACATGGAGCG | |
| CACAGCGATACAGGACAG | AGTGGGCCAAGATTGTACG | ||
| 基因Gene | 5'扩增引物 5' primers | 3'扩增引物3' primers | |
| 实时荧光定量PCR RT-qPCR | EfOctβ1R | CCTGTGGGTATTGTCGTTCGTC | CGTAGCCTGCGTCGTTGGTTA |
| EfOctβ2R | TATCGTTCTGGGTGCCTGGAG | CGGACATAGATGCTGCGTAAC | |
| EfEF1a | CCGCCAACATCACTACTGAAGT | GCAACATAACCACGACGCAAT |
| 蛋白Protein | 登录号Accession number | 物种Species | 蛋白Protein | 登录号Accession number | 物种Species |
|---|---|---|---|---|---|
| ClOctβ1R | XP_014241423.1 | Cimex lectularius | TdOctβ1R | XP_037947986.1 | Teleopsis dalmanni |
| NlOctβ1R | ATY68966.1 | Nilaparvata lugens | DbOctβ1R | XP_017849616.1 | Drosophila busckii |
| CsOctβ1R | XP_033609947.1 | Cryptotermes secundus | DhOctβ1R | XP_023175715.1 | Drosophila hydei |
| ZnOctβ1R | XP_021920523.1 | Zootermopsis nevadensis | DwOctβ1R | EDW85043.1 | Drosophila willistoni |
| ObOctβ1R | XP_011349656.1 | Ooceraea biroi | DmOctβ1R | XP_002000086.1 | Drosophila mojavensis |
| CcOctβ1R | XP_015594309.1 | Cephus cinctus | DaOctβ1R | XP_017855803.1 | Drosophila arizonae |
| TcOctβ1R | NP_001280514.1 | Tribolium castaneum | DvOctβ1R | XP_002054844.1 | Drosophila virilis |
| PxOctβ1R | XP_013165427.1 | Papilio xuthus | DiOctβ1R | XP_034488357.1 | Drosophila innubila |
| PmOctβ1R | XP_014360567.1 | Papilio machaon | DeOctβ1R | XP_001982264.1 | Drosophila erecta |
| GmOctβ1R | XP_031764033.1 | Galleria mellonella | DsOctβ1R | XP_034665037.1 | Drosophila subobscura |
| TnOctβ1R | XP_026729368.1 | Trichoplusia ni | DtOctβ1R | XP_017007379.1 | Drosophila takahashii |
| VtOctβ1R | XP_026501717.1 | Vanessa tameamea | DgOctβ1R | XP_034127656.1 | Drosophila guanche |
| DpOctβ1R | XP_032523541.1 | Danaus plexippus plexippus | DyOctβ1R | XP_002098302.1 | Drosophila yakuba |
| NvOctβ1R | NP_001317478.1 | Nicrophorus vespilloides | CfOctβ1R | XP_026475745.1 | Ctenocephalides felis |
| HaOctβ1R | XP_021187626.1 | Helicoverpa armigera | HhOctβ2R | XP_014285375.1 | Halyomorpha halys |
| PrOctβ1R | XP_022123444.1 | Pieris rapae | ClOctβ2R | XP_014254146.1 | Cimex lectularius |
| BaOctβ1R | XP_023952781.1 | Bicyclus anynana | RpOctβ2R | ATI14906.1 | Rhodnius prolixus |
| BmOctβ1R | XP_004922133.2 | Bombyx mori | NlOctβ2R | ASA47149.1 | Nilaparvata lugens |
| AtOctβ1R | XP_019864623.1 | Aethina tumida | ZnOctβ2R | XP_021931096.1 | Zootermopsis nevadensis |
| MhOctβ1R | XP_034837322.1 | Maniola hyperantus | CsOctβ2R | XP_023721065.1 | Cryptotermes secundus |
| HkOctβ1R | XP_026322929.1 | Hyposmocoma kahamanoa | LsOctβ2R | RZF43302.1 | Laodelphax striatellus |
| LsOctβ1R | VVC99498.1 | Leptidea sinapis | BgOctβ2R | PSN51957.1 | Blattella germanica |
| TpOctβ1R | XP_034238788.1 | Thrips palmi | TsOctβ2R | CAD7258263.1 | Timema shepardi |
| FaOctβ1R | XP_011312392.1 | Fopius arisanus | TdOctβ2R | CAD7194428.1 | Timema douglasi |
| ArOctβ1R | XP_020712470.1 | Athalia rosae | TmOctβ2R | CAD7423498.1 | Timema monikensis |
| OfOctβ1R | XP_028167767.1 | Ostrinia furnacalis | TbOctβ2R | CAD7437944.1 | Timema bartmani |
| PhOctβ1R | XP_002422997.1 | Pediculus humanus corporis | TpOctβ2R | XP_034233045.1 | Thrips palmi |
| MsOctβ1R | XP_037296074.1 | Manduca sexta | NvOctβ2R | XP_017768337.1 | Nicrophorus vespilloides |
| HsOctβ1R | XP_011135559.1 | Harpegnathos saltator | DqOctβ2R | XP_014477130.1 | Dinoponera quadriceps |
| FoOctβ1R | XP_026276499.1 | Frankliniella occidentalis | CfOctβ2R | XP_011263103.1 | Camponotus floridanus |
| PgOctβ1R | XP_020284449.1 | Pseudomyrmex gracilis | SiOctβ2R | XP_011166273.1 | Solenopsis invicta |
| DqOctβ1R | XP_014467276.1 | Dinoponera quadriceps | LhOctβ2R | XP_012226543.1 | Linepithema humile |
| SlOctβ1R | XP_022827519.1 | Spodoptera litura | CcOctβ2R | XP_018400970.1 | Cyphomyrmex costatus |
| ApOctβ1R | XP_025836197.1 | Agrilus planipennis | ObOctβ2R | XP_032684807.1 | Odontomachus brunneus |
| BtOctβ1R | XP_018906333.1 | Bemisia tabaci | TcOctβ2R | XP_024891919.1 | Temnothorax curvispinosus |
| AcOctβ1R | KYM86550.1 | Atta colombica | TzOctβ2R | XP_018309105.1 | Trachymyrmex zeteki |
| HiOctβ1R | XP_037913033.1 | Hermetia illucens | ApOctβ2R | XP_018331973.1 | Agrilus planipennis |
| TsOctβ1R | KYN34595.1 | Trachymyrmex septentrionalis | WaOctβ2R | XP_011702425.1 | Wasmannia auropunctata |
| DcOctβ1R | XP_008477582.2 | Diaphorina citri | MpOctβ2R | XP_012535778.1 | Monomorium pharaonis |
| MpOctβ1R | XP_022179990.1 | Myzus persicae | FeOctβ2R | XP_029669314.1 | Formica exsecta |
| DnOctβ1R | XP_015364764.1 | Diuraphis noxia | AfOctβ2R | XP_003694478.1 | Apis florea |
| RmOctβ1R | XP_026809104.1 | Rhopalosiphum maidis | PbOctβ2R | XP_011643320.1 | Pogonomyrmex barbatus |
| SfOctβ1R | XP_025409207.1 | Sipha flava | BtOctβ2R | XP_033219965.1 | Belonocnema treatae |
| ScOctβ1R | XP_013099911.1 | Stomoxys calcitrans | NcOctβ2R | QID89710.1 | Nephotettix cincticeps |
| GfOctβ1R | XP_037885413.1 | Glossina fuscipes | AdOctβ2R | XP_006621346.1 | Apis dorsata |
| AcOctβ2R | XP_016908111.1 | Apis cerana | MgOctβ2R | XP_033333269.1 | Megalopta genalis |
| DnOctβ2R | XP_015429562.1 | Dufourea novaeangliae | BvOctβ2R | XP_033362706.1 | Bombus vosnesenskii |
| MrOctβ2R | XP_003701983.1 | Megachile rotundata | OaOctβ2R | XP_012281474.1 | Orussus abietinus |
| DcOctβ2R | XP_026679423.1 | Diaphorina citri | CiOctβ2R | XP_034939572.1 | Chelonus insularis |
| HlOctβ2R | XP_017790436.1 | Habropoda laboriosa | CsOctβ2R | XP_011504152.1 | Ceratosolen solmsi marchali |
| AmOctβ2R | XP_006558130.1 | Apis mellifera | ArOctβ2R | XP_012261826.1 | Athalia rosae |
| PcOctβ2R | XP_014616065.1 | Polistes canadensis | OtOctβ2R | XP_022918261.1 | Onthophagus taurus |
| AeOctβ2R | XP_011065978.1 | Acromyrmex echinatior | LyOctβ2R | KAF5287830.1 | Lamprigera yunnana |
| NmOctβ2R | XP_031833977.1 | Nomia melanderi | VmOctβ2R | XP_035736463.1 | Vespa mandarini |
| EmOctβ2R | XP_017763346.1 | Eufriesea mexicana | FaOctβ2R | XP_011300779.1 | Fopius arisanus |
| PdOctβ2R | XP_015180910.1 | Polistes dominula | MsOctβ2R | XP_025204042.1 | Melanaphis sacchari |
| NfOctβ2R | XP_029169862.1 | Nylanderia fulva | RmOctβ2R | XP_026809287.1 | Rhopalosiphum maidis |
| HsOctβ2R | XP_011140150.2 | Harpegnathos saltator | DvOctβ2R | XP_028130069.1 | Diabrotica virgifera virgifera |
| ObOctβ2R | XP_029045046.1 | Osmia bicornis bicornis | LdOctβ2R | XP_023015134.1 | Leptinotarsa decemlineata |
| VeOctβ2R | XP_011876313.1 | Vollenhovia emeryi | DaOctβ2R | XP_015109333.1 | Diachasma alloeum |
| OlOctβ2R | XP_034187840.1 | Osmia lignaria | SfOctβ2R | XP_025423429.1 | Sipha flava |
| BiOctβ2R | XP_003488300.1 | Bombus impatiens | AtOctβ2R | XP_019867648.1 | Aethina tumida |
| MdOctβ2R | XP_008548223.1 | Microplitis demolitor |
表2 系统发育分析所用蛋白信息
Table 2 List sequence information used in phylogenetic analysis
| 蛋白Protein | 登录号Accession number | 物种Species | 蛋白Protein | 登录号Accession number | 物种Species |
|---|---|---|---|---|---|
| ClOctβ1R | XP_014241423.1 | Cimex lectularius | TdOctβ1R | XP_037947986.1 | Teleopsis dalmanni |
| NlOctβ1R | ATY68966.1 | Nilaparvata lugens | DbOctβ1R | XP_017849616.1 | Drosophila busckii |
| CsOctβ1R | XP_033609947.1 | Cryptotermes secundus | DhOctβ1R | XP_023175715.1 | Drosophila hydei |
| ZnOctβ1R | XP_021920523.1 | Zootermopsis nevadensis | DwOctβ1R | EDW85043.1 | Drosophila willistoni |
| ObOctβ1R | XP_011349656.1 | Ooceraea biroi | DmOctβ1R | XP_002000086.1 | Drosophila mojavensis |
| CcOctβ1R | XP_015594309.1 | Cephus cinctus | DaOctβ1R | XP_017855803.1 | Drosophila arizonae |
| TcOctβ1R | NP_001280514.1 | Tribolium castaneum | DvOctβ1R | XP_002054844.1 | Drosophila virilis |
| PxOctβ1R | XP_013165427.1 | Papilio xuthus | DiOctβ1R | XP_034488357.1 | Drosophila innubila |
| PmOctβ1R | XP_014360567.1 | Papilio machaon | DeOctβ1R | XP_001982264.1 | Drosophila erecta |
| GmOctβ1R | XP_031764033.1 | Galleria mellonella | DsOctβ1R | XP_034665037.1 | Drosophila subobscura |
| TnOctβ1R | XP_026729368.1 | Trichoplusia ni | DtOctβ1R | XP_017007379.1 | Drosophila takahashii |
| VtOctβ1R | XP_026501717.1 | Vanessa tameamea | DgOctβ1R | XP_034127656.1 | Drosophila guanche |
| DpOctβ1R | XP_032523541.1 | Danaus plexippus plexippus | DyOctβ1R | XP_002098302.1 | Drosophila yakuba |
| NvOctβ1R | NP_001317478.1 | Nicrophorus vespilloides | CfOctβ1R | XP_026475745.1 | Ctenocephalides felis |
| HaOctβ1R | XP_021187626.1 | Helicoverpa armigera | HhOctβ2R | XP_014285375.1 | Halyomorpha halys |
| PrOctβ1R | XP_022123444.1 | Pieris rapae | ClOctβ2R | XP_014254146.1 | Cimex lectularius |
| BaOctβ1R | XP_023952781.1 | Bicyclus anynana | RpOctβ2R | ATI14906.1 | Rhodnius prolixus |
| BmOctβ1R | XP_004922133.2 | Bombyx mori | NlOctβ2R | ASA47149.1 | Nilaparvata lugens |
| AtOctβ1R | XP_019864623.1 | Aethina tumida | ZnOctβ2R | XP_021931096.1 | Zootermopsis nevadensis |
| MhOctβ1R | XP_034837322.1 | Maniola hyperantus | CsOctβ2R | XP_023721065.1 | Cryptotermes secundus |
| HkOctβ1R | XP_026322929.1 | Hyposmocoma kahamanoa | LsOctβ2R | RZF43302.1 | Laodelphax striatellus |
| LsOctβ1R | VVC99498.1 | Leptidea sinapis | BgOctβ2R | PSN51957.1 | Blattella germanica |
| TpOctβ1R | XP_034238788.1 | Thrips palmi | TsOctβ2R | CAD7258263.1 | Timema shepardi |
| FaOctβ1R | XP_011312392.1 | Fopius arisanus | TdOctβ2R | CAD7194428.1 | Timema douglasi |
| ArOctβ1R | XP_020712470.1 | Athalia rosae | TmOctβ2R | CAD7423498.1 | Timema monikensis |
| OfOctβ1R | XP_028167767.1 | Ostrinia furnacalis | TbOctβ2R | CAD7437944.1 | Timema bartmani |
| PhOctβ1R | XP_002422997.1 | Pediculus humanus corporis | TpOctβ2R | XP_034233045.1 | Thrips palmi |
| MsOctβ1R | XP_037296074.1 | Manduca sexta | NvOctβ2R | XP_017768337.1 | Nicrophorus vespilloides |
| HsOctβ1R | XP_011135559.1 | Harpegnathos saltator | DqOctβ2R | XP_014477130.1 | Dinoponera quadriceps |
| FoOctβ1R | XP_026276499.1 | Frankliniella occidentalis | CfOctβ2R | XP_011263103.1 | Camponotus floridanus |
| PgOctβ1R | XP_020284449.1 | Pseudomyrmex gracilis | SiOctβ2R | XP_011166273.1 | Solenopsis invicta |
| DqOctβ1R | XP_014467276.1 | Dinoponera quadriceps | LhOctβ2R | XP_012226543.1 | Linepithema humile |
| SlOctβ1R | XP_022827519.1 | Spodoptera litura | CcOctβ2R | XP_018400970.1 | Cyphomyrmex costatus |
| ApOctβ1R | XP_025836197.1 | Agrilus planipennis | ObOctβ2R | XP_032684807.1 | Odontomachus brunneus |
| BtOctβ1R | XP_018906333.1 | Bemisia tabaci | TcOctβ2R | XP_024891919.1 | Temnothorax curvispinosus |
| AcOctβ1R | KYM86550.1 | Atta colombica | TzOctβ2R | XP_018309105.1 | Trachymyrmex zeteki |
| HiOctβ1R | XP_037913033.1 | Hermetia illucens | ApOctβ2R | XP_018331973.1 | Agrilus planipennis |
| TsOctβ1R | KYN34595.1 | Trachymyrmex septentrionalis | WaOctβ2R | XP_011702425.1 | Wasmannia auropunctata |
| DcOctβ1R | XP_008477582.2 | Diaphorina citri | MpOctβ2R | XP_012535778.1 | Monomorium pharaonis |
| MpOctβ1R | XP_022179990.1 | Myzus persicae | FeOctβ2R | XP_029669314.1 | Formica exsecta |
| DnOctβ1R | XP_015364764.1 | Diuraphis noxia | AfOctβ2R | XP_003694478.1 | Apis florea |
| RmOctβ1R | XP_026809104.1 | Rhopalosiphum maidis | PbOctβ2R | XP_011643320.1 | Pogonomyrmex barbatus |
| SfOctβ1R | XP_025409207.1 | Sipha flava | BtOctβ2R | XP_033219965.1 | Belonocnema treatae |
| ScOctβ1R | XP_013099911.1 | Stomoxys calcitrans | NcOctβ2R | QID89710.1 | Nephotettix cincticeps |
| GfOctβ1R | XP_037885413.1 | Glossina fuscipes | AdOctβ2R | XP_006621346.1 | Apis dorsata |
| AcOctβ2R | XP_016908111.1 | Apis cerana | MgOctβ2R | XP_033333269.1 | Megalopta genalis |
| DnOctβ2R | XP_015429562.1 | Dufourea novaeangliae | BvOctβ2R | XP_033362706.1 | Bombus vosnesenskii |
| MrOctβ2R | XP_003701983.1 | Megachile rotundata | OaOctβ2R | XP_012281474.1 | Orussus abietinus |
| DcOctβ2R | XP_026679423.1 | Diaphorina citri | CiOctβ2R | XP_034939572.1 | Chelonus insularis |
| HlOctβ2R | XP_017790436.1 | Habropoda laboriosa | CsOctβ2R | XP_011504152.1 | Ceratosolen solmsi marchali |
| AmOctβ2R | XP_006558130.1 | Apis mellifera | ArOctβ2R | XP_012261826.1 | Athalia rosae |
| PcOctβ2R | XP_014616065.1 | Polistes canadensis | OtOctβ2R | XP_022918261.1 | Onthophagus taurus |
| AeOctβ2R | XP_011065978.1 | Acromyrmex echinatior | LyOctβ2R | KAF5287830.1 | Lamprigera yunnana |
| NmOctβ2R | XP_031833977.1 | Nomia melanderi | VmOctβ2R | XP_035736463.1 | Vespa mandarini |
| EmOctβ2R | XP_017763346.1 | Eufriesea mexicana | FaOctβ2R | XP_011300779.1 | Fopius arisanus |
| PdOctβ2R | XP_015180910.1 | Polistes dominula | MsOctβ2R | XP_025204042.1 | Melanaphis sacchari |
| NfOctβ2R | XP_029169862.1 | Nylanderia fulva | RmOctβ2R | XP_026809287.1 | Rhopalosiphum maidis |
| HsOctβ2R | XP_011140150.2 | Harpegnathos saltator | DvOctβ2R | XP_028130069.1 | Diabrotica virgifera virgifera |
| ObOctβ2R | XP_029045046.1 | Osmia bicornis bicornis | LdOctβ2R | XP_023015134.1 | Leptinotarsa decemlineata |
| VeOctβ2R | XP_011876313.1 | Vollenhovia emeryi | DaOctβ2R | XP_015109333.1 | Diachasma alloeum |
| OlOctβ2R | XP_034187840.1 | Osmia lignaria | SfOctβ2R | XP_025423429.1 | Sipha flava |
| BiOctβ2R | XP_003488300.1 | Bombus impatiens | AtOctβ2R | XP_019867648.1 | Aethina tumida |
| MdOctβ2R | XP_008548223.1 | Microplitis demolitor |
| 基因Gene | 开放阅读框ORF/bp | 蛋白Protein/aa | 蛋白分子量MW/kD | 等电点 pI | 亲水系数GRAVY |
|---|---|---|---|---|---|
| EfOctβ1R | 1 245 | 414 | 47.34 | 9.02 | 0.194 |
| EfOctβ2R | 1 230 | 409 | 46.67 | 8.76 | 0.260 |
表3 叉角厉蝽的章鱼胺受体EfOctβ1R和EfOctβ2R信息
Table 3 List of EfOctβ1R and EfOctβ2R in Eocanthecona furcellata
| 基因Gene | 开放阅读框ORF/bp | 蛋白Protein/aa | 蛋白分子量MW/kD | 等电点 pI | 亲水系数GRAVY |
|---|---|---|---|---|---|
| EfOctβ1R | 1 245 | 414 | 47.34 | 9.02 | 0.194 |
| EfOctβ2R | 1 230 | 409 | 46.67 | 8.76 | 0.260 |
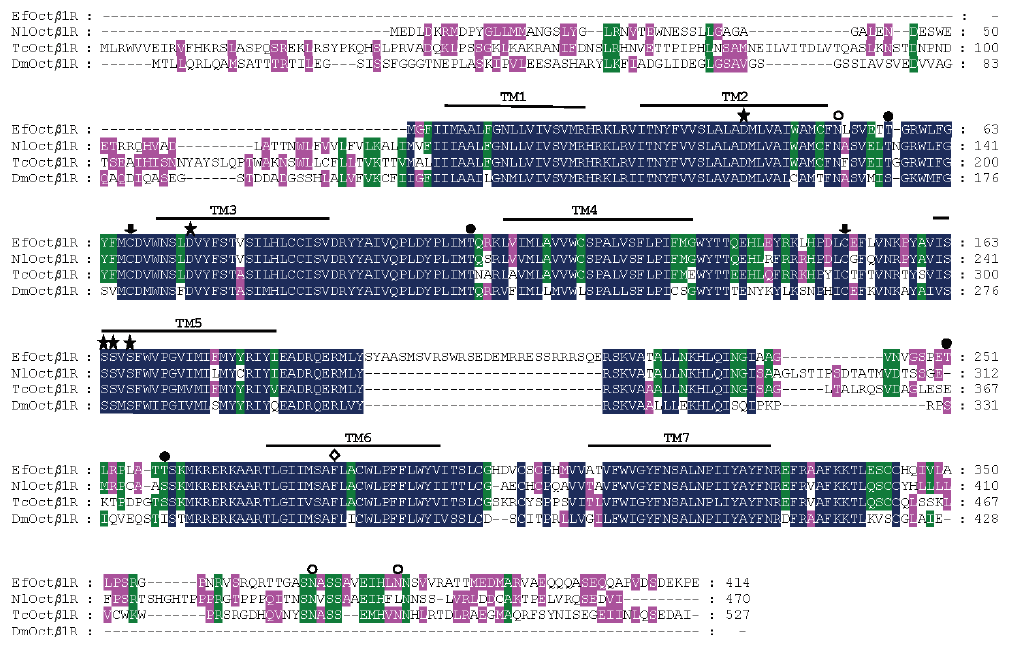
图1 叉角厉蝽EfOctβ1R与褐飞虱、赤拟谷盗、果蝇同类蛋白氨基酸序列比对分析 TM1-TM7 表示跨膜结构域 1-7,潜在的 N-糖基化和蛋白激酶 C 结合位点分别用○和●标注,半胱氨酸残基用↓标注,配体结合位点用★标注, TM6 中 FxxxWxP 用◇标注,下同
Fig. 1 Amino acid sequence alignment of EfOctβ1R in E. furrellata and orthologous receptors from Nilaparvata lugens(NlOctβ1R,ATY68966.1),Tribolium castaneum(TcOctβ1R,NP_001280514.1)and Drosophila melanogaster(DmOctβ1R,NP_001163690.1) The seven transmembrane regions are predicted by TM1-TM7. Potential N-glycosylation sites and protein kinases binding sites by PKC are labelled by○and●,respectively. The filled arrows mark as cysteine residue ligand binding sites are marked with★. The FxxxWxP motif in TM6 is indicated by◇,The same below
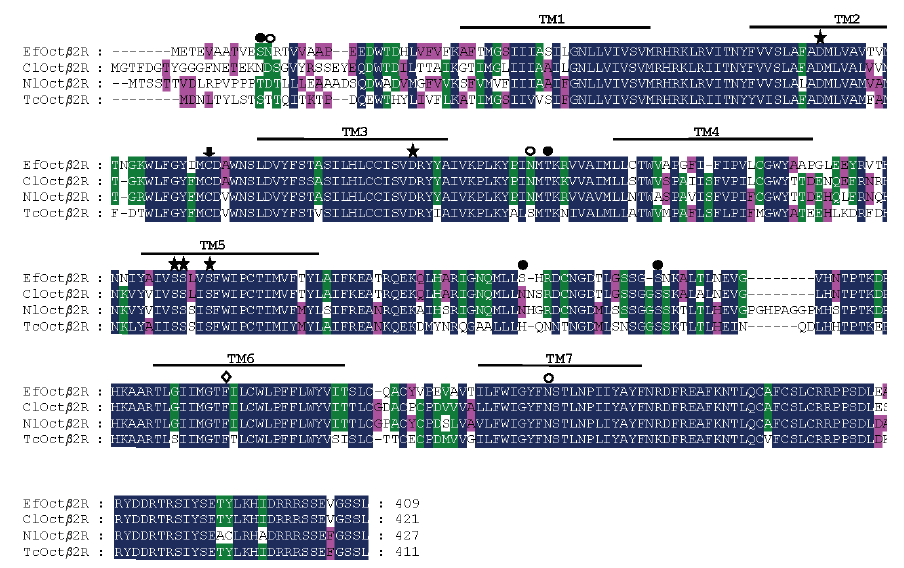
图2 叉角厉蝽EfOctβ2R与跳蚤、褐飞虱、赤拟谷盗同类蛋白氨基酸序列比对分析
Fig. 2 Amino acid sequence alignment of EfOctβ2R and orthologous receptors from Cimex lectularius(ClOctβ2R,XP_014254146.1),Nilaparvata lugens(NlOctβ2R,ASA47149.1)and Tribolium castaneum(TcOctβ2R,NP_001280501.1)
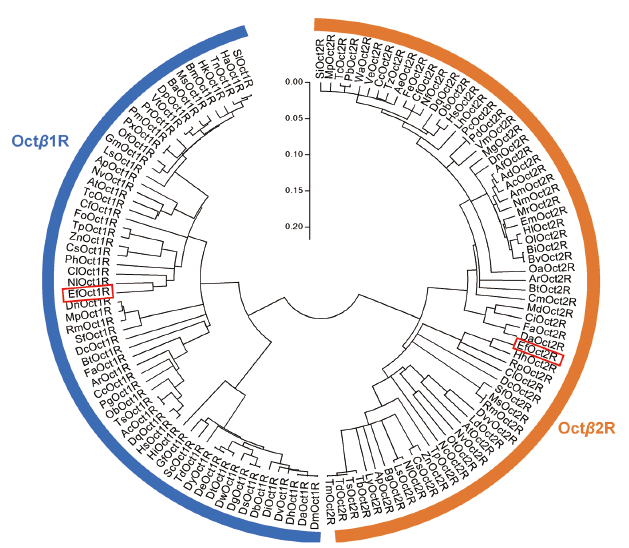
图3 EfOctβ1R和EfOctβ2R与其他昆虫同类蛋白的系统进化树分析 EfOctβ1R 和 EfOctβ2R以红框标明
Fig. 3 Phylogenetic analyses of EfOctβ1R and EfOctβ2 R and their homologs in other insects EfOctβ1R and EfOctβ2R are highlighted in red box
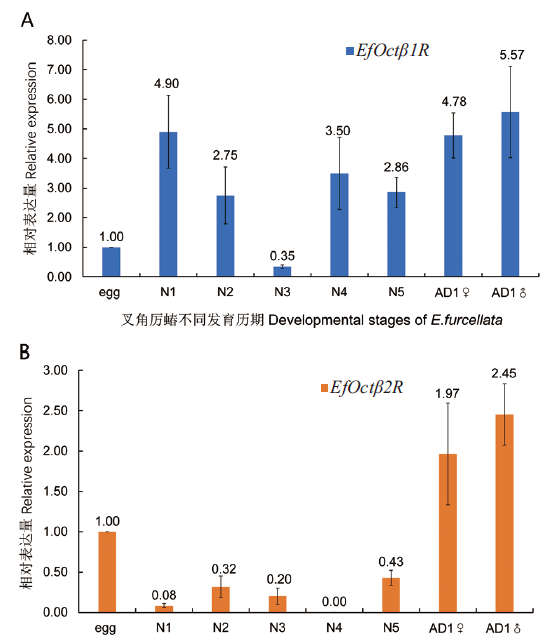
图4 叉角厉蝽EfOctβ1R和EfOctβ2R基因表达模式 图中数据以EfEF1a为内参基因,为4次生物学重复的平均值±标准差,下同
Fig. 4 Expression pattern of EfOctβ1R and EfOctβ2 R in E. furcellata Data are represented as the mean±SD from four independent experiments with EfEF1a as the housekeeping gene,the same below
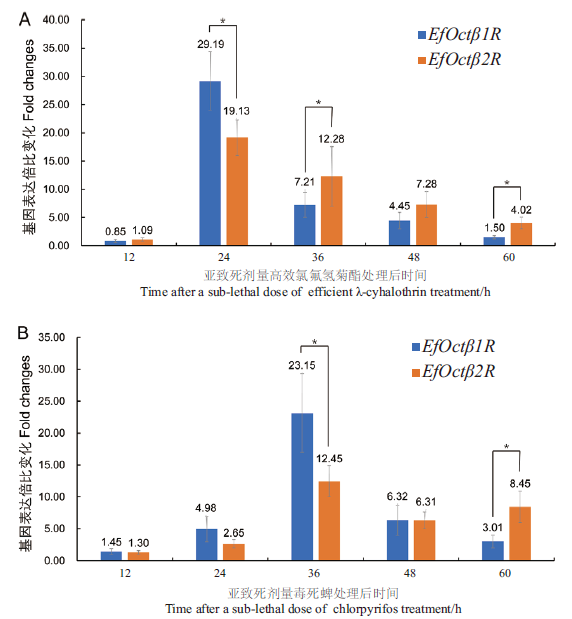
图5 亚致死剂量的高效氯氟氢酯酯和毒死蜱处理后叉角厉蝽EfOctβ1R和EfOctβ2R基因表达量变化
Fig. 5 Change in the gene expressions of EfOctβ1R and EfOctβ2R in E. furellata treated with sublethal doses of efficient r-cyhalothrin and chlorpyrifos
| [1] | 林长春, 王浩杰, 任华东, 等. 叉角厉蝽生物学特性研究[J]. 林业科学研究, 1998, 11(1):92-96. |
| Lin CC, Wang HJ, Ren HD, et al. Study on the biological characteristics of Cantheconidea furcellata(Woff)[J]. Forestry Scientific Research, 1998, 11(1):92-96. | |
| [2] | 姚明勇, 周吕, 王岚, 等. 光周期对叉角厉蝽生长发育及繁殖的影响[J]. 西南师范大学学报, 2020, 45(3):109-114. |
| Yao MY, Zhou L, Wang L, et al. Effects of different photoperiods on development and reproduction of Eocanthecona furcellata[J]. Journal of Southwest China Normal University, 2020, 45(3):109-114. | |
| [3] | 潘灿平, 李维喜, 张卢军, 等. 昆虫体内章鱼胺的分布、功能及其研究进展[J]. 昆虫知识, 2005, 42(4):369-374. |
| Pan CP, Li WX, Zhang LJ, et al. Distrubution, function and research progress of octopamine in insects[J]. Chinese Bulletin of Entomology, 42(4):369-374. | |
| [4] |
Evans PD and Maqueira B. Insect octopamine receptors:a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors[J]. Invertebrate Neuroscience, 2005, 5:111-118.
doi: 10.1007/s10158-005-0001-z URL |
| [5] |
Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors[J]. Journal of Neurochemistry, 2005, 94:547-560.
pmid: 15998303 |
| [6] | 吴顺凡, 郭建洋, 黄佳, 等. 昆虫体内章鱼胺和酪胺的研究进展[J]. 昆虫学报, 2010, 53(10):1157-1166. |
| Wu SF, Guo JY, Huang J, et al. Advances in insect octopamine and tyramine[J]. Acta Entomologica Sinica, 2010, 53(10):1157-1166. | |
| [7] | 申王尚, 陈鹏, 陈萍. 生物胺对昆虫生殖影响的研究进展[J]. 农业生物技术学报, 2018, 26(11):1979-1988. |
| Shen WS, Chen P, Chen P. Research advances in the effects of biogenic amines on insect reproduction[J]. Journal of Agricultural Biotechnology, 2018, 26(11):1979-1988. | |
| [8] | 肖达, 郭晓军, 王甦, 等. 三种杀虫剂对几种昆虫天敌的毒力测定[J]. 环境昆虫学报, 2014, 36(6):951-958. |
| Xiao D, Guo XJ, Wang F, et al. The toxicity of three insecticides to natural enemy[J]. Journal of Enviornmental Entomology, 2014, 36(6):951-958. | |
| [9] | 唐良德, 邱宝利, 任顺祥. 天敌昆虫抗药性研究进展[J]. 应用昆虫学报, 2014, 51(1):13-25. |
| Tang DL, Qui BL, Ren SX. A review of insecticide resistance in the natural enemies of pest insects[J]. Chinese Journal of Applied Entomology, 2014, 51(1):13-25. | |
| [10] | 戴伟. 生物农药对黑肩绿盲蝽的亚致死效应和机制研究[D]. 扬州:扬州大学, 2018. |
| Dai W. Sublethal effects and mechanisms of biopesticides on the predator Cyrtorhinus lividipennis[D]. Yangzhou:Yangzhou University, 2018. | |
| [11] | Ballesteros J, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors[J]. Methods in Neurosciences, 1995, 25:366-428. |
| [12] |
Schmittgen T. Analyzing Real-time PCR data by the comparative CT method[J]. Nature Protocols, 2008, 3(6):1101-1108.
pmid: 18546601 |
| [13] | Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster[J]. Microscopy Reserch and Techique, 1999, 45:106-121. |
| [14] |
Chen X, Ohta H, Ozoe F, et al. Functional and pharmacological characterization of a β-adrenergic-like octopamine receptor from the silkworm Bombyx mori[J]. Insect Biochemistry and Molecular Biology, 2010, 40:476-486.
doi: 10.1016/j.ibmb.2010.04.007 pmid: 20417278 |
| [15] |
Wu SF, Yao Y, Huang J, et al. Characterization of a β-adrenergic-like octopamine receptor from the rice stem borer Chilo suppressalis[J]. Journal of Experimental Biology, 2012, 215:2646-2652.
doi: 10.1242/jeb.068932 URL |
| [16] | 李慧敏. 桔小实蝇章鱼胺受体基因BdOctβ1R和BdOctβ2R的生理功能及药理学特性研究[D]. 重庆:西南大学, 2017. |
| Li HM. Studies on the physiological function and pharmacological properties of BdOctβ1R and BdOctβ2R in Bactrocera dorsalis[D]. Chonqing:Southwest University, 2017. | |
| [17] |
Even N, Devaud JM, Barron AB. General stress responses in the honey bee[J]. Insects, 2012, 3:1271-1298.
doi: 10.3390/insects3041271 URL |
| [18] |
Raushenbakh I. Stress-response in insects:mechanism, genetic control, and role in adaptation[J]. Genetika, 1997, 33:1110-1118.
pmid: 9378303 |
| [19] | Hirashima A, Sukhanova M, Rauschenbach I. Biogenic amines in Drosophila virilis under stress conditions[J]. Bioscence Biotechnology and Biochemistry, 2000, 64:2625-2630. |
| [20] | Mayack C, Phalen N, Carmichael K, et al. Appetite is correlated with octopamine and hemolymph sugar levels in forager honeybees[J]. Journal of Comparative Physiology, Neuroethology sensory Neural and Behavioral Physiology, 2019, 205:609-617. |
| [21] | Iba M, Nagao T and Urano A. Effects of population density on growth, behavior and levels of biogenic amines in the cricket, Gryllus bimaculatus[J]. Zoological Science, 1995, 12. |
| [22] | 毛念. 章鱼胺介导的粘虫幼虫密度依赖的抗病免疫机制研究[D]. 扬州:扬州大学, 2017. |
| Mao N. Octopamine mediate lavae density-dependent diseases resistance in Mythimna separate[D]. Yangzhou:Yangzhou University, 2017. | |
| [23] | Hirashima A, Eto M. Biogenic amines in Periplaneta americana L. :accumulation of octopamine, synephrine, and tyramine by stress[J]. Bioscience Biotechnology and Biochemstry, 1993, 57:172-173. |
| [24] | Rix RR and Christopher CG. Acute exposure to worst-case concentrations of amitraz does not affect honey bee learning, short-term memory, or hemolymph octopamine levels[J]. Journal of Economic Entomology, 2017, 110:127-132. |
| [25] |
Ahmed MA, Vogel CF, Matsumura F. Unique biochemical and molecular biological mechanism of synergistic actions of formamidine compounds on selected pyrethroid and neonicotinoid insecticides on the fourth instar larvae of Aedes aegypti(Diptera:Culicidae)[J]. Pesticide Biochemistry and Physiology, 2015, 120:57-63.
doi: 10.1016/j.pestbp.2015.01.008 URL |
| [26] |
Hong TK, Perumalsamy H, Jang KH, et al. Ovicidal and larvicidal activity and possible mode of action of phenylpropanoids and ketone identified in Syzygium aromaticum bud against Bradysia procera[J]. Pesticide Biochemistry and Physiology, 2018, 145, 29-38.
doi: 10.1016/j.pestbp.2018.01.003 URL |
| [1] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [2] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [3] | 饶紫环, 谢志雄. 一株Olivibacter jilunii 纤维素降解菌株的分离鉴定与降解能力分析[J]. 生物技术通报, 2023, 39(8): 283-290. |
| [4] | 孙明慧, 吴琼, 刘丹丹, 焦小雨, 王文杰. 茶树CsTMFs的克隆与表达分析[J]. 生物技术通报, 2023, 39(7): 151-159. |
| [5] | 潘国强, 吴思源, 刘璐, 郭惠明, 程红梅, 苏晓峰. 大丽轮枝菌(Verticillim dahliae)突变体库的构建与分析[J]. 生物技术通报, 2023, 39(5): 112-119. |
| [6] | 刘奎, 李兴芬, 杨沛欣, 仲昭晨, 曹一博, 张凌云. 青杄转录共激活因子PwMBF1c的功能研究与验证[J]. 生物技术通报, 2023, 39(5): 205-216. |
| [7] | 魏明, 王欣玉, 伍国强, 赵萌. NAD依赖型去乙酰化酶SRT在植物表观遗传调控中的作用[J]. 生物技术通报, 2023, 39(4): 59-70. |
| [8] | 刘思佳, 王浩楠, 付宇辰, 闫文欣, 胡增辉, 冷平生. ‘西伯利亚’百合LiCMK基因克隆及功能分析[J]. 生物技术通报, 2023, 39(3): 196-205. |
| [9] | 张红红, 方晓峰. 相分离调控植物胁迫感知和应答的研究进展[J]. 生物技术通报, 2023, 39(11): 44-53. |
| [10] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [11] | 尤垂淮, 谢津津, 张婷, 崔天真, 孙欣路, 臧守建, 武奕凝, 孙梦瑶, 阙友雄, 苏亚春. 钩吻脂氧合酶基因 GeLOX1 的鉴定及低温胁迫表达分析[J]. 生物技术通报, 2023, 39(11): 318-327. |
| [12] | 侯瑞泽, 鲍悦, 陈启亮, 毛桂玲, 韦博霖, 侯雷平, 李梅兰. 普通白菜PRR5的克隆、表达及功能验证[J]. 生物技术通报, 2023, 39(10): 128-135. |
| [13] | 杨敏, 龙雨青, 曾娟, 曾梅, 周新茹, 王玲, 付学森, 周日宝, 刘湘丹. 灰毡毛忍冬UGTPg17、UGTPg36基因克隆及功能研究[J]. 生物技术通报, 2023, 39(10): 256-267. |
| [14] | 王楠楠, 王文佳, 朱强. 植物胁迫相关microRNA研究进展[J]. 生物技术通报, 2022, 38(8): 1-11. |
| [15] | 李秀青, 胡子曜, 雷建峰, 代培红, 刘超, 邓嘉辉, 刘敏, 孙玲, 刘晓东, 李月. 棉花黄萎病抗性相关基因GhTIFY9的克隆与功能分析[J]. 生物技术通报, 2022, 38(8): 127-134. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||