








生物技术通报 ›› 2021, Vol. 37 ›› Issue (12): 265-273.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1511
收稿日期:2020-12-13
出版日期:2021-12-26
发布日期:2022-01-19
作者简介:王欢禹,女,硕士研究生,研究方向:肿瘤的免疫治疗;E-mail: 基金资助:
WANG Huan-yu( ), CHANG Hao-wan, ZHANG Chong-qi, JIN Wei-lin, WEI Fang(
), CHANG Hao-wan, ZHANG Chong-qi, JIN Wei-lin, WEI Fang( )
)
Received:2020-12-13
Published:2021-12-26
Online:2022-01-19
摘要:
比较检测新型嵌合抗原受体(CAR)的5种方法。通过慢病毒感染得到稳定表达嵌合抗原受体的细胞。使用免疫球蛋白G抗体染色、蛋白质 L染色、绿色荧光蛋白共表达法、实时荧光定量PCR的绝对定量和相对定量法5种方法检测不同嵌合抗原受体在Jurkat细胞中的表达。成功构建6种CAR慢病毒表达载体(Meso-CAR、Met-CAR、R132H-CAR1-2-GFP、R132H-CAR2-4-GFP、Dupixent-HL-CAR-GFP、Dupixent-LH-CAR-GFP),并得到其对应的CAR稳定表达Jurkat细胞。发现Meso-CAR和Met-CAR可同时用免疫球蛋白G抗体和蛋白质 L染色鉴定其表达(免疫球蛋白G抗体染色效果较优),而其余4种CAR均不可以。对荧光法染色不可行的CAR可使用绿色荧光蛋白共表达法、实时荧光定量PCR的绝对定量和相对定量法间接检测CAR的表达,3种方法的结果之间存在一定的对应性。不同的CAR分子,可用不同方法确定细胞中CAR分子的表达情况。
王欢禹, 常昊宛, 章崇祺, 金卫林, 魏芳. 五种检测嵌合抗原受体表达方法的比较[J]. 生物技术通报, 2021, 37(12): 265-273.
WANG Huan-yu, CHANG Hao-wan, ZHANG Chong-qi, JIN Wei-lin, WEI Fang. Comparison of 5 Methods of Evaluating the Expressions of Chimeric Antigen Receptors[J]. Biotechnology Bulletin, 2021, 37(12): 265-273.
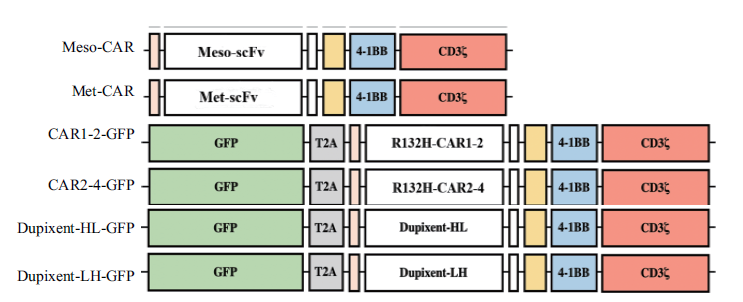
图1 CAR载体示意图 4-1BB和CD3ζ为CAR分子细胞内共刺激信号,scFv 为靶向特定抗原的单链抗体片段,后4种载体的CAR分子通过T2A系统与绿色荧光蛋白共表达。(Dupixent-HL-GFP中scFv部分重链排在轻链前,Dupixent-LH-GFP中scFv部分轻链排在重链前)
Fig. 1 Schematic diagram of CAR(chimeric antigen rec-eptor)vectors 4-1BB and CD3ζ are intracellular costimulatory signals of CAR molecules,and scFv is single-chain antibody fragment targeting specific antigens. The CAR molecules in the latest 4 vectors co-express with green fluorescent protein by the T2A system.(The scFv in Dupixent-HL-GFP is arranged in an order of the heavy chain followed by the light chain,and the scFv in Dupixent-LH-GFP is arranged in an order of the light chain followed by the heavy chain)

图2 CAR载体酶切图 上方条带为载体骨架,下方条带为图1所示的CAR分子部分
Fig. 2 Map of restriction enzyme digestion of CAR vectors The upper band is the vector backbone,and the lower band is the CAR DNA schemed in Fig. 1 M:Marker. 1:Meso-CAR. 2:Met-CAR. 3:CAR1-2-GFP. 4:CAR2-4-GFP. 5:Dupixent-HL-GFP. 6:Dupixent-LH-GFP
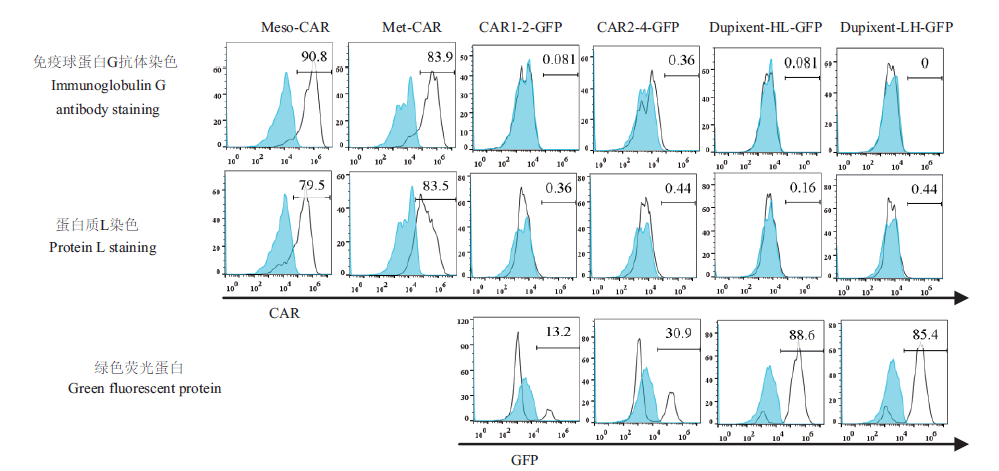
图4 流式细胞仪检测CAR表达结果 在免疫球蛋白G抗体染色中Met-CAR,Dupixent-HL-GFP和Dupixent-HL-GFP细胞使用的一抗为Biotin-SP Rabbit Anti-Human IgG;Meso-CAR,CAR1-2-GFP和CAR2-4-GFP使用的一抗为Biotin-SP Goat Anti-Mouse IgG。在蛋白质 L染色中,6种细胞使用的一抗为Biotin-protein L。这两种染色方法使用的二抗均为PE-Streptavidin。前两行数据中,空心峰为同时使用一抗和荧光二抗的实验组,实心峰为仅加荧光二抗的对照组;第三行数据中,实心峰为Jurkat细胞,空心峰为GFP与CAR共表达的4种细胞。纵坐标为细胞数量,横坐标为荧光强度,数据显示检测到的CAR分子阳性率
Fig. 4 Results of CAR expression detected by flow cytometry The primary antibody used for Met-CAR,Dupixent-HL-GFP and Dupixent-HL-GFP cells in the immunoglobulin G antibody staining assay is Biotin-SP Rabbit anti-Human IgG,while that for Meso-CAR,CAR1-2-GFP and CAR2-4-GFP is Biotin-SP Goat Anti-Mouse IgG. In protein L staining assay,the primary antibody used for the six types of cells is Biotin-protein L. The secondary antibody used in both staining methods is PE-Streptavidin. In the first two rows of data,the experimental group for the primary antibody and fluorescent secondary antibody is represented as a solid black line,and control with only fluorescent secondary antibody is represented as sky blue shade. In the third row of data,the Jurkat cell with lentiviral infection group is represented as a solid black line and the 4 types of cells co-expressed by GFP and CAR are represented as sky blue shade. The X-axis represents the number of cells,and Y-axis represents the fluorescence intensity. The data shows the positive efficiency of CAR molecules
图5 两种染色方法平均荧光强度及阳性率比较 图4流式细胞仪检测结果量化得到平均荧光强度(每个样品值减去仅加荧光二抗的对照品数值)及阳性率,取3次独立重复实验均值进行分析
Fig. 5 Comparison of two staining methods shown as average fluorescence intensity and positive percentage The data measured by the flow cytometry in Fig. 4 are presented as the average fluorescence intensity(the value of each sample - the value of the control sample with only the fluorescent secondary antibody)and the positive percentage. The final data are the average of results from 3 independent experiments
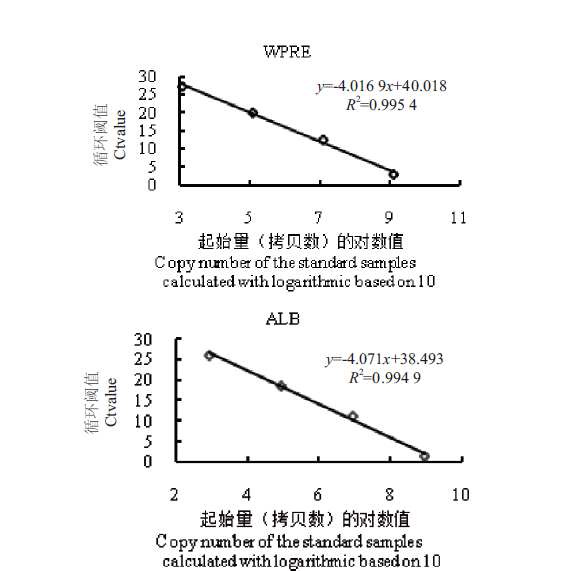
图7 标准品标准曲线图(绝对定量法) 取4个不同拷贝数梯度(标准品WPRE:1.23×103、1.23×105、1.23×107、1.23×109;标准品ALB:8.64×102、8.64×104、8.64×106、8.64×108)的标准品基因片段为模板进行荧光定量PCR。标准品计算所得的拷贝数进行以10为底的对数运算,得到的数值为横坐标,纵坐标为实时定量PCR结果中的循环阈值(Ct值)
Fig. 7 Standard curve diagrams of standard samples(absolute quantitative method) The gene fragments of standard samples with 4 different copy number gradients(standard WPRE:1.23×103,1.23×105,1.23×107,1.23×109;standard ALB:8.64×102,8.64×104,8.64106 and 8.64×108)as templates were in fluorescent quantitative PCR. The copy number of the standard samples was calculated with logarithmic based on 10,shown in X-axis,and the Y-axis is is the cycle threshold(Ct value)in the real-time quantitative PCR
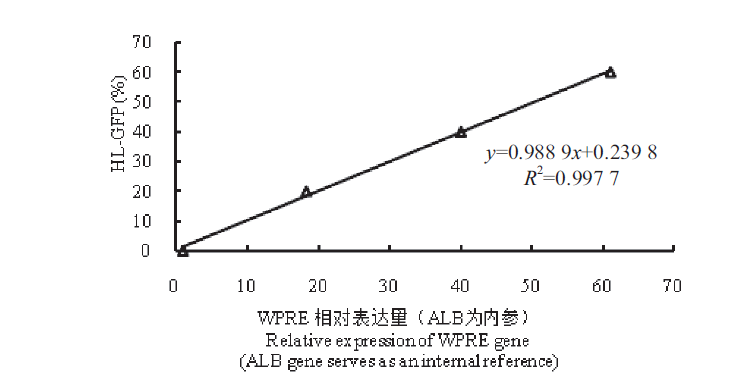
图8 HL-GFP百分比与WPRE相对表达量的标准曲线图(相对定量法) 通过流式细胞仪读取Jurkat-Dupixent-HL-GFP绿色荧光阳性细胞比例。使用未感染的Jurkat细胞与Jurkat-Dupixent-HL-GFP绿色荧光阳性细胞混合,以获得GFP阳性梯度,分别为0(Jurkat)、20%、40%、60%。提取基因组进行qPCR检测,根据WPRE基因相对表达量(ALB基因为内参)和Jurkat-Dupixent-HL-GFP绿色荧光阳性的比例绘制标准曲线图
Fig. 8 Standard curve of the percentage of HL-GFP and the relative expression of WPRE(relative quantitative method) The percentage of Jurkat-Dupixent-HL-GFP positive cells was determined by flow cytometry. Uninfected Jurkat cells were mixed with Jurkat-Dupixent-HL-GFP positive cells to obtain the gradients of GFP positive cells:0(Jurkat),20%,40% and 60%,respectively. The genome DNA was extracted for quantitative PCR detection,and a standard curve was depicted based on the relative expression of WPRE gene(ALB gene serves as an internal reference)and the percentage of Jurkat-Dupixent-HL-GFP positive population

图9 四种新型CAR分子3种不同检测方法的比较图 GFP共表达法为图4流式细胞仪检测结果中绿色荧光蛋白表达阳性率;相对定量法为图8相对定量标准曲线计算;绝对定量法为图7标准曲线计算得到平均每个细胞整合CAR分子的拷贝数
Fig. 9 Comparison of 3 different methods in detecting 4 novel CAR molecules The GFP co-expression refers to the percentage of GFP positive population measured by flow cytometry as shown in Fig. 4. The relative quantitative method refers to calculation via relative quantitative method as shown in Fig. 8. The absolute quantitative method refers to the average copy number of CAR molecules integrated per cell by substituting the standard curve of Fig. 7
| 适用性Applicability | 直观性Intuitiveness | 缺点Disadvantages | 应用Application | |
|---|---|---|---|---|
| GFP流式检测 GFP flow cytometry | 普适 Universal | 间接 Indirect | 不可用于临床 Cannot be used in clinical cases | 无法进行CAR分子染色情况下, 检测临床前CAR表达水平 Preclinical CAR expression levels can be detected when CAR molecular staining is not possible |
| IgG抗体染色 IgG antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| Protein L染色 Protein L antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| 相对定量法(推测CAR阳性率) Relative quantitative method(calculating CAR expression positive rate) | 普适 Universal | 间接 Indirect | 需同时构建含GFP和不含GFP的两种CAR T细胞 Two kinds of CAR T cells with and without GFP need to be constructed at the same time | 无法进行CAR分子染色情况下, 获得大致CAR表达水平 In the absence of CAR molecular staining, approximate CAR expression levels can be obtained |
| 绝对定量法(每个细胞整合CAR拷贝数) Absolute quantitative method (average copy number of CAR molecules integrated per cell) | 普适 Universal | 间接 Indirect | 每个细胞整合CAR分子的拷贝数是一个均值,与实际CAR分子的表达比例存在一定误差 The number of copies of each cell integrated CAR molecule is an average, with errors in the proportion of actual CAR molecule expression | 临床中监测CAR T细胞的扩增,持久性 Clinical monitoring of the amplification and persistence of CAR T cells |
表1 五种方法CAR检测方法的优劣比较表
Table 1 Comparison of the five methods in detecting CAR moleculars
| 适用性Applicability | 直观性Intuitiveness | 缺点Disadvantages | 应用Application | |
|---|---|---|---|---|
| GFP流式检测 GFP flow cytometry | 普适 Universal | 间接 Indirect | 不可用于临床 Cannot be used in clinical cases | 无法进行CAR分子染色情况下, 检测临床前CAR表达水平 Preclinical CAR expression levels can be detected when CAR molecular staining is not possible |
| IgG抗体染色 IgG antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| Protein L染色 Protein L antibody staining | 部分scFv Part of scFv | 直接 Direct | 无法检测双靶点CAR T细胞是否是双阳性 It is not possible to detect whether a dual-target CAR T cell is double-positive | 检测部分单一scFv的CAR分子T细胞 A portion of CAR T cells that express a single scFv can be detected |
| 相对定量法(推测CAR阳性率) Relative quantitative method(calculating CAR expression positive rate) | 普适 Universal | 间接 Indirect | 需同时构建含GFP和不含GFP的两种CAR T细胞 Two kinds of CAR T cells with and without GFP need to be constructed at the same time | 无法进行CAR分子染色情况下, 获得大致CAR表达水平 In the absence of CAR molecular staining, approximate CAR expression levels can be obtained |
| 绝对定量法(每个细胞整合CAR拷贝数) Absolute quantitative method (average copy number of CAR molecules integrated per cell) | 普适 Universal | 间接 Indirect | 每个细胞整合CAR分子的拷贝数是一个均值,与实际CAR分子的表达比例存在一定误差 The number of copies of each cell integrated CAR molecule is an average, with errors in the proportion of actual CAR molecule expression | 临床中监测CAR T细胞的扩增,持久性 Clinical monitoring of the amplification and persistence of CAR T cells |
| [1] | June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy:a race to the finish line[J]. Sci Transl Med, 2015, 7(280):280ps7. |
| [2] |
Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia[J]. N Engl J Med, 2013, 368(16):1509-1518.
doi: 10.1056/NEJMoa1215134 URL |
| [3] |
Panagopoulou TI, Rafiq QA. CAR-T immunotherapies:Biotechnological strategies to improve safety, efficacy and clinical outcome through CAR engineering[J]. Biotechnol Adv, 2019, 37(7):107411.
doi: S0734-9750(19)30101-6 pmid: 31251969 |
| [4] |
Martinez M, Moon EK. CAR T cells for solid tumors:new strategies for finding, infiltrating, and surviving in the tumor microenvironment[J]. Front Immunol, 2019, 10:128.
doi: 10.3389/fimmu.2019.00128 pmid: 30804938 |
| [5] |
Chen D, Yang J. Development of novel antigen receptors for CAR T-cell therapy directed toward solid malignancies[J]. Transl Res, 2017, 187:11-21.
doi: 10.1016/j.trsl.2017.05.006 URL |
| [6] |
Lonez C, Hendlisz A, Shaza L, et al. Celyad’s novel CAR T-cell therapy for solid malignancies[J]. Curr Res Transl Med, 2018, 66(2):53-56.
doi: 10.1016/j.retram.2018.03.001 URL |
| [7] |
Zheng Z, Chinnasamy N, Morgan RA. Protein L:a novel reagent for the detection of chimeric antigen receptor(CAR)expression by flow cytometry[J]. J Transl Med, 2012, 10:29.
doi: 10.1186/1479-5876-10-29 URL |
| [8] |
Lv J, Zhao R, Wu D, et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer[J]. J Hematol Oncol, 2019, 12(1):18.
doi: 10.1186/s13045-019-0704-y URL |
| [9] |
Wang H, Du X, Chen WH, et al. Establishment of a quantitative polymerase chain reaction assay for monitoring chimeric antigen receptor T cells in peripheral blood[J]. Transplant Proc, 2018, 50(1):104-109.
doi: 10.1016/j.transproceed.2017.11.028 URL |
| [10] | Bunse L, Schumacher T, Sahm F, et al. Proximity ligation assay evaluates IDH1R132H presentation in gliomas[J]. J Clin Invest, 2015, 125(2):593-606. |
| [11] |
Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma[J]. The Oncol, 2007, 12(5):569-576.
doi: 10.1634/theoncologist.12-5-569 URL |
| [12] |
Wang C, Zhu C, Wei F, et al. Constitutive activation of interleukin-13/STAT6 contributes to Kaposi’s sarcoma-associated herpesvirus-related primary effusion lymphoma cell proliferation and survival[J]. J Virol, 2015, 89(20):10416-10426.
doi: 10.1128/JVI.01525-15 URL |
| [13] | 张飞飞, 孙文, 耿琦, 等. 一种检测慢病毒滴度的实时荧光定量PCR方法[J]. 生物技术通讯, 2019, 30(4):523-527, 588. |
| Zhang FF, Sun W, Geng Q, et al. A real-time fluorescence quantitative PCR method for detecting Lentivirus titer[J]. Lett Biotechnol, 2019, 30(4):523-527, 588. | |
| [14] |
Hu Y, Huang J. The chimeric antigen receptor detection toolkit[J]. Front Immunol, 2020, 11:1770.
doi: 10.3389/fimmu.2020.01770 URL |
| [15] |
Fehse B, Badbaran A, Berger C, et al. Digital PCR assays for precise quantification of CD19-CAR-T cells after treatment with axicabtagene ciloleucel[J]. Mol Ther Methods Clin Dev, 2020, 16:172-178.
doi: 10.1016/j.omtm.2019.12.018 URL |
| [16] |
Sheih A, Voillet V, Hanafi LA, et al. Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy[J]. Nat Commun, 2020, 11(1):219.
doi: 10.1038/s41467-019-13880-1 URL |
| [17] |
Davenport AJ, Cross RS, Watson KA, et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity[J]. PNAS, 2018, 115(9):E2068-E2076.
doi: 10.1073/pnas.1716266115 URL |
| [1] | 余慧, 王静, 梁昕昕, 辛亚平, 周军, 赵会君. 宁夏枸杞铁镉响应基因的筛选及其功能验证[J]. 生物技术通报, 2023, 39(7): 195-205. |
| [2] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [3] | 宋海娜, 吴心桐, 杨鲁豫, 耿喜宁, 张华敏, 宋小龙. 葱鳞葡萄孢菌诱导下韭菜RT-qPCR内参基因的筛选和验证[J]. 生物技术通报, 2023, 39(3): 101-115. |
| [4] | 穆德添, 万凌云, 章瑶, 韦树根, 陆英, 付金娥, 田艺, 潘丽梅, 唐其. 钩藤管家基因筛选及生物碱合成相关基因的表达分析[J]. 生物技术通报, 2023, 39(2): 126-138. |
| [5] | 曹英芳, 赵新, 刘双, 李瑞环, 刘娜, 徐石勇, 高芳瑞, 马卉, 兰青阔, 檀建新, 王永. 抗除草剂大豆GE-J12实时荧光定量PCR检测方法的建立[J]. 生物技术通报, 2022, 38(7): 146-152. |
| [6] | 徐圆圆, 赵国春, 郝颖颖, 翁学煌, 陈仲, 贾黎明. 无患子RT-qPCR内参基因的筛选与验证[J]. 生物技术通报, 2022, 38(10): 80-89. |
| [7] | 陈建军, 赵怡迪, 曹香林. 脂多糖对鲤肠上皮细胞转录组模式的调控分析[J]. 生物技术通报, 2021, 37(8): 213-220. |
| [8] | 李恬静薇, 邹潇潇, 朱军, 鲍时翔. 长茎葡萄蕨藻胁迫条件下RT-qPCR内参基因的筛选与验证[J]. 生物技术通报, 2021, 37(10): 266-276. |
| [9] | 王彩霞, 杜方原, 林祥梅, GrzegorzWozniakowski, 王勤, 冯春燕, 吴绍强. 稳定表达非洲猪瘟病毒P54蛋白的Vero细胞系的建立[J]. 生物技术通报, 2020, 36(5): 139-144. |
| [10] | 庞鹏湘, 常燕楠, 尉瑞敏, 郜刚. 马铃薯StSRP1的克隆、表达及生物信息学分析[J]. 生物技术通报, 2019, 35(7): 10-16. |
| [11] | 孔春艳, 陈永坤, 王莎莎, 郝大海, 杨宇, 龚明. 小桐子低温胁迫下microRNA实时荧光定量PCR内参的筛选与比较[J]. 生物技术通报, 2019, 35(7): 25-32. |
| [12] | 朱锐, 叶雨情, 王雅欣, 杨晨茹, 王红伟, 孙晓晴, 张研, 李尚琪, 李炯棠. 鲤两种孕激素受体基因克隆、表达及比较分析[J]. 生物技术通报, 2019, 35(7): 46-53. |
| [13] | 赵志文, 李艳娇, 户勋, 范晓静, 卓涛, 邹华松. 用于植物病原细菌标记的pBB-GFP载体构建及应用[J]. 生物技术通报, 2018, 34(3): 136-141. |
| [14] | 侯岚菲, 杨洪, 邓治, 代龙军, 门中华, 李德军. 橡胶树ADC1的克隆、表达及生物信息学分析[J]. 生物技术通报, 2018, 34(11): 111-119. |
| [15] | 孙海烨, 张梁, 李由然, 顾正华, 丁重阳, 石贵阳. 利用增强型绿色荧光蛋白研究不同启动子在乳酸克鲁维酵母中的功能[J]. 生物技术通报, 2017, 33(6): 197-206. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||