








生物技术通报 ›› 2022, Vol. 38 ›› Issue (1): 157-167.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0129
岑潇龙1( ), 雷曦1, 马诗云1, 陈倩茹1, 黄遵锡1,2,3,4, 周峻沛1,2,3,4, 张蕊1,2,3,4(
), 雷曦1, 马诗云1, 陈倩茹1, 黄遵锡1,2,3,4, 周峻沛1,2,3,4, 张蕊1,2,3,4( )
)
收稿日期:2021-02-02
出版日期:2022-01-26
发布日期:2022-02-22
作者简介:岑潇龙,男,硕士研究生,研究方向:微生物学;E-mail: 基金资助:
CEN Xiao-long1( ), LEI Xi1, MA Shi-yun1, CHEN Qian-ru1, HUANG Zun-xi1,2,3,4, ZHOU Jun-pei1,2,3,4, ZHANG Rui1,2,3,4(
), LEI Xi1, MA Shi-yun1, CHEN Qian-ru1, HUANG Zun-xi1,2,3,4, ZHOU Jun-pei1,2,3,4, ZHANG Rui1,2,3,4( )
)
Received:2021-02-02
Published:2022-01-26
Online:2022-02-22
摘要:
透明质酸酶能够将透明质酸聚糖降解成具有抗氧化等生物活性的低分子量寡糖。微生物来源透明质酸酶具有酶学性质多样和易于重组表达等特点,是开发透明质酸酶的研究热点。通过基因组测序获得一个潜在的金黄色葡萄球菌来源透明质酸裂解酶基因hylS,将其进行了大肠杆菌BL21(DE3)异源重组表达,并对重组酶进行了酶学特性和酶解产物抗氧化性分析。纯化后的重组酶rHylS的最适pH和温度分别为5.0和45℃;专一性降解透明质酸,比活是(1.6×105±5.4)U/mg;降解透明质酸产生低分子量的不饱和寡糖,属于内切透明质酸裂解酶;酶解产物对ABTS、DPPH、超氧阴离子和羟自由基清除能力显著高于未酶解的高分子量透明质酸,且与浓度呈正相关。金黄色葡萄球菌来源的透明质酸裂解酶HylS酶学性质优良,可用于生产具有抗氧化性低分子量的不饱和透明质酸寡糖。
岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167.
CEN Xiao-long, LEI Xi, MA Shi-yun, CHEN Qian-ru, HUANG Zun-xi, ZHOU Jun-pei, ZHANG Rui. Heterologous Expression and Characterization of the Hyaluronic Acid Lyase HylS from Staphylococcus aureus[J]. Biotechnology Bulletin, 2022, 38(1): 157-167.
| 透明质酸裂解酶 Hyaluronic acid lyase | HylS | HAase | - | HAase-B | HCLaseM | HCLase |
|---|---|---|---|---|---|---|
| 序列一致性a Sequence identity a | 100 | 27.4 | 24.1 | 22.4 | 20.7 | 19.6 |
| 带电荷氨基酸残基 Charged amino acids(RKHDE) | 31.0 | 26.0 | 25.5 | 24.6 | 21.5 | 21.6 |
| 酸性氨基酸残基 Acidic amino acids(DE) | 14.7 | 12.4 | 11.6 | 13.2 | 10.5 | 9.5 |
| 碱性氨基酸残基 Basic amino acids(KR) | 14.4 | 11.3 | 12.0 | 10.1 | 8.7 | 9.4 |
| 极性氨基酸残基 Polar amino acids(NCQSTY) | 32.5 | 28.7 | 36.0 | 29.8 | 24.0 | 28.6 |
| 疏水氨基酸残基 Hydrophobic amino acids(AILFWV) | 26.4 | 35.4 | 28.9 | 32.4 | 39.2 | 35.8 |
| D Asp | 8.8 | 6.2 | 6.4 | 6.3 | 5.9 | 5.8 |
| E Glu | 5.9 | 6.3 | 5.1 | 6.9 | 4.6 | 3.7 |
| C Cys | 0.2 | 0.5 | 0 | 0.1 | 0.6 | 0.3 |
| Y Tyr | 4.6 | 4.3 | 4.0 | 3.9 | 1.9 | 4.0 |
| H His | 1.9 | 2.3 | 1.9 | 1.3 | 2.4 | 2.7 |
| K Lys | 12.4 | 7.8 | 9.2 | 6.2 | 1.0 | 4.4 |
| R Arg | 2.0 | 3.5 | 2.8 | 3.9 | 7.7 | 4.9 |
| 蛋白序列登陆号Protein accession numbers in GenBank database | AYU99970 | AKM20831 | CAD46929 | AHB61202 | QGL52623 | AIL54323 |
| 来源Source | Staphylococcus aureus STA | Streptococcus zooe-pidemicus MF002 | Streptococcus aga- lactiae NEM316 | Bacillus sp. A50 | Microbacterium sp. H14 | Vibrio sp. FC509 |
表1 PL8家族透明质酸裂解酶的氨基酸残基组成比率
Table 1 Amino acid residues frequencies of hyaluronic acid(HA)lyases in PL8 family
| 透明质酸裂解酶 Hyaluronic acid lyase | HylS | HAase | - | HAase-B | HCLaseM | HCLase |
|---|---|---|---|---|---|---|
| 序列一致性a Sequence identity a | 100 | 27.4 | 24.1 | 22.4 | 20.7 | 19.6 |
| 带电荷氨基酸残基 Charged amino acids(RKHDE) | 31.0 | 26.0 | 25.5 | 24.6 | 21.5 | 21.6 |
| 酸性氨基酸残基 Acidic amino acids(DE) | 14.7 | 12.4 | 11.6 | 13.2 | 10.5 | 9.5 |
| 碱性氨基酸残基 Basic amino acids(KR) | 14.4 | 11.3 | 12.0 | 10.1 | 8.7 | 9.4 |
| 极性氨基酸残基 Polar amino acids(NCQSTY) | 32.5 | 28.7 | 36.0 | 29.8 | 24.0 | 28.6 |
| 疏水氨基酸残基 Hydrophobic amino acids(AILFWV) | 26.4 | 35.4 | 28.9 | 32.4 | 39.2 | 35.8 |
| D Asp | 8.8 | 6.2 | 6.4 | 6.3 | 5.9 | 5.8 |
| E Glu | 5.9 | 6.3 | 5.1 | 6.9 | 4.6 | 3.7 |
| C Cys | 0.2 | 0.5 | 0 | 0.1 | 0.6 | 0.3 |
| Y Tyr | 4.6 | 4.3 | 4.0 | 3.9 | 1.9 | 4.0 |
| H His | 1.9 | 2.3 | 1.9 | 1.3 | 2.4 | 2.7 |
| K Lys | 12.4 | 7.8 | 9.2 | 6.2 | 1.0 | 4.4 |
| R Arg | 2.0 | 3.5 | 2.8 | 3.9 | 7.7 | 4.9 |
| 蛋白序列登陆号Protein accession numbers in GenBank database | AYU99970 | AKM20831 | CAD46929 | AHB61202 | QGL52623 | AIL54323 |
| 来源Source | Staphylococcus aureus STA | Streptococcus zooe-pidemicus MF002 | Streptococcus aga- lactiae NEM316 | Bacillus sp. A50 | Microbacterium sp. H14 | Vibrio sp. FC509 |

图1 HylS与PL8家族透明质酸裂解酶的部分氨基酸序列比对 序列的名称为GenBank数据库蛋白序列登陆号:AAK99090来自天蓝色链霉菌A3(2),PDB登陆号为2WCO、2WDA和2X03;AHB61202来自芽孢杆菌属A50;AAK74491来自肺炎链球菌TIGR4;CAD46929来自无乳链球菌NEM316,PDB登陆号为1F1S、1I8Q和1LXM;QGL52623来自细小杆菌属H14;相似氨基酸用方框标注;一致氨基酸用黑影标注;催化氨基酸残基用“*”标记;结合底物透明质酸的芳香族氨基酸残基用“#”标记
Fig. 1 Partial amino acid sequences alignment of HylS and PL8 HA lyases Sequences are named as the protein accession numbers in GenBank database. AAK99090 from Streptomyces coelicolor A3(2)(PDB ID:2WCO,2WDA and 2X03). AHB61202 from Bacillus sp. A50. AAK74491 from Streptococcus pneumoniae TIGR4. CAD46929 from S. agalactiae NEM316(PDB ID:1F1S,1I8Q and 1LXM). QGL52623 from Microbacterium sp. H14. Identical and similar amino acids are shaded in black and framed,respectively. The catalytic group is marked using “*”. The hydrophobic patch in hyaluronic acid of bound substrate is marked using “#”

图2 纯化的rHylS的SDS-PAGE分析 M:蛋白标准品;1:未诱导的重组菌破碎液上清;2:诱导后的重组菌破碎液上清;3:纯化后的重组酶HylS
Fig. 2 SDS-PAGE analysis of the purified rHylS M:Protein markers. 1:Cell lysate of uninduced transformant. 2:Cell lysate of induced transformant. 3:Purified recombinant HylS
| 试剂 Reagent | 相对酶活力 Relative activity /% | 试剂 Reagent | 相对酶活力 Relative activity/% | |
|---|---|---|---|---|
| None | 100.0±1.4 | CaCl2 | 60.7±2.0 | |
| MnSO4 | 178.3±1.6 | NiSO4 | 40.7±0.9 | |
| KCl | 123.1±2.8 | PbAc | 39.8±0.9 | |
| FeSO4 | 103.8±2.3 | ZnSO4 | 29.8±0.5 | |
| NaCl | 99.9±0.8 | AlCl3 | 0 | |
| MgSO4 | 97.9±1.1 | β-Mercaptoethanol | 162.7±1.8 | |
| LiCl | 93.8±2.4 | SDS | 91.7±2.1 | |
| CuSO4 | 86.5±1.5 | CTAB | 65.2±1.0 | |
| CoCl2 | 77.4±1.0 | EDTA | 42.8±0.7 |
表2 金属离子和化学试剂对纯化rHylS酶活力影响
Table 2 Effects of metal ions and chemical reage-nts on the purified rHylS
| 试剂 Reagent | 相对酶活力 Relative activity /% | 试剂 Reagent | 相对酶活力 Relative activity/% | |
|---|---|---|---|---|
| None | 100.0±1.4 | CaCl2 | 60.7±2.0 | |
| MnSO4 | 178.3±1.6 | NiSO4 | 40.7±0.9 | |
| KCl | 123.1±2.8 | PbAc | 39.8±0.9 | |
| FeSO4 | 103.8±2.3 | ZnSO4 | 29.8±0.5 | |
| NaCl | 99.9±0.8 | AlCl3 | 0 | |
| MgSO4 | 97.9±1.1 | β-Mercaptoethanol | 162.7±1.8 | |
| LiCl | 93.8±2.4 | SDS | 91.7±2.1 | |
| CuSO4 | 86.5±1.5 | CTAB | 65.2±1.0 | |
| CoCl2 | 77.4±1.0 | EDTA | 42.8±0.7 |
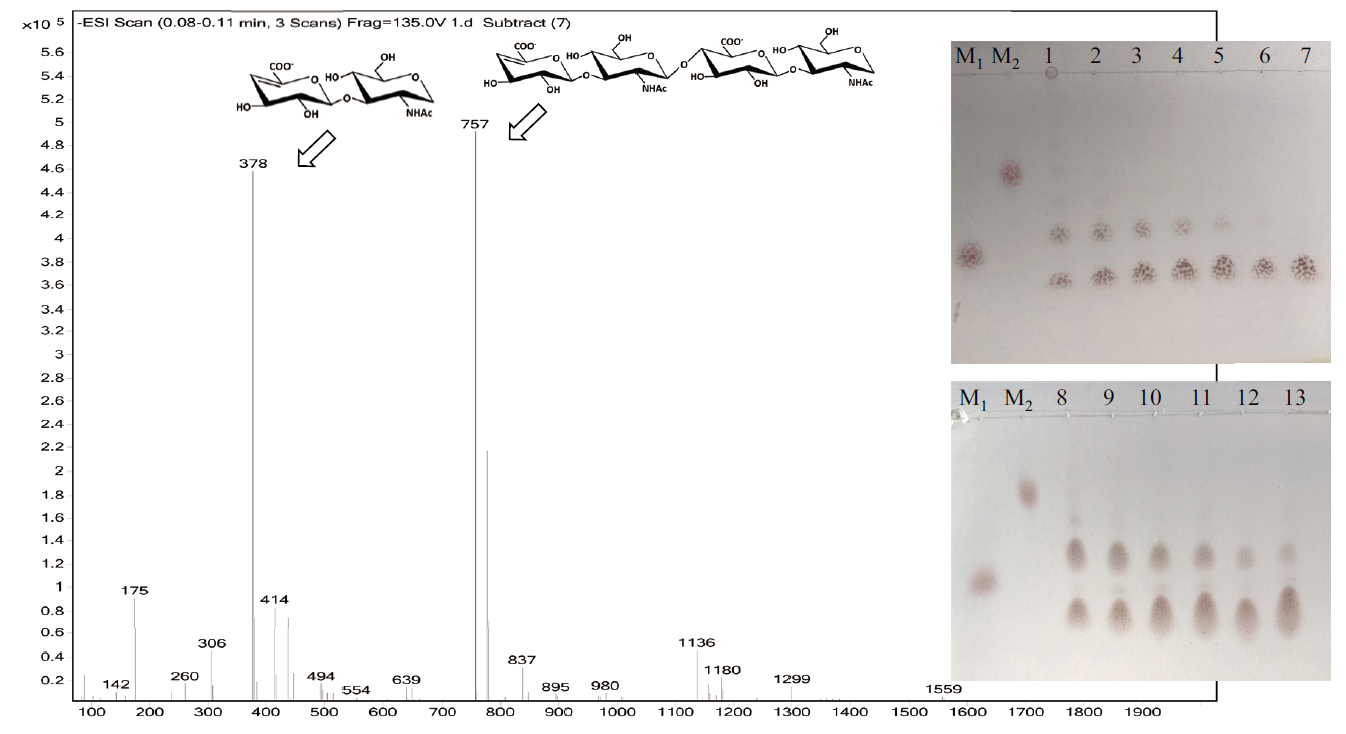
图5 rHylS酶解透明质酸所得产物的TLC和ESI-MS分析 TLC:M1和M2分别为壳二糖和壳四糖;1-7:分别为rHylS在0.5、1、2、4、6、8和10 h下降解2%HA的酶解产物;8-13:分别为rHylS在0.5、2、4、6、8和10 h下降解5% HA的酶解产物;ESI-MS:rHylS在2 h下降解2%HA的酶解产物
Fig. 5 TLC and ESI-MS analysis of the degraded products of HA by rHylS TLC:M1 and M2 are chitobiose and chitotetraose,respectively. 1-7:rHylS degraded 2% HA for 0.5,1,2,4,6,8 and 10 h,respectively. 8-13:rHylS degraded 5% HA for 0.5,2,4,6,8 and 10 h,respectively. ESI-MS:rHylS degraded 2% HA for 2 h

图6 rHylS酶解透明质酸所得产物的抗氧化活性 rHylS酶解产物对各自由基的清除能力采用最小差异检验(P<0.01)进行标记,各自由基实验组分别标记,不同字母代表差异显著
Fig. 6 Antioxidant activity of the degraded products of HA by rHylS The scavenging ability of the degraded products by rHylS to each free radical is labeled individually according to the least significant difference test among values(P<0.01),and different letters refer to significant differences
| 透明质酸裂解酶 HA lyase | HylS | HAase | - | HAase-B | HCLaseM | HCLase |
|---|---|---|---|---|---|---|
| 最适pH Optimal pH | 5.0 | 6.0 | 6.3 | 6.5 | 7.0 | 8.0 |
| pH稳定性a pH stabilitya | 100%/ pH 5.0/60 min | 95% pH 5.0/60 min | - | 75% pH 5.0/60 min | 70% pH 5.0/12 h | - |
| 最适温度 Optimal temperature/℃ | 45 | 37 | 40 | 44 | 35 | 30 |
| 热稳定性b Thermostabilityb | 60%/50℃/60 min | 70%/50℃/60 min | - | 50%/50℃/10 min | 50%/40℃/60 min | 50%/40℃/60 min |
| 激活剂 Activators | Mn2+/K+ β-Mercaptoethanol | Ca2+/Mg2+/Co2+ | Mg2+ | Ca2+/Mg2+/Ni2+ | - | Li+/Na+/K+ |
| 抑制剂 Inhibitors | Ni2+/Zn2+/Al3+/Pb2+ EDTA | Zn2+/Cu2+ | Zn2+/Al3+/Cu2+ /Fe2+/Mn2+ | Zn2+/ Cu2+/ SDS/EDTA | Hg2+/SDS | Ag+/Co2+/Hg2+/ Ni2+/Cu2+/Zn2+/Fe3+/Cr3+ |
| 底物特异性c Substrate specificityc | HA | - | HA/CS/DS | HA/CS | HA/CS/DS | HA/CS |
| 比活d Specific activity / (U·mg-1)d | 1.6×105 | 10.5 | 8.1×104 | 1.0×106 | 278.3 | 4.5×105 |
| 检测方法e Measurement methode | 紫外法 UV spectrophotometry | 二硝基水杨酸比色法 DNS colorimetry | CATB浊度法 CATB turbidimetry | BSA浊度法 BSA turbidimetry | 紫外法 UV spectroph-otometry | 紫外法 UV spectrophotometry |
| 降解模式 Degrading pattern | 内切 Endo | - | 外切 Exo | 内切 Endo | 内切 Endo | 内切 Endo |
| 来源 Source | S. aureus STA | S. zooepidemicus MF002 | S. agalactiae NEM316 | Bacillus sp. A50 | Microbacterium sp.H14 | Vibrio sp. FC509 |
| 参考文献References | This study | [17] | [18-19] | [20] | [21] | [22] |
表3 PL8家族透明质酸裂解酶的酶学特性
Table 3 Characteristics of HA lyases in PL8 family
| 透明质酸裂解酶 HA lyase | HylS | HAase | - | HAase-B | HCLaseM | HCLase |
|---|---|---|---|---|---|---|
| 最适pH Optimal pH | 5.0 | 6.0 | 6.3 | 6.5 | 7.0 | 8.0 |
| pH稳定性a pH stabilitya | 100%/ pH 5.0/60 min | 95% pH 5.0/60 min | - | 75% pH 5.0/60 min | 70% pH 5.0/12 h | - |
| 最适温度 Optimal temperature/℃ | 45 | 37 | 40 | 44 | 35 | 30 |
| 热稳定性b Thermostabilityb | 60%/50℃/60 min | 70%/50℃/60 min | - | 50%/50℃/10 min | 50%/40℃/60 min | 50%/40℃/60 min |
| 激活剂 Activators | Mn2+/K+ β-Mercaptoethanol | Ca2+/Mg2+/Co2+ | Mg2+ | Ca2+/Mg2+/Ni2+ | - | Li+/Na+/K+ |
| 抑制剂 Inhibitors | Ni2+/Zn2+/Al3+/Pb2+ EDTA | Zn2+/Cu2+ | Zn2+/Al3+/Cu2+ /Fe2+/Mn2+ | Zn2+/ Cu2+/ SDS/EDTA | Hg2+/SDS | Ag+/Co2+/Hg2+/ Ni2+/Cu2+/Zn2+/Fe3+/Cr3+ |
| 底物特异性c Substrate specificityc | HA | - | HA/CS/DS | HA/CS | HA/CS/DS | HA/CS |
| 比活d Specific activity / (U·mg-1)d | 1.6×105 | 10.5 | 8.1×104 | 1.0×106 | 278.3 | 4.5×105 |
| 检测方法e Measurement methode | 紫外法 UV spectrophotometry | 二硝基水杨酸比色法 DNS colorimetry | CATB浊度法 CATB turbidimetry | BSA浊度法 BSA turbidimetry | 紫外法 UV spectroph-otometry | 紫外法 UV spectrophotometry |
| 降解模式 Degrading pattern | 内切 Endo | - | 外切 Exo | 内切 Endo | 内切 Endo | 内切 Endo |
| 来源 Source | S. aureus STA | S. zooepidemicus MF002 | S. agalactiae NEM316 | Bacillus sp. A50 | Microbacterium sp.H14 | Vibrio sp. FC509 |
| 参考文献References | This study | [17] | [18-19] | [20] | [21] | [22] |
| [1] |
Tolg C, Telmer P, Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair[J]. PLoS One, 2014, 9(2):e88479.
doi: 10.1371/journal.pone.0088479 URL |
| [2] | 李憬昱, 王凤山. 低分子质量和寡聚透明质酸制备、活性与应用研究进展[J]. 药物生物技术, 2019, 26(1):64-68. |
| Li JY, Wang FS. Research progress in the preparation methods, bioactivities and applications of low molecular weight and oligomeric hyaluronic acid[J]. Pharm Biotechnol, 2019, 26(1):64-68. | |
| [3] | 雷曦, 张蕊, 黄遵锡, 等. 透明质酸酶的研究进展[J]. 微生物学通报, 2021, 48(3):882-895. |
| Lei X, Zhang R, Huang ZX, et al. Research progress of hyaluronidases[J]. Microbiol China, 2021, 48(3):882-895. | |
| [4] | Wang W, Wang J, Li F. Hyaluronidase and chondroitinase[J]. Adv Exp Med Biol, 2017, 925:75-87. |
| [5] |
Duran-Reynals F. Tissue permeability and the spreading factors in infection 1[J]. Bacteriol Rev, 1942, 6(4):197-252.
doi: 10.1128/br.6.4.197-252.1942 pmid: 16350083 |
| [6] |
El-Safory NS, Fazary AE, Lee CK. Hyaluronidases, a group of glycosidases:Current and future perspectives[J]. Carbohydr Polym, 2010, 81(2):165-181.
doi: 10.1016/j.carbpol.2010.02.047 URL |
| [7] | Rungsa P, Janpan P, Saengkun Y, et al. Heterologous expression and mutagenesis of recombinant Vespa affinis hyaluronidase protein(rVesA2)[J]. J Venom Animals Toxins Trop Dis, 2019, 25:e20190030. |
| [8] |
Bordon KC, Wiezel GA, Amorim FG, et al. Arthropod venom Hyaluronidases:biochemical properties and potential applications in medicine and biotechnology[J]. J Venom Anim Toxins Incl Trop Dis, 2015, 21:43.
doi: 10.1186/s40409-015-0042-7 pmid: 26500679 |
| [9] |
Huang H, Liang Q, Wang Y, et al. High-level constitutive expression of leech hyaluronidase with combined strategies in recombinant Pichia pastoris[J]. Appl Microbiol Biotechnol, 2020, 104(4):1621-1632.
doi: 10.1007/s00253-019-10282-7 pmid: 31907577 |
| [10] |
Garron ML, Cygler M. Structural and mechanistic classification of uronic acid-containing polysaccharide lyases[J]. Glycobiology, 2010, 20(12):1547-1573.
doi: 10.1093/glycob/cwq122 URL |
| [11] |
Viborg AH, Terrapon N, Lombard V, et al. A subfamily roadmap of the evolutionarily diverse glycoside hydrolase family 16(GH16)[J]. J Biol Chem, 2019, 294(44):15973-15986.
doi: 10.1074/jbc.RA119.010619 URL |
| [12] | 张海洋. 透明质酸酶的表达及酶法制备低聚透明质酸[D]. 天津:天津科技大学, 2016. |
| Zhang HY. Expression of A hyalronidase and preparation oligomers hyaluronate by enzymolysis[D]. Tianjin:Tianjin University of Science & Technology, 2016. | |
| [13] | 李帅帅. 一种节杆菌来源的透明质酸酶的研究[D]. 济南:山东大学, 2017. |
| Li SS. Study on a hyaluronidase from Arthrobacter sp[D]. Jinan:Shandong University, 2017. | |
| [14] |
Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server[J]. Nucleic Acids Res, 2014, 42(W1):W320-W324.
doi: 10.1093/nar/gku316 URL |
| [15] |
Waterhouse A, Bertoni M, Bienert S, et al. SWISS-MODEL:homology modelling of protein structures and complexes[J]. Nucleic Acids Res, 2018, 46(w1):W296-W303.
doi: 10.1093/nar/gky427 URL |
| [16] |
Zhu YP, Li XT, Sun BG, et al. Properties of an alkaline-tolerant, thermostable xylanase from Streptomyces chartreusis L1105, suitable for xylooligosaccharide production[J]. J Food Sci, 2012, 77(5):C506-C511.
doi: 10.1111/jfds.2012.77.issue-5 URL |
| [17] | 陈怡斐. 重组透明质酸酶的表达、纯化及其酶学性质研究[D]. 上海:上海应用技术学院, 2016. |
| Chen YF. Expression, purification and enzymatic proporties of recombinant hyaluronidase[D]. Shanghai:Shanghai Institute of Technology, 2016. | |
| [18] |
Ozegowski JH, Günther E, Reichardt W. Purification and characterization of hyaluronidase from Streptococcus agalactiae[J]. Zentralbl Bakteriol, 1994, 280(4):497-506.
doi: 10.1016/S0934-8840(11)80509-8 URL |
| [19] |
Li S, Jedrzejas MJ. Hyaluronan binding and degradation by Streptococcus agalactiae hyaluronate lyase[J]. J Biol Chem, 2001, 276(44):41407-41416.
pmid: 11527972 |
| [20] |
Guo X, Shi Y, Sheng J, et al. A novel hyaluronidase produced by Bacillus sp. A50[J]. PLoS One, 2014, 9(4):e94156.
doi: 10.1371/journal.pone.0094156 URL |
| [21] |
Sun JH, Han X, Song GR, et al. Cloning, expression, and characterization of a new glycosaminoglycan lyase from Microbacterium sp. H14[J]. Mar Drugs, 2019, 17(12):681.
doi: 10.3390/md17120681 URL |
| [22] |
Han W, Wang W, Zhao M, et al. A novel eliminase from a marine bacterium that degrades hyaluronan and chondroitin sulfate[J]. J Biol Chem, 2014, 289(40):27886-27898.
doi: 10.1074/jbc.M114.590752 URL |
| [23] |
Zhao YJ, Zhang YH, Cao Y, et al. Structural analysis of alkaline β-mannanase from alkaliphilic Bacillus sp. N16-5:implications for adaptation to alkaline conditions[J]. PLoS One, 2011, 6(1):e14608. DOI: 10.1371/journal.pone.0014608.
doi: 10.1371/journal.pone.0014608 URL |
| [24] |
Liu Q, Yang PL, Luo HY, et al. A novel endo-1, 4-β-mannanase from Bispora antennata with good adaptation and stability over a broad pH range[J]. Appl Biochem Biotechnol, 2012, 166(6):1442-1453.
doi: 10.1007/s12010-011-9537-z URL |
| [25] |
Nukui M, Taylor KB, McPherson DT, et al. The function of hydrophobic residues in the catalytic cleft of Streptococcus pneumoniae hyaluronate lyase. Kinetic characterization of mutant enzyme forms[J]. J Biol Chem, 2003, 278(5):3079-3088.
doi: 10.1074/jbc.M204999200 URL |
| [26] |
Jedrzejas MJ, Mello LV, de Groot BL, et al. Mechanism of hyaluronan degradation by Streptococcus pneumoniae hyaluronate lyase. Structures of complexes with the substrate[J]. J Biol Chem, 2002, 277(31):28287-28297.
pmid: 11991948 |
| [27] | 张晓娟, 宫倩红. 特异性透明质酸酶的研究进展[J]. 青岛大学学报:医学版, 2018, 54(6):750-752, 755. |
| Zhang XJ, Gong QH. Research progress of specific hyaluronidases[J]. J Qingdao Univ:Med Sci, 2018, 54(6):750-752, 755. | |
| [28] | 王晓宇, 杜国荣, 李华. 抗氧化能力的体外测定方法研究进展[J]. 食品与生物技术学报, 2012, 31(3):247-252. |
| Wang XY, Du GR, Li H. Progress of analytical methods for antioxidant capacity in vitro[J]. J Food Sci Biotechnol, 2012, 31(3):247-252. | |
| [29] | 柯春林, 乔德亮, 曾晓雄. 低分子量透明质酸的制备及其抗氧化活性的研究[J]. 食品工业科技, 2010, 31(1):107-111. |
| Ke CL, Qiao DL, Zeng XX. Preparation of the low molecular weight hyaluronic acid and its antioxidant activity[J]. Sci Technol Food Ind, 2010, 31(1):107-111. | |
| [30] | 李密, 李和生, 张丽媛, 等. 乌贼眼透明质酸的体外抗氧化性及对小鼠创愈性质的研究[J]. 中国食品学报, 2017, 17(10):30-36. |
| Li M, Li HS, Zhang LY, et al. Studies on the antioxidant and wound heal properties of hyaluronic acid from cuttlefish[J]. J Chin Inst Food Sci Technol, 2017, 17(10):30-36. | |
| [31] | 高瑞昌, 陈辉, 李来好, 等. 低分子量罗非鱼眼透明质酸的制备及其抗氧化性研究[J]. 食品工业科技, 2015, 36(3):60-64. |
| Gao RC, Chen H, Li LH, et al. Preparation and antioxidant properties of low molecular weigh hyaluronic acid from tilapia eye[J]. Sci Technol Food Ind, 2015, 36(3):60-64. |
| [1] | 游子娟, 陈汉林, 邓辅财. 鱼皮生物活性肽的提取及功能活性研究进展[J]. 生物技术通报, 2023, 39(7): 91-104. |
| [2] | 陈晓萌, 张雪静, 张欢, 张宝江, 苏艳. 重组牛乳源金黄色葡萄球菌GapC蛋白优势B细胞抗原表位的预测和筛选[J]. 生物技术通报, 2023, 39(5): 306-313. |
| [3] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [4] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [5] | 程深伟, 张克强, 梁军锋, 刘福元, 郜兴亮, 杜连柱. 畜禽养殖粪污中典型致病菌的三重微滴式数字PCR定量检测方法的建立[J]. 生物技术通报, 2022, 38(9): 271-280. |
| [6] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [7] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [8] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [9] | 马艳琴, 邱益彬, 李莎, 徐虹. 透明质酸的生物合成及其代谢工程的研究进展[J]. 生物技术通报, 2022, 38(2): 252-262. |
| [10] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [11] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [12] | 张瑶心, 王亮节, 郑文, 徐汉琴, 郑恋, 钟静. 产几丁质酶的无色杆菌ZWW8的发酵产酶及酶学性质研究[J]. 生物技术通报, 2021, 37(4): 96-106. |
| [13] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [14] | 蒋成辉, 曾巧英, 王萌, 潘阳阳, 刘旭明, 尚天甜. CRISPR/Cas9构建srtA基因敲除的金黄色葡萄球菌[J]. 生物技术通报, 2020, 36(9): 253-265. |
| [15] | 赵海燕, 宋晨斌, 刘正亚, 马兴荣, 尚会会, 李安华, 关现军, 王建设. 来源于Laceyella sp.的α-淀粉酶基因克隆、重组表达及酶学性质研究[J]. 生物技术通报, 2020, 36(8): 23-33. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||