








生物技术通报 ›› 2022, Vol. 38 ›› Issue (2): 123-131.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0539
常晴1( ), 束月蓉1, 王文韬1, 蒋昊1, 延泉德1, 钱政1, 高雪纯1, 吴金鸿2, 张勇1(
), 束月蓉1, 王文韬1, 蒋昊1, 延泉德1, 钱政1, 高雪纯1, 吴金鸿2, 张勇1( )
)
收稿日期:2021-04-22
出版日期:2022-02-26
发布日期:2022-03-09
作者简介:常晴,女,硕士,研究方向:应用酶学;E-mail: 基金资助:
CHANG Qing1( ), SHU Yue-rong1, WANG Wen-tao1, JIANG Hao1, YAN Quan-de1, QIAN Zheng1, GAO Xue-chun1, WU Jin-hong2, ZHANG Yong1(
), SHU Yue-rong1, WANG Wen-tao1, JIANG Hao1, YAN Quan-de1, QIAN Zheng1, GAO Xue-chun1, WU Jin-hong2, ZHANG Yong1( )
)
Received:2021-04-22
Published:2022-02-26
Online:2022-03-09
摘要:
褐藻寡糖有着丰富的生物学功能,酶法制备功能性褐藻寡糖具有重要实践应用价值。为发掘高活性及稳定性的褐藻寡糖制备酶,对浅海热液嗜热菌Yeosuana marina sp. JLT21中的海藻酸裂解酶YMA-1的基因在大肠杆菌中进行表达、纯化及酶活鉴定。结果发现YMA-1由306个氨基酸残基构成,是多糖裂解酶家族7(PL7)新成员;重组YMA-1酶的最适催化条件是55℃,pH 9.0,比活力1.3×104U/mg,Cu2+ 可有效促进酶活;在37℃,pH 9.0 条件下,该酶对海藻酸钠、聚甘露糖醛酸和聚古罗糖醛酸的比活力分别达到(5 201.21±86.46)U/mg、(6 399.73±253.12)U/mg和(3 751.68±116.25)U/mg,酶解海藻酸钠终产物多为不饱和三糖和四糖,表现出内切双功能型海藻酸裂解酶活性。YMA-1酶作为PL7家族中较宽底物谱、高活性及稳定性的内切海藻酸裂解酶,在高效绿色生产功能性褐藻寡糖上有着潜在应用价值。
常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131.
CHANG Qing, SHU Yue-rong, WANG Wen-tao, JIANG Hao, YAN Quan-de, QIAN Zheng, GAO Xue-chun, WU Jin-hong, ZHANG Yong. Heterologous Expression and Characterization of Endo-type Alginate Lyase from Yeosuana marina sp. JLT21[J]. Biotechnology Bulletin, 2022, 38(2): 123-131.

图1 海藻酸裂解酶YMA-1的系统进化树及PL7家族酶氨基酸序列比对分析 A:Bootstrap 是从1 000个重复中获得的置信度值(以百分比表示),分支的右侧是海藻酸分解菌的种类和在 GenBank 上的登录号,标尺表示 20%的序列差异;B:FlAlyA来自 Flavobacterium sp. UMI-01(BAP05660),A1-II’ 来自 Sphingomonas sp. A1(BAD16656),AlyA来自Klebsiella pneumoniae(AAA25049),AlyA5来自 Zobellia galactanivorans DsijT(CAZ98266.1),AlyA1来自 Z. galactanivorans DsiJT(CAZ95239),AlgAT5来自 Defluviitalea phaphyphila(WP_058486006.1),PA1167 来自 Pseudomonas aeruginosa PAO1(AAG04556),AlyC3来自 Psychromonas sp.(QOP59290.1)
Fig.1 Phylogenetic tree of alginate lyase YMA-1 and amino acid sequence alignment analysis of PL7 family enzyme A:Bootstraps are the confidence values obtained from 1,000 replications(%). Species of alginolytic bacteria and the accession number from GenBank are listed on the right. Scale bar indicates approximately 20% sequence difference. B:FlAlyA from Flavobacterium sp. UMI-01(BAP05660),A1-II’ from Sphingomonas sp. A1(BAD16656),AlyA from Klebsiella pneumoniae(AAA25049),AlyA5 from Zobellia galactanivorans DsijT(CAZ98266.1),AlyA1 from Z. galactanivorans DsiJT(CAZ95239),AlgAT5 from Defluviitalea phaphyphila(WP_058486006.1),PA1167 from Pseudomonas aeruginosa PAO1(AAG04556),and AlyC3 from Psychromonas sp.(QOP59290.1)

图2 海藻酸裂解酶YMA-1的重组表达及纯化分析 A:SDS-PAGE检测重组酶YMA-1纯度,M:蛋白 Marker;1:粗酶液,2:20 mmol/L 咪唑流穿液,3:30 mmol/L咪唑流穿液,4:50 mmol/L咪唑流穿液,5:80 mmol/L咪唑流穿液,6:纯化后的重组酶YMA-1;B:重组酶YMA-1的分子筛层析色谱图
Fig.2 Recombinant expression,purification and analysis of alginate lyase YMA-1 A:Detecting the purity of recombinase YMA-1 via SDS-PAGE. M:Protein marker. 1:Crude enzyme. 2:Flow-through with 20 mmol/L imidazole buffer;3:Flow-through with 30 mM imidazole buffer. 4:Flow-through with 50 mmol/L imidazole buffer. 5:Flow-through with 80 mmol/L imidazole buffer. 6:Purified recombinant enzyme YMA-1. B:Gel filtration chromatography analysis of recombinant enzyme YMA-1
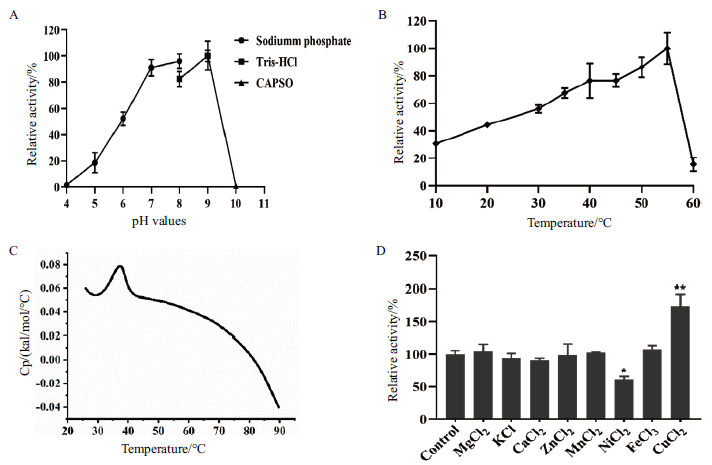
图3 海藻酸裂解酶YMA-1在不同pH、温度和金属离子条件下活性分析 A:重组酶YMA-1在 pH在5.0-10.0条件下活性分析,YMA-1催化最适pH 9.0,在该条件下酶比活力可达4.7×103 U/mg;B:重组酶YMA-1在10-60℃ 度下活性分析,最适温度为55℃,酶比活力高达1.3×104 U/mg;C:差示扫描量热法(DSC)测得重组酶YMA-1 的Tm值37℃;D:不同金属离子对重组酶YMA-1活性的影响分析
Fig.3 Activities of alginate lyase YMA-1 under different pH,temperature and metal ions A:Determination of YMA-1 activity at pH 5.0 to 10.0. YMA-1 catalysis was optimal at pH 9.0,and its specific activity of the enzyme was 4.7×103 U/mg. B:Examination of YMA-1 activity at 10℃ to 60℃. YMA-1 showed the optimal activity of 1.3×104 U/mg at 55℃. C:Measurement of YMA-1 thermostability Tm value was determined by differential scanning calorimetry(DSC). D:Effects of various metal ions on the activity of YMA-1

图4 海藻酸裂解酶YMA-1的底物偏好性分析 A:海藻酸裂解酶催化底物模式;根据海藻酸裂解酶作用的底物结构不同,可以分为 Poly M型裂解酶、Poly G型裂解酶和双功能型裂解酶;B:重组YMA-1酶对3种不同结构海藻酸底物偏好性分析;该酶可对3种不同结构的海藻酸底物表现出高活性,证实了其是一种双功能海藻酸裂解酶
Fig.4 Substrate preference of alginate lyase YMA-1 A:Substrate specificity of alginate lyases. Alginate lyases can be classified into Poly M lyase and Poly G lyase and bi-functional lyase according to chemical structures of substrates acting with alginate lyase. B:Preference of YMA-1 on 3 substrates in different structures,it presents high activity on 3 alginic acid substrates in different structure,confirming it is a bi-functional alginate lyase

图6 海藻酸裂解酶YMA-1催化海藻酸钠降解的寡糖产物分析 A:海藻酸钠降解产物HPLC分析图,YMA-1降解海藻酸钠通过HPLC共检测到4种不同产物,出峰时间分别为3.92,4.04,4.13和4.23 min;B:海藻酸钠降解产物ESI-MS鉴定图谱,重组酶降解海藻酸钠最终产物对应时间分别为不饱和二糖(ΔDP2)([M-H]-=351.0),不饱和三糖(ΔDP3)([M-H]-=527.09),不饱和四糖(ΔDP4)([M-H]-=703.12)和不饱和五糖(ΔDP5)([M-H]-=879.15)
Fig.6 LC-MS analysis of the oligosaccharide products from degrading sodium alginates by YMA-1 catalysis A:HPLC analysis of products of YMA-1 catalysis. Four different end-products were detected,and the peak times were 3.92,4.04,4.13 and 4.23 min,respectively. B:ESI-MST analysis of products of YMA-1 catalysis. The end-products were unsaturated disaccharides(ΔDP2)([M-H]-=351.0),unsaturated trisaccharides(ΔDP3)([M-H]-=527.09),unsaturated tetrasaccharide(ΔDP4)([M-H]-=703.12)and unsaturated pentasaccharide(ΔDP5)([M-H]-=879.15),respectively
| [1] |
Kloareg B, Demarty M, Mabeau S. Ion-exchange properties of isolated cell walls of brown algae:the interstitial solution[J]. J Exp Bot, 1987, 38(10):1652-1662.
doi: 10.1093/jxb/38.10.1652 URL |
| [2] |
Sellimi S, Younes I, Ayed HB, et al. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed[J]. Int J Biol Macromol, 2015, 72:1358-1367.
doi: 10.1016/j.ijbiomac.2014.10.016 URL |
| [3] |
Tang JC, Taniguchi H, Chu H, et al. Isolation and characterization of alginate-degrading bacteria for disposal of seaweed wastes[J]. Lett Appl Microbiol, 2009, 48(1):38-43.
doi: 10.1111/j.1472-765X.2008.02481.x pmid: 19018967 |
| [4] |
Wong TY, Preston LA, Schiller NL. Alginate lyase:review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications[J]. Annu Rev Microbiol, 2000, 54(1):289-340.
doi: 10.1146/micro.2000.54.issue-1 URL |
| [5] |
Jain S, Ohman DE. Role of an alginate lyase for alginate transport in mucoid Pseudomonas aeruginosa[J]. Infect Immun, 2005, 73(10):6429-6436.
doi: 10.1128/IAI.73.10.6429-6436.2005 URL |
| [6] |
Cheng D, Jiang C, Xu J, et al. Characteristics and applications of alginate lyases:a review[J]. Int J Biol Macromol, 2020, 164:1304-1320.
doi: 10.1016/j.ijbiomac.2020.07.199 URL |
| [7] |
Zhu B, Yin H. Alginate lyase:Review of major sources and classification, properties, structure-function analysis and applications[J]. Bioengineered, 2015, 6(3):125-131.
doi: 10.1080/21655979.2015.1030543 URL |
| [8] | Xu F, Wang P, Zhang YZ, et al. Diversity of three-dimensional structures and catalytic mechanisms of alginate lyases[J]. Appl Environ Microbiol, 2018, 84(3):e02040-17. |
| [9] |
Lombard V, Bernard T, Rancurel C, et al. A hierarchical classification of polysaccharide lyases for glycogenomics[J]. Biochem J, 2010, 432(3):437-444.
doi: 10.1042/BJ20101185 URL |
| [10] |
Wang B, Ji SQ, Lu M, et al. Biochemical and structural characterization of alginate lyases:an update[J]. Curr Biotechnol, 2015, 4(3):223-239.
doi: 10.2174/2211550104666150723231423 URL |
| [11] |
Nakata S, Murata K, Hashimoto W, et al. Uncovering the reactive nature of 4-deoxy- l - erythro -5-hexoseulose uronate for the utilization of alginate, a promising marine biopolymer[J]. Sci Rep, 2019, 9:17147.
doi: 10.1038/s41598-019-53597-1 pmid: 31748627 |
| [12] |
Kim HS, Lee CG, Lee EY. Alginate lyase:Structure, property, and application[J]. Biotechnol Bioprocess Eng, 2011, 16(5):843-851.
doi: 10.1007/s12257-011-0352-8 URL |
| [13] |
Wang Y, Han F, Hu B, et al. In vivo prebiotic properties of alginate oligosaccharides prepared through enzymatic hydrolysis of alginate[J]. Nutr Res, 2006, 26(11):597-603.
doi: 10.1016/j.nutres.2006.09.015 URL |
| [14] |
Islan GA, Bosio VE, Castro GR. Alginate lyase and ciprofloxacin co-immobilization on biopolymeric microspheres for cystic fibrosis treatment[J]. Macromol Biosci, 2013, 13(9):1238-1248.
doi: 10.1002/mabi.201300134 URL |
| [15] |
Tang L, Wang Y, Gao S, et al. Biochemical characteristics and molecular mechanism of an exo-type alginate lyase VxAly7D and its use for the preparation of unsaturated monosaccharides[J]. Biotechnol Biofuels, 2020, 13:99.
doi: 10.1186/s13068-020-01738-4 URL |
| [16] |
Lu D, Zhang Q, Wang S, et al. Biochemical characteristics and synergistic effect of two novel alginate lyases from Photobacterium sp. FC615[J]. Biotechnol Biofuels, 2019, 12:260.
doi: 10.1186/s13068-019-1600-y URL |
| [17] |
Yamasaki M, Moriwaki S, Miyake O, et al. Structure and function of a hypothetical Pseudomonas aeruginosa protein PA1167 classified into family PL-7:a novel alginate lyase with a beta-sandwich fold[J]. J Biol Chem, 2004, 279(30):31863-31872.
doi: 10.1074/jbc.M402466200 URL |
| [18] |
Kobayashi T, Uchimura K, Miyazaki M, et al. A new high-alkaline alginate lyase from a deep-sea bacterium Agarivorans sp[J]. Extremophiles, 2009, 13(1):121-129.
doi: 10.1007/s00792-008-0201-7 pmid: 19002649 |
| [19] |
Inoue A, Anraku M, Nakagawa S, et al. Discovery of a novel alginate lyase from Nitratiruptor sp. SB155-2 thriving at deep-sea hydrothermal vents and identification of the residues responsible for its heat stability[J]. J Biol Chem, 2016, 291(30):15551-15563.
doi: 10.1074/jbc.M115.713230 URL |
| [20] |
Li S, Wang L, Chen X, et al. Cloning, expression, and biochemical characterization of two new oligoalginate lyases with synergistic degradation capability[J]. Mar Biotechnol:NY, 2018, 20(1):75-86.
doi: 10.1007/s10126-017-9788-y URL |
| [1] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [2] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [3] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [4] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [5] | 王小桃, 邹杭, 吴怡, 向省维, 吕华, 刘超兰, 林家富, 王欣荣, 褚以文, 宋涛. Paraglaciecola hydrolytica中新型β-琼胶酶Aga2的异源表达及酶学性质分析[J]. 生物技术通报, 2022, 38(11): 258-268. |
| [6] | 岑潇龙, 雷曦, 马诗云, 陈倩茹, 黄遵锡, 周峻沛, 张蕊. 金黄色葡萄球菌透明质酸裂解酶HylS的异源表达与特性研究[J]. 生物技术通报, 2022, 38(1): 157-167. |
| [7] | 曹汝菲, 李泽轩, 许欢, 张莎, 张敏敏, 戴枫, 段晓雷. 脆弱拟杆菌Pif1解旋酶的表达纯化与晶体生长[J]. 生物技术通报, 2021, 37(9): 180-190. |
| [8] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [9] | 段绪果, 张玉华, 黄婷婷, 丁乾, 栾舒越, 朱秋雨. 化学分子伴侣及诱导条件协同强化Thermotoga maritima α-葡聚糖磷酸化酶可溶性表达[J]. 生物技术通报, 2021, 37(8): 233-242. |
| [10] | 张瑶心, 王亮节, 郑文, 徐汉琴, 郑恋, 钟静. 产几丁质酶的无色杆菌ZWW8的发酵产酶及酶学性质研究[J]. 生物技术通报, 2021, 37(4): 96-106. |
| [11] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [12] | 赵海燕, 宋晨斌, 刘正亚, 马兴荣, 尚会会, 李安华, 关现军, 王建设. 来源于Laceyella sp.的α-淀粉酶基因克隆、重组表达及酶学性质研究[J]. 生物技术通报, 2020, 36(8): 23-33. |
| [13] | 王惠兰, 吴金勇, 陈祥松, 袁丽霞, 朱薇薇, 姚建铭. N-乙酰神经氨酸醛缩酶的固定化及固定化酶性质研究[J]. 生物技术通报, 2020, 36(6): 165-173. |
| [14] | 赵震, 王莎莎, 吕星星, 陶妍, 谢晶, 钱韻芳. 重组毕赤酵母产青蛤Mytimacin抗菌肽的表达研究[J]. 生物技术通报, 2020, 36(5): 150-158. |
| [15] | 李卫娜, 申冬玲, 张煜星, 刘学通, 伊日布斯. Mangrovibacterium sp. SH-52耐热内切型海藻酸裂解酶基因的克隆及酶学鉴定[J]. 生物技术通报, 2020, 36(12): 82-90. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||