








生物技术通报 ›› 2022, Vol. 38 ›› Issue (2): 150-157.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0390
收稿日期:2021-03-30
出版日期:2022-02-26
发布日期:2022-03-09
作者简介:谢果珍,女,博士研究生,研究方向:中医药微生态;E-mail: 基金资助:
XIE Guo-zhen( ), TANG Yuan, NING Xiao-mei, QIU Ji-hui, TAN Zhou-jin(
), TANG Yuan, NING Xiao-mei, QIU Ji-hui, TAN Zhou-jin( )
)
Received:2021-03-30
Published:2022-02-26
Online:2022-03-09
摘要:
为考察铁皮石斛多糖对高脂饮食小鼠肠黏膜屏障的影响,采用水提醇沉法提取铁皮石斛多糖,联合高脂饲料给予小鼠8周后观察肠黏膜结构及肠黏膜菌群的变化。结果显示高脂饮食显著破坏了肠黏膜结构,表现为肠黏膜萎缩,上皮细胞脱落并伴有炎性渗出,Corynebacterium_1及Staphylococcus等与感染及炎症相关的菌属大量增殖。铁皮石斛多糖对肠黏膜结构有较好的保护作用,并可减少Corynebacterium_1的丰度,同时提高肠黏膜共生菌Candidatus_Arthromitus的丰度,促进了 Muribaculaceae、Bacteroides、Lachnospiraceae_NK4A136_group等碳水化合物代谢、短链脂肪酸产生相关菌的增殖。研究表明铁皮石斛多糖对肠黏膜屏障的保护作用或与其维持肠黏膜结构完整,调节肠黏膜菌群组成及促进碳水化合物代谢,生成短链脂肪酸有关。
谢果珍, 唐圆, 宁晓妹, 邱集慧, 谭周进. 铁皮石斛多糖对高脂饮食小鼠肠黏膜结构及菌群的影响[J]. 生物技术通报, 2022, 38(2): 150-157.
XIE Guo-zhen, TANG Yuan, NING Xiao-mei, QIU Ji-hui, TAN Zhou-jin. Effects of Dendrobium officinale Polysaccharides on the Intestinal Mucosal Structure and Microbiota in Mice Fed a High-fat Diet[J]. Biotechnology Bulletin, 2022, 38(2): 150-157.

图1 铁皮石斛多糖对高脂饮食小鼠肠黏膜结构的影响 N:正常组;H:高脂饮食组;HP:铁皮石斛多糖组,下同
Fig. 1 Effects of DOP on the intestinal mucosal structure of mice fed a high fat diet N:Normal group. H:High fat diet group. HP:DOP group. The same below
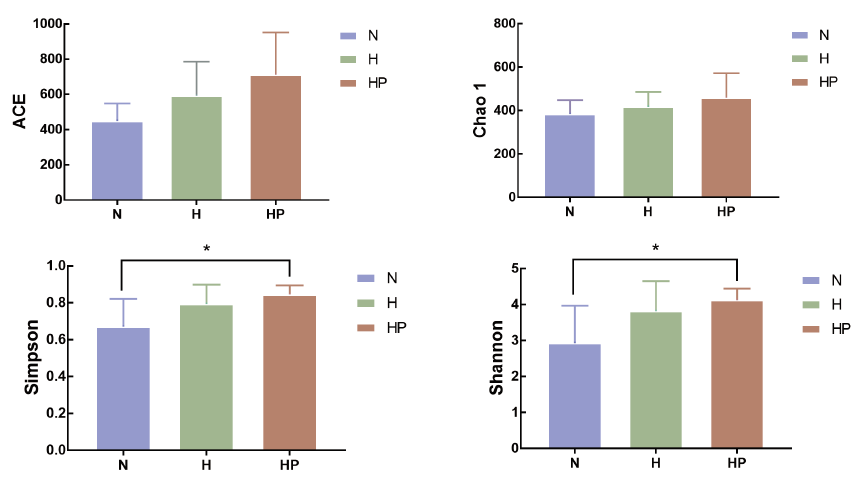
图3 铁皮石斛多糖对高脂饮食小鼠肠黏膜菌群Alpha多样性的影响(±s,N=5) *表示铁皮石斛多糖组与正常组相比,P< 0.05
Fig. 3 Effect of DOP on the Alpha diversity of mucosa-associated microbiota of mice fed a high fat diet(±s,N = 5) * refers to HP compared with N,P<0.05

图5 铁皮石斛多糖对高脂饮食小鼠肠黏膜菌群结构的影响 A:门水平;B:属水平
Fig. 5 Effect of DOP on the structure of mucosa-associated microbiota of mice fed a high fat diet A:Phylum. B:Genus
| [1] |
Wan MLY, Ling KH, El-Nezami H, et al. Influence of functional food components on gut health[J]. Crit Rev Food Sci Nutr, 2019, 59(12):1927-1936.
doi: 10.1080/10408398.2018.1433629 URL |
| [2] |
Gulhane M, Murray L, Lourie R, et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22[J]. Sci Rep, 2016, 6:28990.
doi: 10.1038/srep28990 pmid: 27350069 |
| [3] |
Proctor C, Thiennimitr P, Chattipakorn N, et al. Diet, gut microbiota and cognition[J]. Metab Brain Dis, 2017, 32(1):1-17.
doi: 10.1007/s11011-016-9917-8 pmid: 27709426 |
| [4] |
Zhang M, Yang XJ. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases[J]. World J Gastroenterol, 2016, 22(40):8905-8909.
doi: 10.3748/wjg.v22.i40.8905 URL |
| [5] |
Nguyen SG, Kim J, Guevarra RB, et al. Laminarin favorably modulates gut microbiota in mice fed a high-fat diet[J]. Food Funct, 2016, 7(10):4193-4201.
pmid: 27713958 |
| [6] |
Shang QS, Wang Y, Pan L, et al. Dietary polysaccharide from Enteromorpha clathrata modulates gut microbiota and promotes the growth of Akkermansia muciniphila, Bifidobacterium spp. and Lactobacillus spp[J]. Mar Drugs, 2018, 16(5):167.
doi: 10.3390/md16050167 URL |
| [7] |
Shi HJ, Chang YG, Gao Y, et al. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide[J]. Food Funct, 2017, 8(9):3383-3393.
doi: 10.1039/C7FO00932A URL |
| [8] |
Kanwal S, Joseph TP, Owusu L, et al. A polysaccharide isolated from Dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model[J]. Nutrients, 2018, 10(8):1003.
doi: 10.3390/nu10081003 URL |
| [9] | 蔡光先, 李娟, 李顺祥, 等. 铁皮石斛古代与现代的应用概况[J]. 湖南中医药大学学报, 2011, 31(5):77-81. |
| Cai GX, Li J, Li SX, et al. Applications of Dendrobium officinale in ancient and modern times[J]. J Tradit Chin Med Univ Hunan, 2011, 31(5):77-81. | |
| [10] |
Zeng Q, Ko CH, Siu WS, et al. Polysaccharides of Dendrobium officinale Kimura & Migo protect gastric mucosal cell against oxidative damage-induced apoptosis in vitro and in vivo[J]. J Ethnopharmacol, 2017, 208:214-224.
doi: 10.1016/j.jep.2017.07.006 URL |
| [11] |
Liang J, Chen S, Chen J, et al. Therapeutic roles of polysaccharides from Dendrobium Officinale on colitis and its underlying mechanisms[J]. Carbohydr Polym, 2018, 185:159-168.
doi: 10.1016/j.carbpol.2018.01.013 URL |
| [12] | 中华人民共和国药典委员会. 中华人民共和国药典一部[S]. 北京: 中国医药科技出版社, 2020:295-296. |
| Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China(No. 1). Beijing: China Medical Science and Technology Press, 2020:295-296. | |
| [13] |
Vancamelbeke M, Vermeire S. The intestinal barrier:a fundamental role in health and disease[J]. Expert Rev Gastroenterol Hepatol, 2017, 11(9):821-834.
doi: 10.1080/17474124.2017.1343143 URL |
| [14] |
Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease[J]. Neurogastroenterol Motil, 2012, 24(6):503-512.
doi: 10.1111/nmo.2012.24.issue-6 URL |
| [15] |
Schroeder BO, Birchenough GMH, Ståhlman M, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration[J]. Cell Host Microbe, 2018, 23(1):27-40. e7.
doi: S1931-3128(17)30498-5 pmid: 29276171 |
| [16] |
Ding SL, Lund PK. Role of intestinal inflammation as an early event in obesity and insulin resistance[J]. Curr Opin Clin Nutr Metab Care, 2011, 14(4):328-333.
doi: 10.1097/MCO.0b013e3283478727 URL |
| [17] |
Borgo F, Garbossa S, Riva A, et al. Body mass index and sex affect diverse microbial niches within the gut[J]. Front Microbiol, 2018, 9:213.
doi: 10.3389/fmicb.2018.00213 URL |
| [18] |
Chen WG, Liu FL, Ling ZX, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer[J]. PLoS One, 2012, 7(6):e39743.
doi: 10.1371/journal.pone.0039743 URL |
| [19] |
Liguori G, Lamas B, Richard ML, et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients[J]. J Crohns Colitis, 2016, 10(3):296-305.
doi: 10.1093/ecco-jcc/jjv209 URL |
| [20] |
Galley JD, Nelson MC, Yu Z, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota[J]. BMC Microbiol, 2014, 14:189.
doi: 10.1186/1471-2180-14-189 URL |
| [21] |
Dalen G, Rachah A, Nørstebø H, et al. Transmission dynamics of intramammary infections caused by Corynebacterium species[J]. J Dairy Sci, 2018, 101(1):472-479.
doi: 10.3168/jds.2017-13162 URL |
| [22] |
Costales J, Alsyouf M, Napolitan P, et al. Corynebacterium urealyt-icum:rare urinary tract infection with serious complications[J]. Can J Urol, 2019, 26(1):9680-9682.
pmid: 30797252 |
| [23] |
Abbott Y, Efstratiou A, Brennan G, et al. Toxigenic Corynebacterium ulcerans associated with upper respiratory infections in cats and dogs[J]. J Small Anim Pract, 2020, 61(9):554-560.
doi: 10.1111/jsap.13185 pmid: 32734615 |
| [24] |
Das S, Rao AS, Sahu SK, et al. Corynebacterium spp as causative agents of microbial keratitis[J]. Br J Ophthalmol, 2016, 100(7):939-943.
doi: 10.1136/bjophthalmol-2015-306749 URL |
| [25] |
Ogasawara M, Matsuhisa T, Kondo T, et al. Pyogenic spondylitis with acute course caused by Corynebacterium simulans[J]. J Infect Chemother, 2020, 26(3):294-297.
doi: S1341-321X(19)30329-0 pmid: 31735633 |
| [26] |
Ondusko DS, Nolt D. Staphylococcus aureus[J]. Pediatr Rev, 2018, 39(6):287-298.
doi: 10.1542/pir.2017-0224 URL |
| [27] |
Hedblom GA, Reiland HA, Sylte MJ, et al. Segmented filamentous bacteria - metabolism meets immunity[J]. Front Microbiol, 2018, 9:1991.
doi: 10.3389/fmicb.2018.01991 pmid: 30197636 |
| [28] |
Chung YW, Gwak HJ, Moon S, et al. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses[J]. PLoS One, 2020, 15(1):e0227886.
doi: 10.1371/journal.pone.0227886 URL |
| [29] |
Tan HZ, Zhai QX, Chen W. Investigations of Bacteroides spp. towards next-generation probiotics[J]. Food Res Int, 2019, 116:637-644.
doi: 10.1016/j.foodres.2018.08.088 URL |
| [30] |
Li ZR, Jia RB, Wu J, et al. Sargassum fusiforme polysaccharide partly replaces acarbose against type 2 diabetes in rats[J]. Int J Biol Macromol, 2021, 170:447-458.
doi: 10.1016/j.ijbiomac.2020.12.126 URL |
| [31] |
Dong J, Liang Q, Niu Y, et al. Effects of Nigella sativa seed polysaccharides on type 2 diabetic mice and gut microbiota[J]. Int J Biol Macromol, 2020, 159:725-738.
doi: 10.1016/j.ijbiomac.2020.05.042 URL |
| [32] |
Wang L, Li C, Huang Q, et al. Polysaccharide from Rosa roxburghii tratt fruit attenuates hyperglycemia and hyperlipidemia and regulates colon microbiota in diabetic db/db mice[J]. J Agric Food Chem, 2020, 68(1):147-159.
doi: 10.1021/acs.jafc.9b06247 URL |
| [33] |
Stadlbauer V, Engertsberger L, Komarova I, et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia:a pilot study[J]. BMC Geriatr, 2020, 20(1):248.
doi: 10.1186/s12877-020-01644-2 pmid: 32690030 |
| [1] | 沙珊珊, 董世荣, 杨玉菊. 肠道菌群及代谢物调控宿主肠道免疫的研究进展[J]. 生物技术通报, 2023, 39(8): 126-136. |
| [2] | 余洋, 刘天海, 刘理旭, 唐杰, 彭卫红, 陈阳, 谭昊. 羊肚菌菌种生产车间气溶胶微生物群落研究[J]. 生物技术通报, 2023, 39(5): 267-275. |
| [3] | 李善家, 雷雨昕, 孙梦格, 刘海锋, 王兴敏. 种子内生细菌多样性与植物互馈作用研究进展[J]. 生物技术通报, 2023, 39(4): 166-175. |
| [4] | 熊淑琪. 胆汁酸生理功能及其与肠道微生物互作研究进展[J]. 生物技术通报, 2023, 39(4): 187-200. |
| [5] | 徐小文, 李金仓, 海都, 查玉平, 宋菲, 王义勋. 核桃炭疽菌携带病毒种类鉴定及多样性分析[J]. 生物技术通报, 2023, 39(3): 278-289. |
| [6] | 孙海航, 官会林, 王旭, 王童, 李泓霖, 彭文洁, 刘柏桢, 樊芳玲. 生物炭对三七连作土壤性质及真菌群落的影响[J]. 生物技术通报, 2023, 39(2): 221-231. |
| [7] | 李颖, 龙长梅, 蒋标, 韩丽珍. 两株PGPR菌株的花生定殖及对根际细菌群落结构的影响[J]. 生物技术通报, 2022, 38(9): 237-247. |
| [8] | 王松, 简晓平, 潘婉舒, 张永光, 王涛, 游玲. 玉米小曲酒糟发酵饲料对育肥猪肠道菌群的影响[J]. 生物技术通报, 2022, 38(9): 248-257. |
| [9] | 王子夜, 王志刚, 阎爱华. 不同树龄桑根际土壤原生生物群落组成多样性[J]. 生物技术通报, 2022, 38(8): 206-215. |
| [10] | 陈天赐, 武少兰, 杨国辉, 江丹霞, 江玉姬, 陈炳智. 无柄灵芝醇提物对小鼠睡眠及肠道菌群的影响[J]. 生物技术通报, 2022, 38(8): 225-232. |
| [11] | 高小宁, 刘睿, 吴自林, 吴嘉云. 宿根矮化病抗感甘蔗品种茎部内生真菌和细菌群落特征分析[J]. 生物技术通报, 2022, 38(6): 166-173. |
| [12] | 徐扬, 张冠初, 丁红, 秦斐斐, 张智猛, 戴良香. 土壤类型对花生根际土壤细菌群落多样性和产量的影响[J]. 生物技术通报, 2022, 38(6): 221-234. |
| [13] | 钟辉, 刘亚军, 王滨花, 和梦洁, 吴兰. 分析方法对细菌群落16S rRNA基因扩增测序分析结果的影响[J]. 生物技术通报, 2022, 38(6): 81-92. |
| [14] | 何亚伦, 曾丽荣, 刘雄, 张铃, 王琼. 高剂量单宁酸对小鼠肠道屏障和肠道菌群的影响[J]. 生物技术通报, 2022, 38(4): 278-287. |
| [15] | 周晓楠, 徐金青, 雷雨晴, 王海庆. 基于GBS-seq的青藏扁蓿豆SNP标记开发[J]. 生物技术通报, 2022, 38(4): 303-310. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||