








生物技术通报 ›› 2022, Vol. 38 ›› Issue (6): 187-197.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1089
收稿日期:2021-08-24
出版日期:2022-06-26
发布日期:2022-07-11
作者简介:陈虎,男,博士,正高级工程师,研究方向:林木遗传育种;E-mail: 基金资助:
CHEN Hu1( ), YANG Zhang-qi1(
), YANG Zhang-qi1( ), SUN Shuang2, LI Peng1, XU Hui-lan1
), SUN Shuang2, LI Peng1, XU Hui-lan1
Received:2021-08-24
Published:2022-06-26
Online:2022-07-11
摘要:
MAPK(mitogen-actived protein kinase)在响应和调控生物与非生物胁迫、激素调节等方面发挥重要作用。马尾松MAPK级联途径7个基因被鉴定,并进行分子特性和外源信号物质处理表达模式分析。结果表明,马尾松与火炬松、北美云杉等针叶裸子植物的关系较近,不同类型基因具有该类基因特有的MAPK结构域以及典型催化氨基酸基序,D(I/L/V)K是马尾松MAPK基因共有的活性基序,PmMAP4K与MAPK级联途径的3个成员未直接互作,MAPK级联途径基因可能通过磷酸化调控各种激素生物合成来响应非生物胁迫。7个基因均响应了MeJA、GA、SA、ABA、Ca2+处理,但模式不同。PmMAP4K基因对Ca2+处理响应明显,其次是PmMAP2K基因,其它基因对单独Ca2+处理响应不明显,4种激素和Ca2+共同处理均提高了基因表达量,MeJA+Ca2+处理MAPK基因表达量高于单独MeJA处理,而GA、SA、ABA单独处理基因的表达量高于其与Ca2+一起处理。以上结果为马尾松MAPK基因与信号物质途径互作应答非生物胁迫提供参考。
陈虎, 杨章旗, 孙爽, 李鹏, 徐慧兰. 马尾松MAPK级联途径应答信号物质基因的表达分析[J]. 生物技术通报, 2022, 38(6): 187-197.
CHEN Hu, YANG Zhang-qi, SUN Shuang, LI Peng, XU Hui-lan. Expressions and of Genes Response to Signal Substances in MAPK Cascade Pathway Genes in Pinus massoniana[J]. Biotechnology Bulletin, 2022, 38(6): 187-197.
| Gene | Forward primer(5'-3') | Reverse primer(5'-3') |
|---|---|---|
| PmCYP | CAAGGGTTCGTCGTTCCAC | TACGGCGAGAAGTTTGCC |
| PmMAPK2 | CCATGGCGGTCAGTATGTGCAG | CATATGGCATTGTCTGCTCGGCA |
| PmMAPK6 | CCGCAGCAGCTCCAGTTCAATC | CTCAGTCACGGCGGCCTCTT |
| PmMAPK9 | GATCCGCAATGAGAAGGCCAGAAG | CCTTCGTCTGCTTGAGCGTATGC |
| PmMAPK15 | CCTCATCAGAGCAGGCATCTTCAG | GAGAAGCAGCACCAAGACAGGAG |
| PmMAP2K | GTTGGAGTACATGGACGGTGGAAC | GGTTGCCAGACAGGTGCTGAAG |
| PmMAP3K | AGCAGCAGTCCTCCAGCGATC | GAGGTCTTGATTGCCGCCAACAA |
| PmMAP4K | ATGCTTCTCTGCCTCCTCTTCTCC | GACGATTCCGAAGTTCCGCAACA |
表1 荧光定量所用基因引物
Table 1 Primer used in the qRT-PCR
| Gene | Forward primer(5'-3') | Reverse primer(5'-3') |
|---|---|---|
| PmCYP | CAAGGGTTCGTCGTTCCAC | TACGGCGAGAAGTTTGCC |
| PmMAPK2 | CCATGGCGGTCAGTATGTGCAG | CATATGGCATTGTCTGCTCGGCA |
| PmMAPK6 | CCGCAGCAGCTCCAGTTCAATC | CTCAGTCACGGCGGCCTCTT |
| PmMAPK9 | GATCCGCAATGAGAAGGCCAGAAG | CCTTCGTCTGCTTGAGCGTATGC |
| PmMAPK15 | CCTCATCAGAGCAGGCATCTTCAG | GAGAAGCAGCACCAAGACAGGAG |
| PmMAP2K | GTTGGAGTACATGGACGGTGGAAC | GGTTGCCAGACAGGTGCTGAAG |
| PmMAP3K | AGCAGCAGTCCTCCAGCGATC | GAGGTCTTGATTGCCGCCAACAA |
| PmMAP4K | ATGCTTCTCTGCCTCCTCTTCTCC | GACGATTCCGAAGTTCCGCAACA |
| 基因 Gene | 开放阅读框 Open reading frame | 氨基酸序列Amino acid sequence/aa | 蛋白分子量Protein mass/kD | 等电点 Protein isoelectric point | 亚细胞定位Subcellular localization | 跨膜结构Transmembrane | 磷酸化位点Kinase phosphorylation(Ser,Thr,Tyr) | 二级结构Secondary structure/% | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α螺旋 Alpha helix | 延伸链 Extended strand | β转角 Beta turn | 无规卷曲 Random coil | ||||||||
| PmMAPK6 | 1 173 | 390 | 44.49 | 5.38 | 细胞核 | 无 | 8,10,8 | 42.31 | 13.59 | 4.36 | 39.74 |
| PmMAPK9 | 1 839 | 612 | 69.41 | 9.11 | 细胞核 | 无 | 44,23,8 | 44.77 | 10.46 | 4.25 | 40.52 |
| PmMAPK2 | 1 119 | 372 | 42.95 | 5.28 | 细胞核 | 无 | 11,11,9 | 43.82 | 14.25 | 5.91 | 36.02 |
| PmMAPK15 | 1 731 | 576 | 65.7 | 9.22 | 细胞核 | 无 | 30,16,11 | 38.54 | 12.85 | 5.73 | 42.88 |
| PmMAP2K | 1 014 | 337 | 37.62 | 9.02 | 细胞核 | 无 | 15,4,3 | 34.42 | 16.02 | 5.64 | 43.92 |
| PmMAP3K | 1 962 | 653 | 72.33 | 5.86 | 细胞核 | 无 | 56,26,7 | 28.18 | 9.8 | 4.44 | 57.58 |
| PmMAP4K | 2 748 | 915 | 100.62 | 5.12 | 细胞核 | 无 | 77,41,19 | 29.4 | 12.13 | 4.92 | 53.55 |
表2 MAPKs蛋白生物信息学分析
Table 2 Bioinformatics analysis of MAPKs protein in P. massoniana
| 基因 Gene | 开放阅读框 Open reading frame | 氨基酸序列Amino acid sequence/aa | 蛋白分子量Protein mass/kD | 等电点 Protein isoelectric point | 亚细胞定位Subcellular localization | 跨膜结构Transmembrane | 磷酸化位点Kinase phosphorylation(Ser,Thr,Tyr) | 二级结构Secondary structure/% | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α螺旋 Alpha helix | 延伸链 Extended strand | β转角 Beta turn | 无规卷曲 Random coil | ||||||||
| PmMAPK6 | 1 173 | 390 | 44.49 | 5.38 | 细胞核 | 无 | 8,10,8 | 42.31 | 13.59 | 4.36 | 39.74 |
| PmMAPK9 | 1 839 | 612 | 69.41 | 9.11 | 细胞核 | 无 | 44,23,8 | 44.77 | 10.46 | 4.25 | 40.52 |
| PmMAPK2 | 1 119 | 372 | 42.95 | 5.28 | 细胞核 | 无 | 11,11,9 | 43.82 | 14.25 | 5.91 | 36.02 |
| PmMAPK15 | 1 731 | 576 | 65.7 | 9.22 | 细胞核 | 无 | 30,16,11 | 38.54 | 12.85 | 5.73 | 42.88 |
| PmMAP2K | 1 014 | 337 | 37.62 | 9.02 | 细胞核 | 无 | 15,4,3 | 34.42 | 16.02 | 5.64 | 43.92 |
| PmMAP3K | 1 962 | 653 | 72.33 | 5.86 | 细胞核 | 无 | 56,26,7 | 28.18 | 9.8 | 4.44 | 57.58 |
| PmMAP4K | 2 748 | 915 | 100.62 | 5.12 | 细胞核 | 无 | 77,41,19 | 29.4 | 12.13 | 4.92 | 53.55 |
| 基因名/结构域Gene/Domain | MAPK | MAP2K | MAP3K | MAP4K | SPS1 | PknB | BREX-PgiW |
|---|---|---|---|---|---|---|---|
| PmMAPK6 | 50-386 | 63-377 | 111-260 | ||||
| PmMAPK9 | 91-428 | 92-384 | 89-380 | ||||
| PmMAPK2 | 32-368 | 42-344 | 95-242 | ||||
| PmMAPK15 | 24-361 | 25-317 | 24-233 | 71-246 | |||
| PmMAP2K | 56-323 | 59-336 | 112-258 | 64-258 | |||
| PmMAP3K | 40-303 | 41-396 | 40-298 | ||||
| PmMAP4K | 299-554 | 299-558 | 300-549 |
表3 MAPKs蛋白功能结构域分析
Table 3 Analysis functional domain of MAPKs protein in P. massoniana
| 基因名/结构域Gene/Domain | MAPK | MAP2K | MAP3K | MAP4K | SPS1 | PknB | BREX-PgiW |
|---|---|---|---|---|---|---|---|
| PmMAPK6 | 50-386 | 63-377 | 111-260 | ||||
| PmMAPK9 | 91-428 | 92-384 | 89-380 | ||||
| PmMAPK2 | 32-368 | 42-344 | 95-242 | ||||
| PmMAPK15 | 24-361 | 25-317 | 24-233 | 71-246 | |||
| PmMAP2K | 56-323 | 59-336 | 112-258 | 64-258 | |||
| PmMAP3K | 40-303 | 41-396 | 40-298 | ||||
| PmMAP4K | 299-554 | 299-558 | 300-549 |
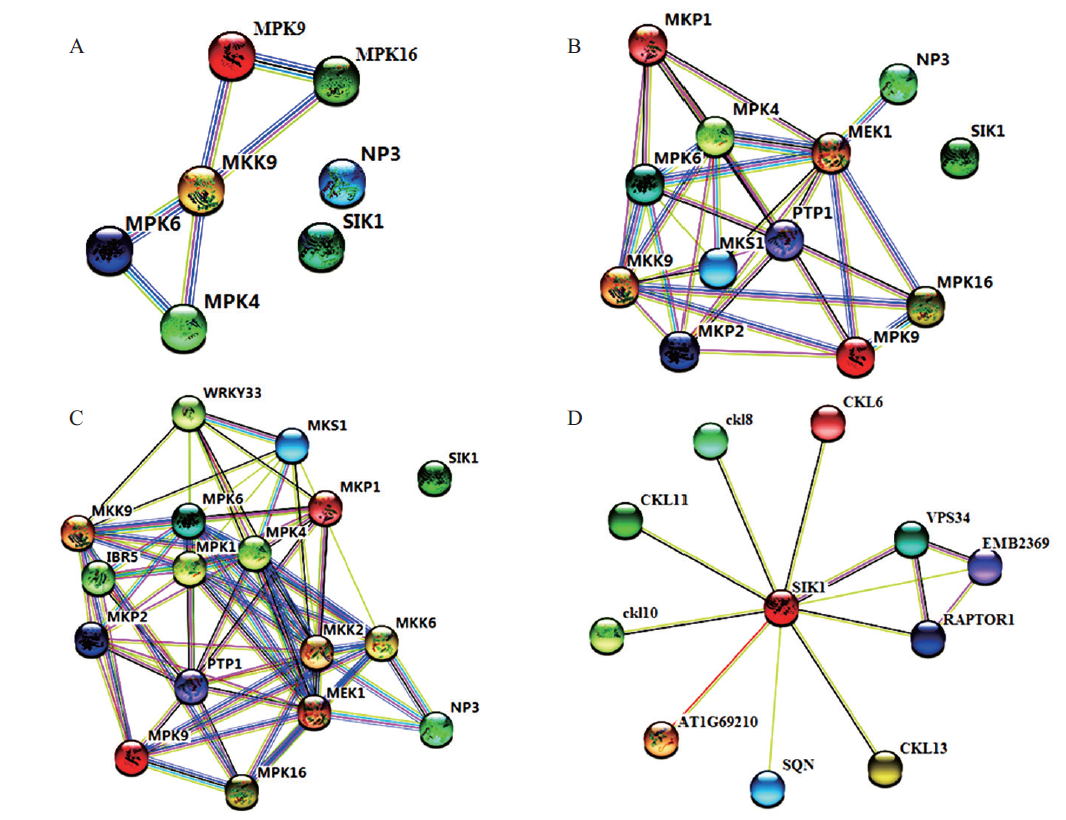
图3 马尾松与拟南芥相关MAPKs基因互作网络分析 A:马尾松MAPK级联途径相关基因一级互作网络;B:马尾松MAPK级联途径相关基因二级互作网络;C:马尾松MAPK级联途径相关基因三级互作网络;D:PmMAP4K基因互作网络
Fig. 3 Interaction network analysis of MAPKs proteins identified in P. massoniana and Arabidopsis A:A first-order interaction network of genes related to MAPK cascade pathway in P. massoniana;B:secondary interaction network of genes related to MAPK cascade pathway in P. massoniana;C:a three-level interaction network of genes related to MAPK cascade pathway in P. massoniana;D:the PmMAP4K gene interaction network
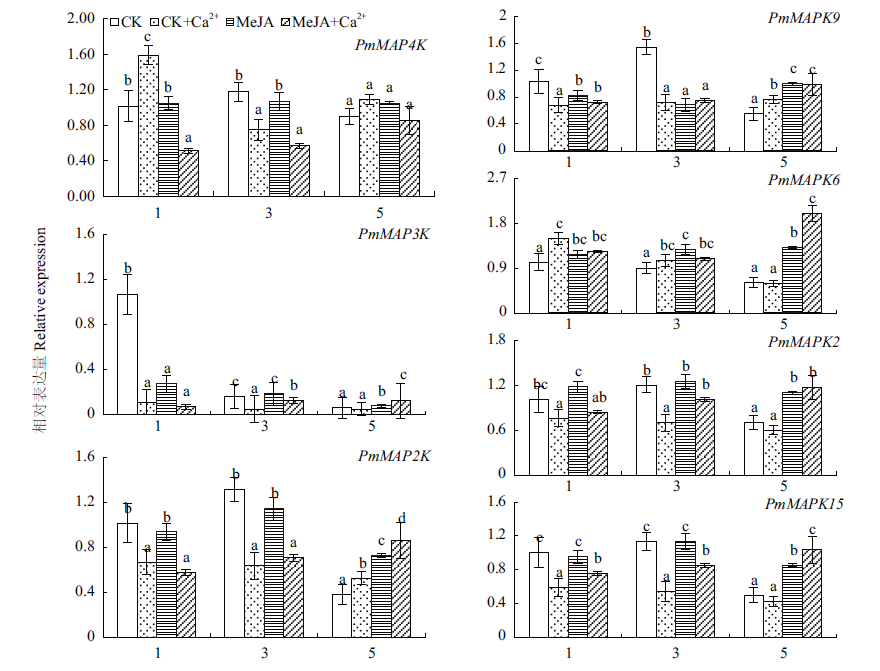
图4 马尾松MAPK级联途径基因在MeJA处理下表达模式 不同字母表示0.05水平上有显著差异,下同
Fig. 4 Expressions of MAPKs genes in response to MeJA treatments in P. massoniana Different letters indicate significant differences at the 0.05 level. The same below
| [1] |
Nakagami H, et al. Emerging MAP kinase pathways in plant stress signalling[J]. Trends Plant Sci, 2005, 10(7):339-346.
doi: 10.1016/j.tplants.2005.05.009 URL |
| [2] |
Ichimura K, Mizoguchi T, Yoshida R, et al. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6[J]. Plant J, 2000, 24(5):655-665.
pmid: 11123804 |
| [3] | Joshi R, Wani SH, Singh B, et al. Transcription factors and plants response to drought stress:current understanding and future directions[J]. Front Plant Sci, 2016, 7:1029. |
| [4] |
Xiong L, Yang Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase[J]. Plant Cell, 2003, 15(3):745-759.
doi: 10.1105/tpc.008714 URL |
| [5] |
Ludwig AA, et al. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants[J]. PNAS, 2005, 102(30):10736-10741.
doi: 10.1073/pnas.0502954102 URL |
| [6] | Sun TJ, Nitta Y, Zhang Q, et al. Antagonistic interactions between two MAP kinase cascades in plant development and immune signaling[J]. EMBO Rep, 2018, 19(7):e45324. |
| [7] |
Jagodzik P, Tajdel-Zielinska M, Ciesla A, et al. Mitogen-activated protein kinase cascades in plant hormone signaling[J]. Front Plant Sci, 2018, 9:1387.
doi: 10.3389/fpls.2018.01387 URL |
| [8] | Wersch RV, Gao F, Zhang Y. Mitogen-activated protein kinase kinase 6 negatively regulates anthocyanin induction in Arabidopsis[J]. Plant Signal Behav, 2018, 13(10):e1526000. |
| [9] |
Takahashi F, Yoshida R, Ichimura K, et al. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis[J]. Plant Cell, 2007, 19(3):805-818.
pmid: 17369371 |
| [10] | 杨章旗, 冯源恒, 谭健晖, 等. 广西马尾松高世代育种策略研究[J]. 广西林业科学, 2018, 47(3):251-256. |
| Yang ZQ, Feng YH, Tan JH, et al. Advanced generation breeding strategy of Pinus massoniana in Guangxi[J]. Guangxi For Sci, 2018, 47(3):251-256. | |
| [11] |
Xiao F, Zhao Y, Wang XR, et al. Transcriptome analysis of needle and root of Pinus massoniana in response to continuous drought stress[J]. Plants, 2021, 10(4):769.
doi: 10.3390/plants10040769 URL |
| [12] | Yang ZQ, Chen H, Jia J, et al. De novo assembly and discovery of metabolic pathways and genes that are involved in defense against pests in Songyun Pinus massoniana Lamb[J]. Bangladesh J Bot, 2016, 45:855-863. |
| [13] |
Chen H, Tan J, Liang X, et al. Molecular mechanism of lateral bud differentiation of Pinus massoniana based on high-throughput sequencing[J]. Sci Rep, 2021, 11(1):9033.
doi: 10.1038/s41598-021-87787-7 pmid: 33907200 |
| [14] |
Chen H, Yang ZQ, Hu Y, et al. Reference genes selection for quantitative gene expression studies in Pinus massoniana L[J]. Trees, 2016, 30(3):685-696.
doi: 10.1007/s00468-015-1311-3 URL |
| [15] |
Zhang X, Wang L, Xu X, et al. Genome-wide identification of mitogen-activated protein kinase gene family in Gossypium raimondii and the function of their corresponding orthologs in tetraploid cultivated cotton[J]. BMC Plant Biol, 2014, 14:345.
doi: 10.1186/s12870-014-0345-9 URL |
| [16] |
Jonak C, Okrész L, Bögre L, et al. Complexity, cross talk and integration of plant MAP kinase signalling[J]. Curr Opin Plant Biol, 2002, 5(5):415-424.
doi: 10.1016/S1369-5266(02)00285-6 URL |
| [17] | 王海波, 等. 小桐子MAPKKKK基因家族的全基因组鉴定及表达分析[J]. 植物生理学报, 2019, 55(3):367-377. |
| Wang HB, Guo JY, Tang LZ. Genome-wide identification and expression analysis of MAPKKKK gene family in Jatropha curcas[J]. Plant Physiol J, 2019, 55(3):367-377. | |
| [18] |
Jia CG, Zhang LP, et al. Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternata f. sp. lycopersici[J]. J Exp Bot, 2013, 64(2):637-650.
doi: 10.1093/jxb/ers360 URL |
| [19] |
Wasternack C, Strnad M. Jasmonate signaling in plant stress responses and development - active and inactive compounds[J]. N Biotechnol, 2016, 33(5 pt b):604-613.
doi: 10.1016/j.nbt.2015.11.001 URL |
| [20] |
Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis[J]. Plant Cell, 2004, 16(12):3386-3399.
doi: 10.1105/tpc.104.026609 URL |
| [21] | Brader G, Djamei A, Teige M, et al. The MAP kinase kinase MKK2 affects disease resistance in Arabidopsis[J]. Mol Plant Microbe Interactions®, 2007, 20(5):589-596. |
| [22] |
Kandoth PK, et al. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects[J]. PNAS, 2007, 104(29):12205-12210.
doi: 10.1073/pnas.0700344104 URL |
| [23] |
Wang J, Pan C, Wang Y, et al. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber[J]. BMC Genomics, 2015, 16:386.
doi: 10.1186/s12864-015-1621-2 pmid: 25976104 |
| [24] |
Ding X, Richter T, Chen M, et al. A rice kinase-protein interaction map[J]. Plant Physiol, 2009, 149(3):1478-1492.
doi: 10.1104/pp.108.128298 URL |
| [25] |
Burnett EC, Desikan R, Moser RC, et al. ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA[J]. J Exp Bot, 2000, 51(343):197-205.
pmid: 10938826 |
| [26] |
Seo S, Katou S, Seto H, et al. The mitogen-activated protein kinases WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants[J]. Plant J, 2007, 49(5):899-909.
doi: 10.1111/j.1365-313X.2006.03003.x URL |
| [27] | Han Y, et al. Correction:the suppression of WRKY44 by GIGA-NTEA-miR172 pathway is involved in drought response of Arabi-dopsis thaliana[J]. PLoS One, 2015, 10(4):e0124854. |
| [28] |
Enders TA, Frick EM, Strader LC. An Arabidopsis kinase cascade influences auxin-responsive cell expansion[J]. Plant J, 2017, 92(1):68-81.
doi: 10.1111/tpj.13635 URL |
| [29] |
Danquah A, de Zélicourt A, Boudsocq M, et al. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana[J]. Plant J, 2015, 82(2):232-244.
doi: 10.1111/tpj.12808 URL |
| [30] |
Chardin C, Schenk ST, Hirt H, et al. Review:mitogen-activated protein kinases in nutritional signaling in Arabidopsis[J]. Plant Sci, 2017, 260:101-108.
doi: 10.1016/j.plantsci.2017.04.006 URL |
| [31] |
Ortiz-Masia D, Perez-Amador MA, Carbonell J, et al. Diverse stress signals activate the C1 subgroup MAP kinases of Arabidopsis[J]. FEBS Lett, 2007, 581(9):1834-1840.
pmid: 17433310 |
| [32] |
Ghawana S, Kumar S, Ahuja PS. Early low-temperature responsive mitogen activated protein kinases RaMPK1 and RaMPK2 from Rheum australe D. Don respond differentially to diverse stresses[J]. Mol Biol Rep, 2010, 37(2):933-938.
doi: 10.1007/s11033-009-9726-9 pmid: 19688272 |
| [33] |
Hill RD, Liu JH, Durnin D, et al. Abscisic acid structure-activity relationships in barley aleurone layers and protoplasts(biological activity of optically active, oxygenated abscisic acid analogs)[J]. Plant Physiol, 1995, 108(2):573-579.
pmid: 12228494 |
| [1] | 刘畅宇, 陈勋, 龙雨青, 陈娅, 刘湘丹, 周日宝. 乙烯生物合成及信号转导途径中介导花衰老相关基因的研究进展[J]. 生物技术通报, 2019, 35(3): 171-182. |
| [2] | 董维鹏,王君实,张少华,燕炯. CRISPR系统及其应用于小鼠的研究进展[J]. 生物技术通报, 2018, 34(5): 57-63. |
| [3] | 田晓明, 颜立红, 向光锋, 蒋利媛. 植物4香豆酸:辅酶A连接酶研究进展[J]. 生物技术通报, 2017, 33(4): 19-26. |
| [4] | 郑育青. 螺旋霉素生物合成中的分子、基因及发酵调控技术[J]. 生物技术通报, 2016, 32(8): 62-68. |
| [5] | 陈珍珠,李蕊,田飞,沈彦婷,葛芹玉. 高选择性和高灵敏度的microRNA检测技术的研究进展[J]. 生物技术通报, 2016, 32(4): 39-47. |
| [6] | 王以斌, 张爱军, 刘芳明, 郑洲, 缪锦来. 南极冰藻对南极极端环境的适应性研究进展[J]. 生物技术通报, 2016, 32(10): 128-134. |
| [7] | 王维,张玉娟,陈洁,刘聚波,夏民旋,沈法富. 植物逆境胁迫相关miRNA研究进展[J]. 生物技术通报, 2015, 31(1): 1-10. |
| [8] | 李杏春, 何双辉. 大伏革菌防治针叶树根腐病的研究进展[J]. 生物技术通报, 2014, 0(7): 26-32. |
| [9] | 李敬, 谷慧英, 王志敏, 汤青林, 宋明. 拟南芥成花关键基因调控网络研究进展[J]. 生物技术通报, 2014, 0(12): 1-8. |
| [10] | 魏运荣;卢清显;卢清君;. MicroRNA与细胞信号转导通路研究进展[J]. , 2010, 0(02): 28-32. |
| [11] | 曾(杰)邦哲;. 应用系统遗传学与细胞发生的基因调控网络序列标记显示分析[J]. , 2010, 0(01): 63-67. |
| [12] | 张清国;杨文华;韩素英;齐力旺;梁国鲁;. 针叶树多倍体及其在遗传育种中的应用研究[J]. , 2009, 0(S1): 58-61. |
| [13] | 刘俊杰;魏小春;齐树森;史为民;. 反义基因技术及其在植物研究上的应用[J]. , 2008, 0(04): 78-84. |
| [14] | 张雪荣;谭四军;吴韩英;胡鸢雷;慈忠玲;林忠平;. 植物腺体的萜类代谢工程[J]. , 2007, 0(03): 49-51. |
| [15] | 高原;陈信波;张志扬;. 木质素生物合成途径及其基因调控的研究进展[J]. , 2007, 0(02): 47-51. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||