








生物技术通报 ›› 2022, Vol. 38 ›› Issue (6): 235-244.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1105
收稿日期:2021-08-27
出版日期:2022-06-26
发布日期:2022-07-11
作者简介:赵子玉,女,硕士,研究方向:细菌耐药性;E-mail: 基金资助:
ZHAO Zi-yu( ), WANG Chun-guang, LV Jian-cun, LI Ji-kai, ZHANG Tie(
), WANG Chun-guang, LV Jian-cun, LI Ji-kai, ZHANG Tie( )
)
Received:2021-08-27
Published:2022-06-26
Online:2022-07-11
摘要:
β-内酰胺酶的产生是细菌产生耐药的常见机制,其主要代表为超广谱β-内酰胺酶,其中CTX-M-14在全世界呈现流行趋势,为寻找一种CTX-M-14抑制剂,使细菌恢复对β-内酰胺类抗生素的敏感性,本试验首先构建了CTX-M-14的原核表达载体,并以CTX-M-14为靶点借助虚拟筛选工具Autodock Vina进行筛选,得到结合作用较好的中药单体抑制剂芸香苷,通过DS Visualizer分析其相关作用力;通过联合抑菌试验验证芸香苷与抗生素的联合抑菌作用;通过酶动力学试验比较抑酶剂芸香苷与克拉维酸的抑酶作用与方式。结果表明,芸香苷与超广谱β-内酰胺酶CTX-M-14结合部位形成多个氢键和范德华力,其结合作用力为9.9 kcal/mol;芸香苷与头孢噻肟钠对大肠杆菌E320呈协同作用(FICI=0.236);0.312 5 mg/mL的芸香苷与头孢噻肟钠对CTX-M-14蛋白阳性重组菌呈协同作用(FICI≤0.375);1.25 mg/mL的芸香苷与头孢噻肟钠对导入空质粒pET-28a(+)的BL-21呈无关作用(FICI=1.5);抑酶剂芸香苷与克拉维酸均为竞争性抑制剂,但克拉维酸抑酶作用优于芸香苷。本试验证明,芸香苷可以竞争性抑制CTX-M-14活性,具有β-内酰胺酶抑制剂潜质。
赵子玉, 王春光, 吕建存, 李继开, 张铁. 超广谱β-内酰胺酶CTX-M-14中药抑制剂的筛选及芸香苷抑酶作用研究[J]. 生物技术通报, 2022, 38(6): 235-244.
ZHAO Zi-yu, WANG Chun-guang, LV Jian-cun, LI Ji-kai, ZHANG Tie. Screening of β-lactamase CTX-M-14 Chinese Medicine Inhibitor and Enzyme Inhibition of Rutin[J]. Biotechnology Bulletin, 2022, 38(6): 235-244.

图4 重组蛋白的表达检测 A:CTX-M-14重组蛋白的诱导表达(M:蛋白marker;1:诱导前;2:诱导后);B:重组蛋白的可溶性检测(M:蛋白marker;1:上清;2:沉淀)
Fig. 4 Expression detection of recombinant protein A:CTX-M-14 recombinant protein(M:Protein marker;1:before induction;2:after induction). B:Soluble detection of recombinant protein(M:Protein marker;1:supernatant;2:precipitation)
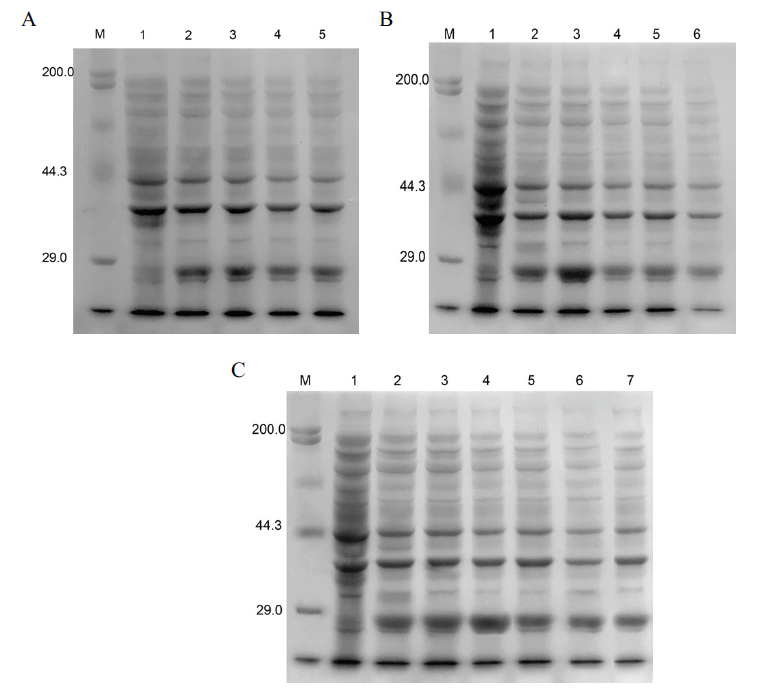
图5 蛋白表达条件的优化 A:诱导时间的优化(M:蛋白marker;1:诱导前;2:3 h;3:4 h;4:5 h;5:6 h);B:诱导温度的优化(M:蛋白marker;1:诱导前;2:26℃;3:31℃;4:34℃;5:37℃);C:IPTG浓度的优化(M:蛋白marker;1:诱导前;2:0.2 mmol/L;3:0.4 mmol/L;4:0.6 mmol/L;5:0.8 mmol/L;6:1.0 mmol/L;7:1.2 mmol/L)
Fig. 5 Optimization of protein expression conditions A:Optimization of induction time(M:Protein marker;1:before induction;2:3 h;3:4 h;4:5 h;5:6 h). B:Optimization of induction temperature(M:Protein marker;1:before induction;2:26℃;3:31℃;4:34℃;5:37℃). C:Optimization of IPTG concentration(M:Protein marker;1:before induction;2:0.2 mmol/L;3:0.4 mmol/L;4:0.6 mmol/L;5:0.8 mmol/L;6:1.0 mmol/L;7:1.2 mmol/L)
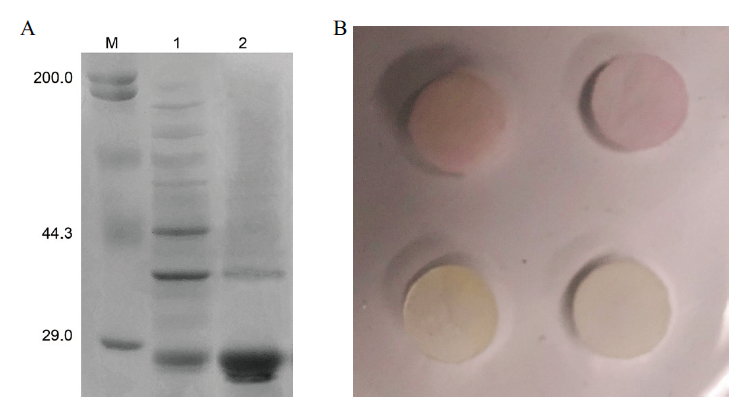
图6 重组蛋白纯化及活性鉴定 A:蛋白纯化鉴定(M:蛋白marker;1:纯化前;2:纯化后);B:蛋白活性鉴定,上排:加入CTX-M-14重组蛋白组;下排:空白对照组
Fig. 6 Purification and activity identification of recomb-inant protein A:Protein purification and identification(M:Protein marker;1:before purification);2:after purification. B:Identification of protein activity. Top row:CTX-M-14 recombinant protein group is added. Lower row:Blank control group
| 配体Ligand | 结合能Binding affinity/(kcal·mol-1) | 均方根偏差/最大值RMSD/UB | 均方根偏差/最小值RMSD/LB |
|---|---|---|---|
| Rhein diglucoside | -11.5 | 0 | 0 |
| Agastachin | -10.5 | 0 | 0 |
| Glycyrrhizin | -10 | 0 | 0 |
| Timosaponin A3 | -10 | 0 | 0 |
| Biepiasterolide | -10 | 0 | 0 |
| Sennidin A | -9.9 | 0 | 0 |
| Rutin | -9.9 | 0 | 0 |
| Ciwujianoside | -9.9 | 0 | 0 |
| Galloylpaeoniflorin | -9.8 | 0 | 0 |
| Acaciin | -9.8 | 0 | 0 |
表1 Autodock Vina虚拟筛选的部分中药成分评分结果
Table 1 Scoring results of some Chinese herbal medicinal ingredients screened virtually by AutoDock Vina
| 配体Ligand | 结合能Binding affinity/(kcal·mol-1) | 均方根偏差/最大值RMSD/UB | 均方根偏差/最小值RMSD/LB |
|---|---|---|---|
| Rhein diglucoside | -11.5 | 0 | 0 |
| Agastachin | -10.5 | 0 | 0 |
| Glycyrrhizin | -10 | 0 | 0 |
| Timosaponin A3 | -10 | 0 | 0 |
| Biepiasterolide | -10 | 0 | 0 |
| Sennidin A | -9.9 | 0 | 0 |
| Rutin | -9.9 | 0 | 0 |
| Ciwujianoside | -9.9 | 0 | 0 |
| Galloylpaeoniflorin | -9.8 | 0 | 0 |
| Acaciin | -9.8 | 0 | 0 |

图7 芸香苷与超广谱β-内酰胺酶CTX-M-14作用力分析 A:芸香苷与CTX-M-14结合的2D结构示意图;B:芸香苷与CTX-M-14结合的3D结构示意图
Fig. 7 Interaction analysis of rutin with extended-spectrum β-lactamases CTX-M-14 A:2D structure diagram of the binding of rutin with CTX-M-14;B:3D schematic diagram of the binding structure of rutin with CTX-M-14
| 药物Drug | E320 | 重组蛋白阳性菌BL-21 Recombinant protein-positive bacterium BL-21 |
|---|---|---|
| 芸香苷/(mg·mL-1) | 2.5 | 2.5 |
| 苯唑西林钠/(μg·mL-1) | >256(R) | 64(R) |
| 氨苄西林钠/(μg·mL-1) | >256(R) | 64(R) |
| 头孢唑啉钠/(μg·mL-1) | >256(R) | 16(R) |
| 头孢他啶/(μg·mL-1) | >256(R) | 8(I) |
| 头孢呋辛/(μg·mL-1) | 4(S) | 16(I) |
| 头孢曲松钠/(μg·mL-1) | >256(R) | 32(R) |
| 头孢噻肟钠/(μg·mL-1) | 1024(R) | >1024(R) |
表2 大肠杆菌E320、重组蛋白阳性菌BL-21的MIC
Table 2 MIC of E. coli E320 and recombinant protein positive BL-21
| 药物Drug | E320 | 重组蛋白阳性菌BL-21 Recombinant protein-positive bacterium BL-21 |
|---|---|---|
| 芸香苷/(mg·mL-1) | 2.5 | 2.5 |
| 苯唑西林钠/(μg·mL-1) | >256(R) | 64(R) |
| 氨苄西林钠/(μg·mL-1) | >256(R) | 64(R) |
| 头孢唑啉钠/(μg·mL-1) | >256(R) | 16(R) |
| 头孢他啶/(μg·mL-1) | >256(R) | 8(I) |
| 头孢呋辛/(μg·mL-1) | 4(S) | 16(I) |
| 头孢曲松钠/(μg·mL-1) | >256(R) | 32(R) |
| 头孢噻肟钠/(μg·mL-1) | 1024(R) | >1024(R) |
| 菌株 Strain | 药物 Drug | 单药MIC MIC of single drug | 联合用药MIC MIC of combination drugs | FICI | 结果判定 Result determination |
|---|---|---|---|---|---|
| E320 | 芸香苷/(mg·mL-1) | 2.5 | 0.625 | 0.236 | 协同作用 |
| 头孢噻肟钠/(μg·mL-1) | 1 024 | 16 | |||
| CTX-M-14蛋白阳性重组菌 | 芸香苷/(mg·mL-1) | 2.5 | 0.312 5 | <0.375 | 协同作用 |
| 头孢噻肟钠/(μg·mL-1) | >1 024 | 256 | |||
| 导入空白质粒pET-28a(+)的BL-21 | 芸香苷/(mg·mL-1) | 2.5 | 1.25 | 1.5 | 无关作用 |
| 头孢噻肟钠/(μg·mL-1) | 8 | 8 |
表3 芸香苷和头孢噻肟钠联合抑菌试验
Table 3 Combined antibacterial test of rutin and cefotaxime sodium
| 菌株 Strain | 药物 Drug | 单药MIC MIC of single drug | 联合用药MIC MIC of combination drugs | FICI | 结果判定 Result determination |
|---|---|---|---|---|---|
| E320 | 芸香苷/(mg·mL-1) | 2.5 | 0.625 | 0.236 | 协同作用 |
| 头孢噻肟钠/(μg·mL-1) | 1 024 | 16 | |||
| CTX-M-14蛋白阳性重组菌 | 芸香苷/(mg·mL-1) | 2.5 | 0.312 5 | <0.375 | 协同作用 |
| 头孢噻肟钠/(μg·mL-1) | >1 024 | 256 | |||
| 导入空白质粒pET-28a(+)的BL-21 | 芸香苷/(mg·mL-1) | 2.5 | 1.25 | 1.5 | 无关作用 |
| 头孢噻肟钠/(μg·mL-1) | 8 | 8 |
| 组别Group | Km | Vmax | Ki | Km/Vmax |
|---|---|---|---|---|
| 空白对照组 | 7.13 | 21.33 | - | 0.33 |
| 溶剂对照组 | 7.11 | 20.97 | - | 0.34 |
| 芸香苷组 | 8.76 | 17.63 | 11.38 | 0.50 |
| 克拉维酸组 | 8.45 | 17.79 | 3.78 | 0.47 |
表4 酶动力学参数
Table 4 Enzyme kinetic parameters
| 组别Group | Km | Vmax | Ki | Km/Vmax |
|---|---|---|---|---|
| 空白对照组 | 7.13 | 21.33 | - | 0.33 |
| 溶剂对照组 | 7.11 | 20.97 | - | 0.34 |
| 芸香苷组 | 8.76 | 17.63 | 11.38 | 0.50 |
| 克拉维酸组 | 8.45 | 17.79 | 3.78 | 0.47 |
| 组别 Group | 初始OD值 Initial OD value | 处理后OD值 OD value after treatment | OD值变化量 OD value change amount | 抑酶保护率 Enzyme inhibition protection rate/% |
|---|---|---|---|---|
| 空白对照组 | 1.15±0.035 | 0.33±0.015 | 0.82±0.026 | - |
| 溶剂对照组 | 1.14±0.020 | 0.32±0.025 | 0.81±0.015 | - |
| 克拉维酸组 | 1.19±0.025 | 0.87±0.020 | 0.32±0.020* | 60.98 |
| 芸香苷组 | 1.50±0.030 | 1.14±0.010 | 0.35±0.020* | 57.31 |
表5 抑酶保护率
Table 5 Inhibition rate of enzyme
| 组别 Group | 初始OD值 Initial OD value | 处理后OD值 OD value after treatment | OD值变化量 OD value change amount | 抑酶保护率 Enzyme inhibition protection rate/% |
|---|---|---|---|---|
| 空白对照组 | 1.15±0.035 | 0.33±0.015 | 0.82±0.026 | - |
| 溶剂对照组 | 1.14±0.020 | 0.32±0.025 | 0.81±0.015 | - |
| 克拉维酸组 | 1.19±0.025 | 0.87±0.020 | 0.32±0.020* | 60.98 |
| 芸香苷组 | 1.50±0.030 | 1.14±0.010 | 0.35±0.020* | 57.31 |
| [1] | 李昕, 曾洁, 王岱, 等. 细菌耐药耐受性机制的最新研究进展[J]. 中国抗生素杂志, 2020, 45(2):113-121. |
| Li X, Zeng J, Wang D, et al. Recent advances in the mechanism of bacterial resistance and tolerance[J]. Chin J Antibiot, 2020, 45(2):113-121. | |
| [2] | 牛琛, 武果桃, 王嘉翼. 细菌耐药性及抗菌新药物研究进展[J]. 中兽医医药杂志, 2015, 34(6):78-80. |
| Niu C, Wu GT, Wang JY. Advance in the study of bacteria drug resistance and new antibacterial medicines[J]. J Tradit Chin Vet Med, 2015, 34(6):78-80. | |
| [3] | 刘昌孝. 当代抗生素发展的挑战与思考[J]. 中国抗生素杂志, 2017, 42(1):1-12. |
| Liu CX. Challenges and thinking of current antibiotic development[J]. Chin J Antibiot, 2017, 42(1):1-12. | |
| [4] |
Varela MF, Stephen J, Lekshmi M, et al. Bacterial resistance to antimicrobial agents[J]. Antibiotics, 2021, 10(5):593.
doi: 10.3390/antibiotics10050593 URL |
| [5] |
Yang YJ, Rasmussen BA, Shlaes DM. Class A β-lactamases—enzyme-inhibitor interactions and resistance[J]. Pharmacol Ther, 1999, 83(2):141-151.
doi: 10.1016/S0163-7258(99)00027-3 URL |
| [6] | 韦志友, 丁军颖, 刘清泉. β-内酰胺酶耐药机制及其中医药相关研究进展[J]. 解放军医药杂志, 2015, 27(10):82-87. |
| Wei ZY, Ding JY, Liu QQ. Advances in β-lactamase resistance mechanism and its TCM-related research[J]. Med Pharm J Chin People’s Liberation Army, 2015, 27(10):82-87. | |
| [7] |
Willyard C. The drug-resistant bacteria that pose the greatest health threats[J]. Nature, 2017, 543(7643):15.
doi: 10.1038/nature.2017.21550 URL |
| [8] |
Bonnet R. Growing group of extended-spectrum beta-lactamases:the CTX-M enzymes[J]. Antimicrob Agents Chemother, 2004, 48(1):1-14.
doi: 10.1128/AAC.48.1.1-14.2004 pmid: 14693512 |
| [9] |
Bush K, et al. Updated functional classification of beta-lactamases[J]. Antimicrob Agents Chemother, 2010, 54(3):969-976.
doi: 10.1128/AAC.01009-09 URL |
| [10] | 黄攀. 产超广谱β-内酰胺酶大肠杆菌耐药性及其流行特征分析[D]. 扬州: 扬州大学, 2019. |
| Huang P. Analysis of drug resistance and epidemiological characteristics of extended-spectrum β-lactamase producing Escherichia coli[D]. Yangzhou: Yangzhou University, 2019. | |
| [11] |
Agga GE, Silva PJ, Martin RS. Detection of extended-spectrum beta-lactamase-producing and carbapenem-resistant bacteria from mink feces and feed in the United States[J]. Foodborne Pathog Dis, 2021, 18(7):497-505.
doi: 10.1089/fpd.2020.2898 URL |
| [12] |
Bradford PA. Extended-spectrum beta-lactamases in the 21st century:characterization, epidemiology, and detection of this important resistance threat[J]. Clin Microbiol Rev, 2001, 14(4):933-951, table of contents.
pmid: 11585791 |
| [13] | Prokesch BC, TeKippe M, Kim J, et al. Primary osteomyelitis caused by hypervirulent Klebsiella pneumoniae[J]. Lancet Infect Dis, 2016, 16(9):e190-e195. |
| [14] | Bandyopadhyay S, Bhattacharyya D, et al. Characterization of multidrug-resistant biofilm-producing Escherichia coli and Klebsiella pneumoniae in healthy cattle and cattle with diarrhea[J]. Microb Drug Resist, 2021:mdr. 2020. 0298. |
| [15] |
Denis B, Lafaurie M, Donay JL, et al. Prevalence, risk factors, and impact on clinical outcome of extended-spectrum beta-lactamase-producing Escherichia coli bacteraemia:a five-year study[J]. Int J Infect Dis, 2015, 39:1-6.
doi: 10.1016/j.ijid.2015.07.010 pmid: 26189774 |
| [16] | 徐建敏, 等. 广州市鼠形动物肠道产ESBL大肠埃希菌分布及病原特征分析[J]. 中国人兽共患病学报, 2020, 36(7):561-565, 571. |
| Xu JM, et al. Study on the antimicrobial resistance mechanism of ESBL-producing Escherichia coli isolated from rodents in Guangzhou[J]. Chin J Zoonoses, 2020, 36(7):561-565, 571. | |
| [17] | Jia P, Zhu Y, Li X, et al. High prevalence of extended-spectrum beta-lactamases in Escherichia coli strains collected from strictly defined community-acquired urinary tract infections in adults in China:a multicenter prospective clinical microbiological and molecular study[J]. Front Microbiol, 2021, 12:663033. |
| [18] |
Hayashi W, Ohsaki Y, Taniguchi Y, et al. High prevalence of blaCTX-M-14 among genetically diverse Escherichia coli recovered from retail raw chicken meat portions in Japan[J]. Int J Food Microbiol, 2018, 284:98-104.
doi: S0168-1605(18)30469-0 pmid: 30096596 |
| [19] | Po KHL, et al. Functional characterization of CTX-M-14 and CTX-M-15 β-lactamases by in vitro DNA shuffling[J]. Antimicrob Agents Chemother, 2017, 61(12):e00891-17. |
| [20] |
Biondi S, Long S, et al. Current trends in β-lactam based β-lactamases inhibitors[J]. Curr Med Chem, 2011, 18(27):4223-4236.
pmid: 21838683 |
| [21] |
Docquier JD, et al. An update on β-lactamase inhibitor discovery and development[J]. Drug Resist Updat, 2018, 36:13-29.
doi: 10.1016/j.drup.2017.11.002 URL |
| [22] |
Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors[J]. Clin Microbiol Rev, 2010, 23(1):160-201.
doi: 10.1128/CMR.00037-09 URL |
| [23] |
Toussaint KA, Gallagher JC. Β-lactam/β-lactamase inhibitor combinations:from then to now[J]. Ann Pharmacother, 2015, 49(1):86-98.
doi: 10.1177/1060028014556652 URL |
| [24] | 汪怡倩, 田晔, 乔石, 等. 中药抗产ESBLs的大肠埃希菌和肺炎克雷伯菌感染的研究进展[J]. 基层医学论坛, 2019, 23(26):3821-3824. |
| Wang YQ, Tian Y, Qiao S, et al. Research progress of Chinese herbal medicine against ESBLs-producing Escherichia coli and ESBLs-producing Klebsiella pneumoniae[J]. Med Forum, 2019, 23(26):3821-3824. | |
| [25] | 马俊利, 李春钢, 张博男, 等. 甜荞麦花叶化学成分研究[J]. 中国实验方剂学杂志, 2010, 16(13):94-96. |
| Ma JL, Li CG, Zhang BN, et al. Study on chemical constituents from flower and leaves of Fagopyrum esculentum[J]. Chin J Exp Tradit Med Formulae, 2010, 16(13):94-96. | |
| [26] | 袁倩倩, 赵海洲, 马延红, 等. 芦丁对MPP+诱导的SH-SY5Y细胞损伤的保护作用[J]. 中国实验方剂学杂志, 2018, 24(16):109-114. |
| Yuan QQ, Zhao HZ, Ma YH, et al. Protective effect of rutin on SH-SY5Y cells injured by MPP+[J]. Chin J Exp Tradit Med Formulae, 2018, 24(16):109-114. | |
| [27] | 潘然, 丁滨, 胡林峰, 等. 芦丁通过调控TG代谢途径抑制肝细胞脂肪变性的作用机制研究[J]. 云南中医学院学报, 2015, 38(5):1-6. |
| Pan R, Ding B, Hu LF, et al. Rutin inhibits oleic acid induced lipogenesis in hepatocyte cells via regulating TG metabolic pathway[J]. J Yunnan Univ Tradit Chin Med, 2015, 38(5):1-6. | |
| [28] |
Cushnie TPT, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids[J]. Int J Antimicrob Agents, 2011, 38(2):99-107.
doi: 10.1016/j.ijantimicag.2011.02.014 URL |
| [29] | Danciu C, Pinzaru IA, Dehelean CA, et al. Antiproliferative and antimicrobial properties of pure and encapsulated rutin[J]. Farmacia, 2018, 66(2):302-308. |
| [30] | 吴琼, 等. 细菌β-内酰胺酶快速检测及分型试剂盒的应用评估[J]. 检验医学, 2010, 25(1):36-37. |
| Wu Q, et al. Evaluation of a novel kit for identifying and differentiating of beta-lactamase[J]. Lab Med, 2010, 25(1):36-37. | |
| [31] | 刘保光, 等. CTX-M型超广谱β-内酰胺酶研究进展[J]. 河南农业科学, 2019, 48(12):1-7. |
| Liu BG, et al. Research progress of CTX-M type extended-spectrum β-lactamases[J]. J Henan Agric Sci, 2019, 48(12):1-7. | |
| [32] | 胡君茹, 姜华, 李喜香. 三种非水溶性供试品溶剂对抑菌试验效果的影响[J]. 西部中医药, 2013, 26(11):30-31. |
| Hu JR, Jiang H, Li XX. Study on three non-water-soluble solvents influencing of inhibitory activity in antibacterial test[J]. West J Tradit Chin Med, 2013, 26(11):30-31. | |
| [33] | 李勇湧, 等. β-内酰胺酶抑制剂抑酶动力学研究[J]. 重庆医科大学学报, 2010, 35(7):1040-1042. |
| Li YY, et al. Study of inhibitory kinetics of lactamase inhibitors[J]. J Chongqing Med Univ, 2010, 35(7):1040-1042. | |
| [34] | 刘保光. β-内酰胺酶CTX-M-14基因的基因环境及TEM-57型酶特性研究[D]. 郑州: 河南农业大学, 2011. |
| Liu BG. Genetic environment of CTX-M-14 gene β-lactamase and characteristics of TEM-57 type enzyme[D]. Zhengzhou: Henan Agricultural University, 2011. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||