








生物技术通报 ›› 2022, Vol. 38 ›› Issue (8): 92-100.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1499
收稿日期:2021-12-03
出版日期:2022-08-26
发布日期:2022-09-14
作者简介:陈光,男,博士,副研究员,研究方向:植物营养与环境胁迫;E-mail: 基金资助:
CHEN Guang1( ), LI Jia2, DU Rui-ying1, WANG Xu1(
), LI Jia2, DU Rui-ying1, WANG Xu1( )
)
Received:2021-12-03
Published:2022-08-26
Online:2022-09-14
摘要:
糖的合成和转运是植物响应干旱胁迫的关键过程。利用干旱诱导的钾转运蛋白基因OsHAK1的启动子驱动OsFLN2基因的异位表达,可以在干旱胁迫下改善水稻的糖代谢水平,进而促进根系生长。pOsHAK1:OsFLN2转基因植株叶片净光合速率、蔗糖磷酸合成酶活性、韧皮部蔗糖转运速率提高,叶片、根系中的蔗糖含量分别显著减少和增加。pOsHAK1:OsFLN2转基因株系的抗旱性优于野生型(WT)水稻,与WT相比,转基因植株脯氨酸积累量较高,而脂质过氧化水平较低。进一步研究发现,干旱处理的转基因株系中胁迫响应基因显著上调,而衰老相关基因的表达量低于WT。结果表明,通过pOsHAK1:OsFLN2的表达,促进糖分代谢,有利于提高水稻的抗旱性和在干旱胁迫下的生产力。
陈光, 李佳, 杜瑞英, 王旭. pOsHAK1:OsFLN2提高水稻的糖代谢水平和抗旱性[J]. 生物技术通报, 2022, 38(8): 92-100.
CHEN Guang, LI Jia, DU Rui-ying, WANG Xu. pOsHAK1:OsFLN2 Expression Enhances the Drought Tolerance by Altering Sugar Metabolism in Rice[J]. Biotechnology Bulletin, 2022, 38(8): 92-100.
| 基因名称Gene name | 正向引物Forward primer(5'-3') | 反向引物Reverse primer(5'-3') |
|---|---|---|
| UBQ5 | CTCGCCGACTACAACATCCA | TCTTGGGCTTGGTGTACGTCTT |
| OsFLN2 | CCGAATGGCTTCTCTTCTTCTC | GGCTCCTGATTGAGTTGGTACTACA |
| SGR | GCAATGTCGCCAAATGACG | GCTCACCACACTCATTCCCTAAAG |
| OsATG8a | AGCCCAGAAAAGGCCATCTT | CATCCTTGTTCTCTTCGTAGATTGC |
| OsSNAC1 | GTCAAGACTGATTGGATCATGC | CCAATCATCCAACCTGAGAGA |
| OsMYB2 | GAGCAGCGAGGAGGAGGT | TGTAGTTGACGAGCAGGAGGT |
表1 荧光定量PCR所用引物
Table 1 Primers used for RT-qPCR assays
| 基因名称Gene name | 正向引物Forward primer(5'-3') | 反向引物Reverse primer(5'-3') |
|---|---|---|
| UBQ5 | CTCGCCGACTACAACATCCA | TCTTGGGCTTGGTGTACGTCTT |
| OsFLN2 | CCGAATGGCTTCTCTTCTTCTC | GGCTCCTGATTGAGTTGGTACTACA |
| SGR | GCAATGTCGCCAAATGACG | GCTCACCACACTCATTCCCTAAAG |
| OsATG8a | AGCCCAGAAAAGGCCATCTT | CATCCTTGTTCTCTTCGTAGATTGC |
| OsSNAC1 | GTCAAGACTGATTGGATCATGC | CCAATCATCCAACCTGAGAGA |
| OsMYB2 | GAGCAGCGAGGAGGAGGT | TGTAGTTGACGAGCAGGAGGT |
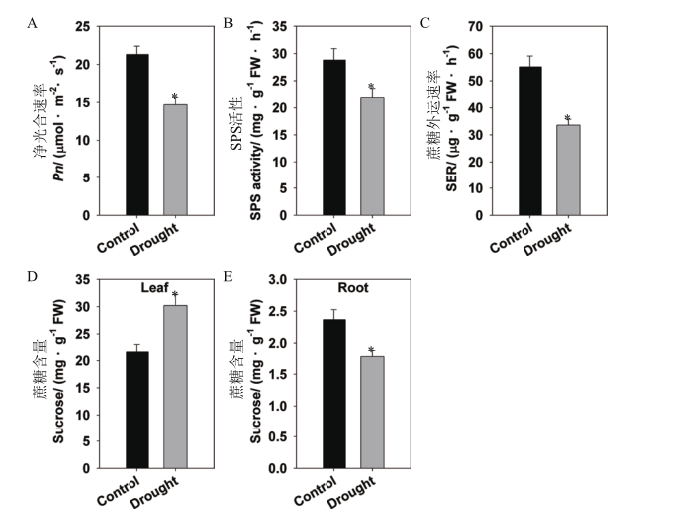
图1 干旱胁迫对水稻糖分代谢的影响 水稻在正常IRRI营养液中生长6周,再用15% PEG处理10 d。A:净光合速率(Pn);B:蔗糖磷酸合成酶(SPS)活性;C:叶片蔗糖外运速率(SER);D-E:叶片(D)和根(E)中蔗糖含量。数值显示的是平均值±SE(n=5),星号(*)表示正常和15% PEG处理之间在P<0.05水平下具有显著差异。FW:鲜重
Fig. 1 Effects of drought stress on sugar metabolism in rice Rice seedlings were growing in normal IRRI solution for 6 weeks,then treated with 15% PEG for 10 d. A:Net photosynthetic rate(Pn). B:Sucrose phosphate synthase(SPS)activity. C:Rate of sucrose export(SER)from the leaf. D-E:Sucrose contents of the leaf(D)and root(E). The values are means ± SE of 5 replicates. Significant differences between normal and 15% PEG treatment are indicated with asterisks(P < 0.05). FW:Fresh weight
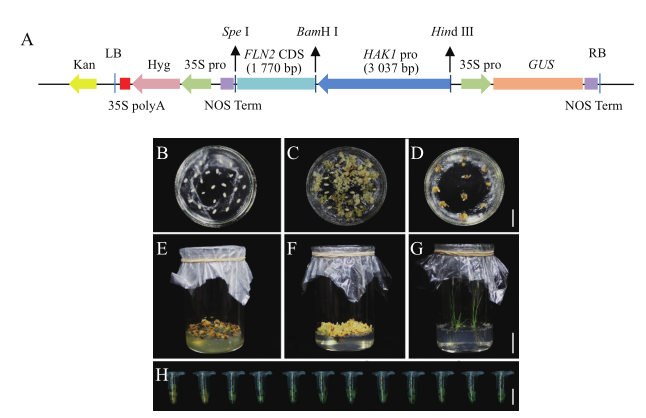
图2 表达载体的构建及水稻遗传转化过程 A:表达载体构建示意图;B-G:愈伤组织诱导(B,C)、选择(D)、分化(E,F)以及生根和炼苗(G),B-D 标尺= 2 cm,E-G标尺 = 3 cm;H:GUS染色鉴定T0代转基因阳性株系,标尺= 1 cm
Fig. 2 Construction of expression vector and process of genetic transformation in rice A:Construction map of expression vector. B-G:Callus induction(B,C),sele-ction of transformed calli(D),shoot regeneration from resistant calli(E,F),and hardening of transgenic plants(G),bar in B-D=2 cm,bar in E-G=3 cm. H:Iden-tification of positive transgenic lines by GUS staining in T0 generation,bar=1 cm
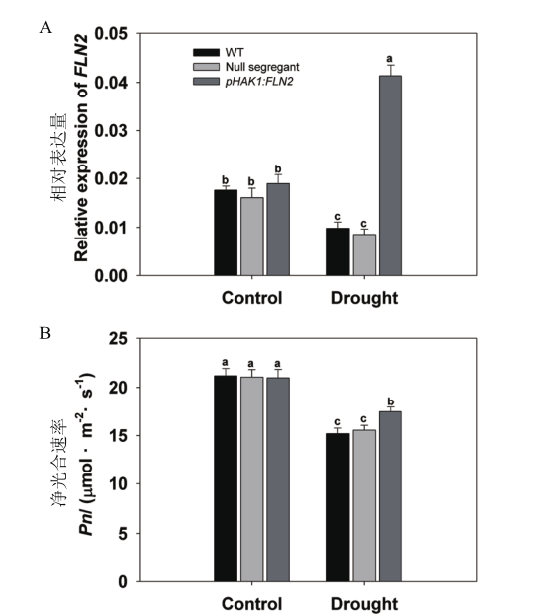
图3 干旱胁迫下T1代pHAK1:FLN2转基因株系与WT的FLN2表达量和净光合速率差异分析 水稻在正常IRRI营养液中生长2周,再用15% PEG处理7 d。A:地上部OsFLN2的RT-qPCR分析,UBQ5基因作为内参;B:净光合速率。将5个不含目的片段的分离株系合并为第2个对照,5个转基因阳性株系合并为pHAK1:FLN2。数值显示的是平均值±SE,不同的字母表示在P<0.05水平下具有显著差异
Fig. 3 Differential analysis of FLN2 expression and net photosynthetic rate between T1 generation of pHAK1:FLN2 transgenic lines and WT in response to drought stress Rice seedlings were growing in normal IRRI solution for 2 weeks,then treated with 15% PEG for 7 d. A:RT-qPCR analysis of endogenous OsFLN2 in the shoots of seedlings. UBQ5 was chosen as the reference sequence. B:Net photosynthetic rate. Results for the five null segregants were combined together as a second control(null segregant),while five transgenic lines were combined together as pHAK1:FLN2. The values are means±SE. Significant differences at P < 0.05 are indicated with different letters
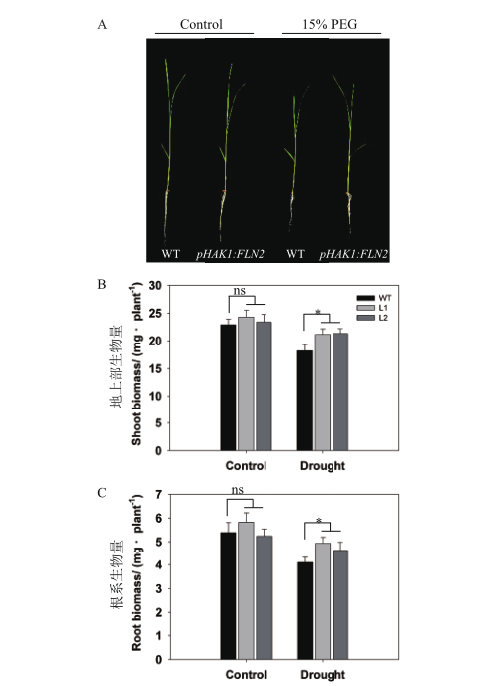
图4 干旱胁迫下pHAK1:FLN2转基因株系与WT的苗期生长差异分析 A:幼苗期植株在正常和15% PEG处理下的生长情况,标尺=5 cm;B-C:地上部(B)和根系(C)生物量(干重)。数值显示的是平均值±SE(n=5),星号和ns分别表示WT和转基因株系之间在P<0.05水平下具有和没有显著差异。下同
Fig. 4 Seedling growth of pHAK1:FLN2 transgenic lines compared with WT in response to drought stress A:Growth performance of the seedlings under normal and 15% PEG treatment,bar = 5 cm. B-C:Shoot(B)and root(C)biomass(dry weight). The values are means ± SE of 5 replicates. Significant differences between WT and transgenic lines are indicated with asterisks(P < 0.05)and ns indicates non-significant differences at that level of significance. The same below
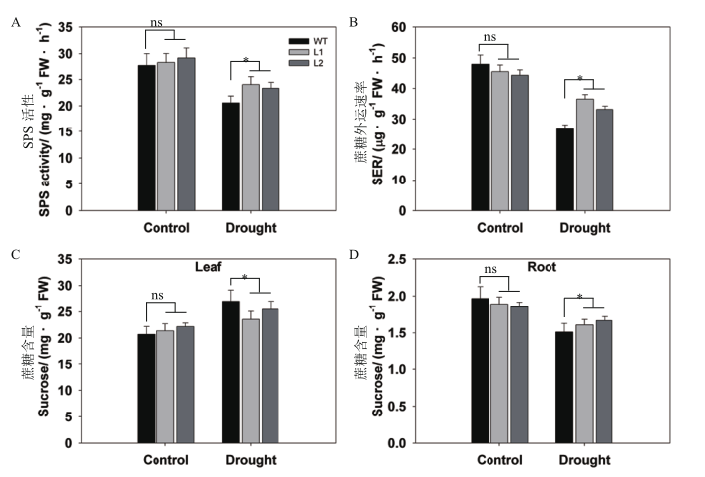
图5 干旱胁迫下pHAK1:FLN2转基因株系与WT的糖代谢差异分析 A:蔗糖磷酸合成酶(SPS)活性;B:蔗糖外运速率(SER);C-D:叶片(C)和根(D)中蔗糖含量。数值显示的是平均值±SE(n=5)
Fig. 5 Differential analysis of sugar metabolism between pHAK1:FLN2 transgenic lines and WT in response to drought stress A:Sucrose phosphate synthase(SPS)activity. B:Rate of sucrose export(SER)from the leaf. C-D:Sucrose contents of the leaf(C)and root(D).The values are means±SE of 5 replicates

图6 干旱胁迫下pHAK1:FLN2转基因株系与WT的根系构型差异分析 A:总根长;B:根表面积。数值显示的是平均值±SE(n=5)
Fig. 6 Differential analysis of root system architecture be-tween pHAK1:FLN2 transgenic lines and WT in response to drought stress A:Total root length. B:Root surface area. The values are means ± SE of 5 replicates
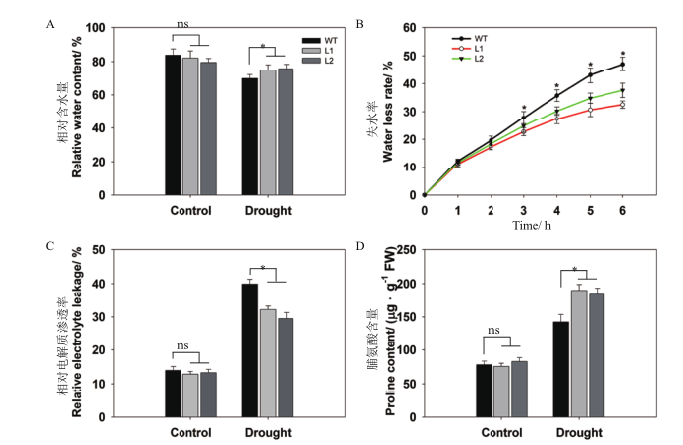
图7 干旱胁迫下pHAK1:FLN2转基因株系与WT的保水能力和脂质过氧化程度差异分析 A:相对含水量;B:叶片失水率;C:电解质渗透率;D:脯氨酸含量。数值显示的是平均值±SE(n=5)
Fig. 7 Differential analysis of water-content ability and lipid peroxidation between pHAK1:FLN2 trans-genic lines and WT in response to drought stress A:Relative water content. B:Water loss rate. C:Relative electrolyte leakage. D:Proline content. The values are means±SE of 5 replicates
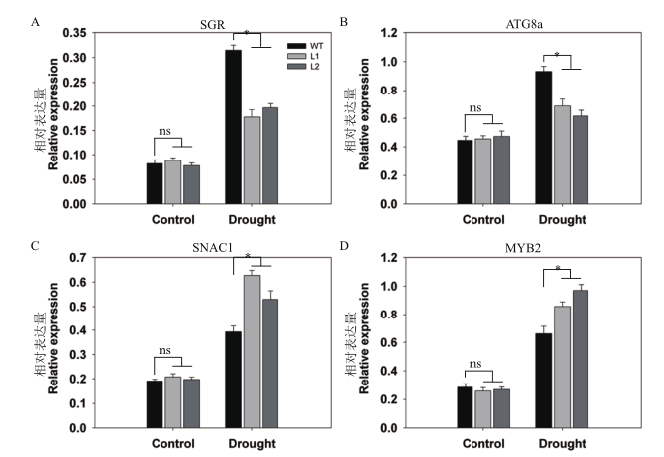
图8 干旱胁迫下pHAK1:FLN2转基因株系与WT中衰老和胁迫响应相关基因的表达差异分析 定量检测的基因有SGR(A),ATG8a(B),SNAC1(C)和MYB2(D)。UBQ5基因作为内参。数值显示的是平均值±SE(n=3)
Fig. 8 Differential analysis of expressions of senescence-associated genes and stress-responsive genes between pHAK1:FLN2 transgenic lines and WT in response to drought stress The genes assayed are(A)SGR,(B)ATG8a,(C)SNAC1 and(D)MYB2. UBQ5 is chosen as the reference gene. The values are means±SE of 3 replicates
| [1] |
Khan MIR, Palakolanu SR, Chopra P, et al. Improving drought tolerance in rice:Ensuring food security through multi-dimensional approaches[J]. Physiol Plant, 2021, 172(2):645-668.
doi: 10.1111/ppl.13223 URL |
| [2] |
Ganie SA, Ahammed GJ. Dynamics of cell wall structure and related genomic resources for drought tolerance in rice[J]. Plant Cell Rep, 2021, 40(3):437-459.
doi: 10.1007/s00299-020-02649-2 URL |
| [3] | 张磊, 许泉. 水稻干旱胁迫相关转录因子研究进展[J]. 江西农业学报, 2014, 26(10):5-11. |
| Zhang L, Xu Q. Research progress in transcription factors related to drought tolerance of rice[J]. Acta Agric Jiangxi, 2014, 26(10):5-11. | |
| [4] | Sahebi M, Hanafi MM, Rafii MY, et al. Improvement of drought tolerance in rice(Oryza sativa L.):genetics, genomic tools, and the WRKY gene family[J]. Biomed Res Int, 2018, 2018:3158474. |
| [5] |
Mathan J, Singh A, Ranjan A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice[J]. Physiol Plant, 2021, 171(4):620-637.
doi: 10.1111/ppl.13210 URL |
| [6] |
Verslues PE, Agarwal M, Katiyar-Agarwal S, et al. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status[J]. Plant J, 2006, 45(4):523-539.
pmid: 16441347 |
| [7] |
Zhang C, Li X, He Y, et al. Physiological investigation of C4-phosphoenolpyruvate-carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance[J]. Plant Physiol Biochem, 2017, 115:328-342.
doi: 10.1016/j.plaphy.2017.03.019 URL |
| [8] |
Joshi R, Sahoo KK, Singh AK, et al. Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions[J]. J Exp Bot, 2020, 71(2):653-668.
doi: 10.1093/jxb/erz462 URL |
| [9] |
Cui LH, Byun MY, Oh HG, et al. Poaceae type II galactinol synthase 2 from Antarctic flowering plant Deschampsia antarctica and rice improves cold and drought tolerance by accumulation of raffinose family oligosaccharides in transgenic rice plants[J]. Plant Cell Physiol, 2020, 61(1):88-104.
doi: 10.1093/pcp/pcz180 URL |
| [10] |
Chen G, Hu J, Lian J, et al. Functional characterization of OsHAK1 promoter in response to osmotic/drought stress by deletion analysis in transgenic rice[J]. Plant Growth Regul, 2019, 88(3):241-251.
doi: 10.1007/s10725-019-00504-3 URL |
| [11] | Chen G, Hu J, Dong LL, et al. The tolerance of salinity in rice requires the presence of a functional copy of FLN2[J]. Biomolecules, 2019, 10(1):E17. |
| [12] |
Chen G, Feng H, Hu Q, et al. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development[J]. Plant Biotechnol J, 2015, 13(6):833-848.
doi: 10.1111/pbi.12320 URL |
| [13] |
Chen G, Liu C, Gao Z, et al. Variation in the abundance of OsHAK1 transcript underlies the differential salinity tolerance of an indica and a Japonica rice cultivar[J]. Front Plant Sci, 2017, 8:2216.
doi: 10.3389/fpls.2017.02216 URL |
| [14] |
Chen G, Zhang Y, Ruan B, et al. OsHAK1 controls the vegetative growth and panicle fertility of rice by its effect on potassium-mediated sugar metabolism[J]. Plant Sci, 2018, 274:261-270.
doi: 10.1016/j.plantsci.2018.05.034 URL |
| [15] |
Chen G, Hu Q, Luo L, et al. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges[J]. Plant Cell Environ, 2015, 38(12):2747-2765.
doi: 10.1111/pce.12585 URL |
| [16] |
Chen G, Wu C, He L, et al. Knocking out the gene RLS1 induces hypersensitivity to oxidative stress and premature leaf senescence in rice[J]. Int J Mol Sci, 2018, 19(10):2853.
doi: 10.3390/ijms19102853 URL |
| [17] |
Chen G, Liu CL, Gao ZY, et al. Driving the expression of RAA1 with a drought-responsive promoter enhances root growth in rice, its accumulation of potassium and its tolerance to moisture stress[J]. Environ Exp Bot, 2018, 147:147-156.
doi: 10.1016/j.envexpbot.2017.12.008 URL |
| [18] |
Chen G, Liu C, Gao Z, et al. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice[J]. Front Plant Sci, 2017, 8:1885.
doi: 10.3389/fpls.2017.01885 URL |
| [19] |
Gupta AK, Kaur N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants[J]. J Biosci, 2005, 30(5):761-776.
doi: 10.1007/BF02703574 URL |
| [20] |
Lemoine R, La Camera S, Atanassova R, et al. Source-to-sink transport of sugar and regulation by environmental factors[J]. Front Plant Sci, 2013, 4:272.
doi: 10.3389/fpls.2013.00272 pmid: 23898339 |
| [21] | Cui Y, Wang M, Zhou H, et al. OsSGL, a novel DUF1645 domain-containing protein, confers enhanced drought tolerance in transgenic rice and Arabidopsis[J]. Front Plant Sci, 2016, 7:2001. |
| [22] |
Jung H, Chung PJ, Park SH, et al. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance[J]. Plant Biotechnol J, 2017, 15(10):1295-1308.
doi: 10.1111/pbi.12716 URL |
| [23] |
Lee DK, Jung H, Jang G, et al. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance[J]. Plant Physiol, 2016, 172(1):575-588.
doi: 10.1104/pp.16.00379 URL |
| [24] |
Aliche EB, Theeuwen TPJM, Oortwijn M, et al. Carbon partitioning mechanisms in POTATO under drought stress[J]. Plant Physiol Biochem, 2020, 146:211-219.
doi: 10.1016/j.plaphy.2019.11.019 URL |
| [25] | Jiang YJ, Qiu YP, Hu YR, et al. Heterologous expression of AtWRKY57 confers drought tolerance in Oryza sativa[J]. Front Plant Sci, 2016, 7:145. |
| [26] |
Song SY, Chen Y, Zhao MG, et al. A novel Medicago truncatula HD-Zip gene, MtHB2, is involved in abiotic stress responses[J]. Environ Exp Bot, 2012, 80:1-9.
doi: 10.1016/j.envexpbot.2012.02.001 URL |
| [27] |
Székely G, Abrahám E, Cséplo A, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis[J]. Plant J, 2008, 53(1):11-28.
doi: 10.1111/j.1365-313X.2007.03318.x URL |
| [28] |
Wang Z, Wang Y, Hong X, et al. Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1(UAP1)induces early leaf senescence and defence responses in rice[J]. J Exp Bot, 2015, 66(3):973-987.
doi: 10.1093/jxb/eru456 URL |
| [29] |
Xia K, Liu T, Ouyang J, et al. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice(Oryza sativa L.)[J]. DNA Res, 2011, 18(5):363-377.
doi: 10.1093/dnares/dsr024 URL |
| [30] |
Zhou QY, Yu Q, Wang ZQ, et al. Knockdown of GDCH gene reveals reactive oxygen species-induced leaf senescence in rice[J]. Plant Cell Environ, 2013, 36(8):1476-1489.
doi: 10.1111/pce.12078 URL |
| [31] |
Shen J, Lv B, Luo L, et al. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice[J]. Sci Rep, 2017, 7:40641.
doi: 10.1038/srep40641 URL |
| [32] |
Hu H, Dai M, Yao J, et al. Overexpressing a NAM, ATAF, and CUC(NAC)transcription factor enhances drought resistance and salt tolerance in rice[J]. PNAS, 2006, 103(35):12987-12992.
doi: 10.1073/pnas.0604882103 URL |
| [33] |
Yang A, Dai X, Zhang WH. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice[J]. J Exp Bot, 2012, 63(7):2541-2556.
doi: 10.1093/jxb/err431 pmid: 22301384 |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 黄小龙, 孙贵连, 马丹丹, 闫慧清. 水稻幼苗酵母单杂文库构建及LAZY1上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 126-135. |
| [3] | 李雪琪, 张素杰, 于曼, 黄金光, 周焕斌. 基于CRISPR/CasX介导的水稻基因组编辑技术的建立[J]. 生物技术通报, 2023, 39(9): 40-48. |
| [4] | 吴元明, 林佳怡, 柳雨汐, 李丹婷, 张宗琼, 郑晓明, 逄洪波. 基于BSA-seq和RNA-seq挖掘水稻株高相关QTL[J]. 生物技术通报, 2023, 39(8): 173-184. |
| [5] | 姚莎莎, 王晶晶, 王俊杰, 梁卫红. 植物激素信号通路调控水稻粒型的分子机制[J]. 生物技术通报, 2023, 39(8): 80-90. |
| [6] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [7] | 翟莹, 李铭杨, 张军, 赵旭, 于海伟, 李珊珊, 赵艳, 张梅娟, 孙天国. 异源表达大豆转录因子GmNF-YA19提高转基因烟草抗旱性[J]. 生物技术通报, 2023, 39(5): 224-232. |
| [8] | 任沛东, 彭健玲, 刘圣航, 姚姿婷, 朱桂宁, 陆光涛, 李瑞芳. 沙福芽孢杆菌GX-H6的分离鉴定及对水稻细菌性条斑病的防病效果[J]. 生物技术通报, 2023, 39(5): 243-253. |
| [9] | 李怡君, 吴晨晨, 李睿, 王喆, 何山文, 韦善君, 张晓霞. 水稻内生细菌新资源分离培养方案探究[J]. 生物技术通报, 2023, 39(4): 201-211. |
| [10] | 卢振万, 李雪琪, 黄金光, 周焕斌. 利用胞嘧啶碱基编辑技术创制耐草甘膦水稻[J]. 生物技术通报, 2023, 39(2): 63-69. |
| [11] | 杨茂, 林宇丰, 戴阳朔, 潘素君, 彭伟业, 严明雄, 李魏, 王冰, 戴良英. OsDIS1通过抗氧化途径负调控水稻耐旱性[J]. 生物技术通报, 2023, 39(2): 88-95. |
| [12] | 蒋铭轩, 李康, 罗亮, 刘建祥, 芦海平. 植物表达外源蛋白研究进展及展望[J]. 生物技术通报, 2023, 39(11): 110-122. |
| [13] | 姜南, 石杨, 赵志慧, 李斌, 赵熠辉, 杨俊彪, 闫家铭, 靳雨璠, 陈稷, 黄进. 镉胁迫下水稻OsPT1的表达及功能分析[J]. 生物技术通报, 2023, 39(1): 166-174. |
| [14] | 陈光, 李佳, 杜瑞英, 王旭. 水稻盐敏感突变体ss2的鉴定与基因功能分析[J]. 生物技术通报, 2022, 38(9): 158-166. |
| [15] | 高晓蓉, 丁尧, 吕军. 芘降解菌Pseudomonas sp. PR3的植物促生特性及其对芘胁迫下水稻生长的影响[J]. 生物技术通报, 2022, 38(9): 226-236. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||