








生物技术通报 ›› 2023, Vol. 39 ›› Issue (2): 254-262.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0421
任思雨1( ), 姜聪一1, 于涛2, 康瑞1, 姜晓冰1(
), 姜聪一1, 于涛2, 康瑞1, 姜晓冰1( )
)
收稿日期:2022-04-07
出版日期:2023-02-26
发布日期:2023-03-07
作者简介:任思雨,女,硕士研究生,研究方向:食品微生物污染与控制;E-mail: 基金资助:
REN Si-yu1( ), JIANG Cong-yi1, YU Tao2, KANG Rui1, JIANG Xiao-bing1(
), JIANG Cong-yi1, YU Tao2, KANG Rui1, JIANG Xiao-bing1( )
)
Received:2022-04-07
Published:2023-02-26
Online:2023-03-07
摘要:
研究agr系统在单核增生细胞李斯特菌(Lm)对不同抗菌剂耐药及生物被膜形成中的作用。构建agr基因缺失突变株,测定突变株对抗菌药物的敏感性;微孔板定量法检测Lm在不同温度下生物被膜的形成量并利用倒置显微镜观察生物被膜结构;采用软平板法测定细菌的群集及泳动运动能力;通过实时荧光定量PCR检测运动相关基因的转录水平。缺失agr系统导致Lm对头孢噻肟、环丙沙星及苯扎氯铵的耐药性降低。与野生株EGD-e相比,突变株在37℃、20℃以及4℃条件下生物被膜形成量均明显降低;∆agrC的群集运动能力增强。RT-qPCR结果显示,缺失agr系统后flaA的转录水平下降。agr系统参与Lm耐药及生物被膜的形成。
任思雨, 姜聪一, 于涛, 康瑞, 姜晓冰. agr系统在单核增生细胞李斯特菌耐药及生物被膜形成中的作用[J]. 生物技术通报, 2023, 39(2): 254-262.
REN Si-yu, JIANG Cong-yi, YU Tao, KANG Rui, JIANG Xiao-bing. Role of agr System in the Antimicrobial Resistance and Biofilm Formation of Listeria monocytogenes[J]. Biotechnology Bulletin, 2023, 39(2): 254-262.
| 引物名称 Primer name | 序列 Sequence(5'-3') | 长度 Length/bp |
|---|---|---|
| agrA-P1 | NNNNNNGGATCCGCTCTTACTGTCTTA- GCGTTCT | 882 |
| agrA-P2 | CCTGTGGCACCGATAAAATTCGCTGC- ATTCTGTTATCTTC | |
| agrA-P3 | GAAGATAACAGAATGCAGCGAATTTT- ATCGGTGCCACAGG | 568 |
| agrA-P4 | NNNNNNACGCGTAGTATTCCCATCGG- CATTT | |
| agrB/D-P1 | NNNNNNGGATCCGTCTACAAAGTTGA- TGGGATT | 680 |
| agrB/D-P2 | ACTCATAGAAGAATCCGCAAATCATCT- TTCCAGCGGTC | |
| agrB/D-P3 | GACCGCTGGAAAGATGATTTGCGGATT- CTTCTATGAGT | 608 |
| agrB/D-P4 | NNNNNNACGCGTGCTAAGACAGTAAG- AGCAACG |
表1 PCR扩增引物序列
Table 1 Primer sequences for PCR amplification
| 引物名称 Primer name | 序列 Sequence(5'-3') | 长度 Length/bp |
|---|---|---|
| agrA-P1 | NNNNNNGGATCCGCTCTTACTGTCTTA- GCGTTCT | 882 |
| agrA-P2 | CCTGTGGCACCGATAAAATTCGCTGC- ATTCTGTTATCTTC | |
| agrA-P3 | GAAGATAACAGAATGCAGCGAATTTT- ATCGGTGCCACAGG | 568 |
| agrA-P4 | NNNNNNACGCGTAGTATTCCCATCGG- CATTT | |
| agrB/D-P1 | NNNNNNGGATCCGTCTACAAAGTTGA- TGGGATT | 680 |
| agrB/D-P2 | ACTCATAGAAGAATCCGCAAATCATCT- TTCCAGCGGTC | |
| agrB/D-P3 | GACCGCTGGAAAGATGATTTGCGGATT- CTTCTATGAGT | 608 |
| agrB/D-P4 | NNNNNNACGCGTGCTAAGACAGTAAG- AGCAACG |

图2 agrA和agrB/D基因缺失株的构建 A:扩增上下游同源臂(M:DL2000 Maker;1:agrA上游同源臂;2:agrA下游同源臂;3:agrA融合片段;4:agrB/D上游同源臂;5:agrB/D下游同源臂;6:agrB/D融合片段);B:双酶切验证(M:DL2000;1,2:pMAD-agrA双酶切验证;3,4:pMAD-agrB/D双酶切验证);C:敲除验证(M:DL2000;1,2:ΔagrA;3:ΔagrB/D)
Fig. 2 Construction of ?agrA and ?agrB/D A: Amplification of upstream and downstream homology arms(M: DL2000 maker; 1: agrA upstream homology arm; 2: agrA downstream homology arm; 3: agrA fusion fragment; 4: agrB/D upstream homology arm; 5: agrB/D downstream homology arm; 6: agrB/D fusion fragment). B: Double digestion verification(M: DL2000; 1, 2: pMAD-agrA double digestion verification; 3, 4: pMAD-agrB/D double digestion verification). C: Knockout validation(M: DL2000; 1, 2: ΔagrA; 3: ΔagrB/D)
| 抗菌剂Antibacterial agents | EGD-e | ΔagrA | ΔagrB/D | ΔagrC | |
|---|---|---|---|---|---|
| 抗生素 Antibiotic | 氨苄西林 Ampicillin | 1 | 1 | 1 | 1 |
| 头孢噻肟 Cefotaxime | 12 | 8 | 12 | 12 | |
| 头孢噻吩 Cephalothin | 12 | 12 | 12 | 12 | |
| 红霉素 Erythromycin | 0.125 | 0.125 | 0.125 | 0.125 | |
| 卡那霉素 Kanamycin | 4 | 4 | 4 | 4 | |
| 四环素 Tetracycline | 0.5 | 0.5 | 0.5 | 0.5 | |
| 氯霉素 Chloramphenicol | 4 | 4 | 4 | 4 | |
| 环丙沙星 Ciprofloxacin | 1 | 1 | 0.5 | 0.5 | |
| 抗菌肽 Antimicrobial peptides | 乳酸链球菌素 Nisin | 10 | 10 | 10 | 10 |
| 消毒剂 Disinfectant | BC Benzalkonium chloride | 6 | 6 | 6 | 6 |
表2 抗菌物质对Lm菌株的MIC值
Table 2 MICs of antimicrobial agents against L. monocytogenes
| 抗菌剂Antibacterial agents | EGD-e | ΔagrA | ΔagrB/D | ΔagrC | |
|---|---|---|---|---|---|
| 抗生素 Antibiotic | 氨苄西林 Ampicillin | 1 | 1 | 1 | 1 |
| 头孢噻肟 Cefotaxime | 12 | 8 | 12 | 12 | |
| 头孢噻吩 Cephalothin | 12 | 12 | 12 | 12 | |
| 红霉素 Erythromycin | 0.125 | 0.125 | 0.125 | 0.125 | |
| 卡那霉素 Kanamycin | 4 | 4 | 4 | 4 | |
| 四环素 Tetracycline | 0.5 | 0.5 | 0.5 | 0.5 | |
| 氯霉素 Chloramphenicol | 4 | 4 | 4 | 4 | |
| 环丙沙星 Ciprofloxacin | 1 | 1 | 0.5 | 0.5 | |
| 抗菌肽 Antimicrobial peptides | 乳酸链球菌素 Nisin | 10 | 10 | 10 | 10 |
| 消毒剂 Disinfectant | BC Benzalkonium chloride | 6 | 6 | 6 | 6 |
| 菌株 Strain | 迟缓期LPD Lag phase duration/h | 平均最大生长速率MMGR Mean maximum growth rate/(Units·h-1) | 平均最大光密度值MMOD Mean maximum optical density/Units | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BHI | BC/(1 μg·mL-1) | BHI | BC/(1 μg·mL-1) | BHI | BC/(1 μg·mL-1) | ||||
| EGDe | 3.488±0.116 | 22.246±0.249 | 0.205±0.0115 | 0.0842±0.00641 | 0.7125±0.00200 | 0.615±0.045 | |||
| ΔagrA | 3.348±0.196 | 16.774±0.352*** | 0.185±0.0167 | 0.1300±0.01970* | 0.7108±0.00062 | 0.566±0.005 | |||
| ΔagrB/D | 3.311±0.143 | 27.401±0.208** | 0.199±0.0129 | 0.0484±0.00171** | 0.7047±0.00960 | 0.577±0.063 | |||
| ΔagrC | 3.503±0.154 | 40.803±0.111*** | 0.196±0.0147 | 0.0646±0.00321* | 0.7005±0.00710 | 0.211±0.155* | |||
表3 生长曲线分析
Table 3 Growth curve analysis
| 菌株 Strain | 迟缓期LPD Lag phase duration/h | 平均最大生长速率MMGR Mean maximum growth rate/(Units·h-1) | 平均最大光密度值MMOD Mean maximum optical density/Units | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BHI | BC/(1 μg·mL-1) | BHI | BC/(1 μg·mL-1) | BHI | BC/(1 μg·mL-1) | ||||
| EGDe | 3.488±0.116 | 22.246±0.249 | 0.205±0.0115 | 0.0842±0.00641 | 0.7125±0.00200 | 0.615±0.045 | |||
| ΔagrA | 3.348±0.196 | 16.774±0.352*** | 0.185±0.0167 | 0.1300±0.01970* | 0.7108±0.00062 | 0.566±0.005 | |||
| ΔagrB/D | 3.311±0.143 | 27.401±0.208** | 0.199±0.0129 | 0.0484±0.00171** | 0.7047±0.00960 | 0.577±0.063 | |||
| ΔagrC | 3.503±0.154 | 40.803±0.111*** | 0.196±0.0147 | 0.0646±0.00321* | 0.7005±0.00710 | 0.211±0.155* | |||

图5 Lm菌株在37℃下形成的生物被膜 A:生物被膜形成量;B:倒置显微镜观察生物被膜(37℃培养2 d)
Fig. 5 Biofilm formation of L. monocytogenes strains at 37℃ A: The biofilm biomass. B: Biofilm assay by inverted microscope(incubated at 37℃ for 2 d)
| 菌株 Strain | 生物被膜量Biofilm mass | ||
|---|---|---|---|
| 1 d | 2 d | 3 d | |
| EGDe | 0.219±0.044 | 1.408±0.114 | 0.611±0.086 |
| ?agrA | 0.106±0.017 | 0.968±0.041 | 0.342±0.040 |
| ?agrB/D | 0.103±0.025 | 1.043±0.070 | 0.330±0.057 |
| ?agrC | 0.162±0.052 | 1.509±0.102 | 0.263±0.030 |
表4 野生株和突变株在37℃下形成的生物被膜量
Table 4 Biofilm biomasses of wild type and mutant strains at 37℃
| 菌株 Strain | 生物被膜量Biofilm mass | ||
|---|---|---|---|
| 1 d | 2 d | 3 d | |
| EGDe | 0.219±0.044 | 1.408±0.114 | 0.611±0.086 |
| ?agrA | 0.106±0.017 | 0.968±0.041 | 0.342±0.040 |
| ?agrB/D | 0.103±0.025 | 1.043±0.070 | 0.330±0.057 |
| ?agrC | 0.162±0.052 | 1.509±0.102 | 0.263±0.030 |

图6 Lm菌株在20℃下形成的生物被膜 A:生物被膜形成量;B:倒置显微镜观察生物被膜(20℃培养6 d)
Fig. 6 Biofilm formation of L. monocytogenes strains at 20℃ A: The biofilm biomass. B: Biofilm assay by inverted microscope(incubated at 20℃ for 6 d)
| 菌株 Strain | 生物被膜量Biofilm mass | ||
|---|---|---|---|
| 3 d | 6 d | 9 d | |
| EGDe | 0.145±0.012 | 0.630±0.070 | 0.436±0.023 |
| ?agrA | 0.102±0.004 | 0.612±0.033 | 0.425±0.019 |
| ?agrB/D | 0.109±0.007 | 0.706±0.064 | 0.389±0.017 |
| ?agrC | 0.099±0.003 | 0.621±0.009 | 0.474±0.013 |
表5 野生株和突变株在20℃下形成的生物被膜量
Table 5 Biofilm biomasses of wild type and mutant strains at 20℃
| 菌株 Strain | 生物被膜量Biofilm mass | ||
|---|---|---|---|
| 3 d | 6 d | 9 d | |
| EGDe | 0.145±0.012 | 0.630±0.070 | 0.436±0.023 |
| ?agrA | 0.102±0.004 | 0.612±0.033 | 0.425±0.019 |
| ?agrB/D | 0.109±0.007 | 0.706±0.064 | 0.389±0.017 |
| ?agrC | 0.099±0.003 | 0.621±0.009 | 0.474±0.013 |
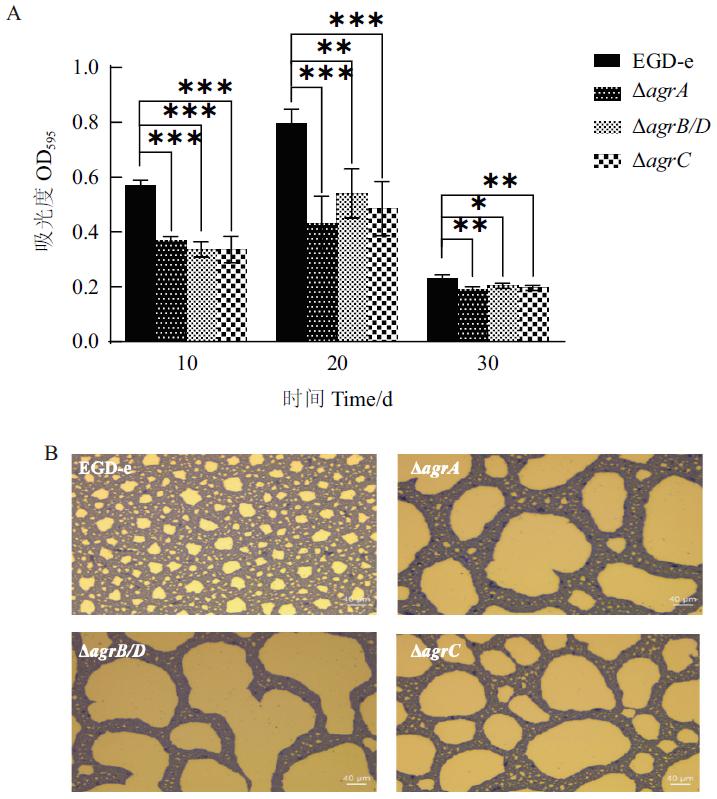
图7 Lm菌株在4℃下形成的生物被膜 A:生物被膜形成量;B:倒置显微镜观察生物被膜(4℃培养20 d)
Fig. 7 Biofilm formations of L. monocytogenes strains at 4℃ 20 d A: The biofilm biomass. B: Biofilm assay by inverted microscope(incubated at 4℃ for 20 d)
| 菌株 Strain | 生物被膜量Biofilm mass | ||
|---|---|---|---|
| 10 d | 20 d | 30 d | |
| EGDe | 0.568±0.017 | 0.794±0.043 | 0.229±0.012 |
| ?agrA | 0.371±0.011 | 0.430±0.082 | 0.191±0.007 |
| ?agrB/D | 0.336±0.022 | 0.540±0.073 | 0.204±0.008 |
| ?agrC | 0.336±0.039 | 0.485±0.080 | 0.196±0.007 |
表6 野生株和突变株在4℃下形成的生物被膜量
Table 6 Biofilm biomasses of wild type and mutant strains at 4℃
| 菌株 Strain | 生物被膜量Biofilm mass | ||
|---|---|---|---|
| 10 d | 20 d | 30 d | |
| EGDe | 0.568±0.017 | 0.794±0.043 | 0.229±0.012 |
| ?agrA | 0.371±0.011 | 0.430±0.082 | 0.191±0.007 |
| ?agrB/D | 0.336±0.022 | 0.540±0.073 | 0.204±0.008 |
| ?agrC | 0.336±0.039 | 0.485±0.080 | 0.196±0.007 |
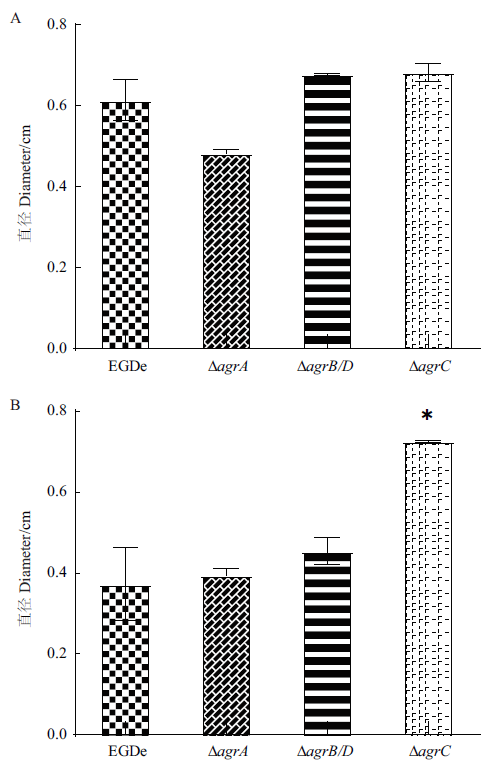
图8 野生株与agr基因缺失突变株的泳动和群集运动性 A:泳动运动性;B:群集运动性
Fig. 8 Swimming and swarming of the wild-type strain and agr gene deletion mutants A: Swimming motilities;B: Swarming motilities
| [1] | 杨丽玉, 杨诗怡, 林巍, 等. 单核细胞增生李斯特菌膜囊泡的制备及生物活性[J]. 微生物学通报, 2021, 48(1): 145-155. |
| Yang LY, Yang SY, Lin W, et al. Isolation and activity of Listeria monocytogenes-derived membrane vesicles[J]. Microbiol China, 2021, 48(1): 145-155. | |
| [2] | 耿忆敏, 任思雨, 于涛, 等. VirAB在单核细胞增生李斯特菌耐药性及生物被膜形成中的作用[J]. 微生物学通报, 2021, 48(2): 471-479. |
| Geng YM, Ren SY, Yu T, et al. Role of VirAB in antimicrobial resistance and biofilm formation of Listeria monocytogene[J]. Microbiol China, 2021, 48(2): 471-479. | |
| [3] |
Karygianni L, Ren Z, Koo H, et al. Biofilm matrixome: extracellular components in structured microbial communities[J]. Trends Microbiol, 2020, 28(8): 668-681.
doi: S0966-842X(20)30087-1 pmid: 32663461 |
| [4] |
Prescott RD, Decho AW. Flexibility and adaptability of quorum sensing in nature[J]. Trends Microbiol, 2020, 28(6): 436-444.
doi: S0966-842X(19)30319-1 pmid: 32001099 |
| [5] |
Bruger EL, Snyder DJ, Cooper VS, et al. Quorum sensing provides a molecular mechanism for evolution to tune and maintain investment in cooperation[J]. ISME J, 2021, 15(4): 1236-1247.
doi: 10.1038/s41396-020-00847-0 pmid: 33342998 |
| [6] | 刘蕾, 桂萌, 武瑞赟, 等. LuxS/AI-2型群体感应系统调控细菌生物被膜形成研究进展[J]. 食品科学, 2016, 37(19): 254-262. |
| Liu L, Gui M, Wu RY, et al. Progress in research on biofilm formation regulated by LuxS/AI-2 quorum sensing[J]. Food Sci, 2016, 37(19): 254-262. | |
| [7] |
Autret N, Raynaud C, Dubail I, et al. Identification of the agr locus of Listeria monocytogenes: role in bacterial virulence[J]. Infect Immun, 2003, 71(8): 4463-4471.
doi: 10.1128/IAI.71.8.4463-4471.2003 URL |
| [8] |
Vivant AL, Garmyn D, Gal L, et al. Survival of Listeria monocytogenes in soil requires AgrA-mediated regulation[J]. Appl Environ Microbiol, 2015, 81(15): 5073-5084.
doi: 10.1128/AEM.04134-14 URL |
| [9] | Vivant AL, Garmyn D, Gal L, et al. The Agr communication system provides a benefit to the populations of Listeria monocytogenes in soil[J]. Front Cell Infect Microbiol, 2014, 4: 160. |
| [10] |
Paspaliari DK, Mollerup MS, Kallipolitis BH, et al. Chitinase expression in Listeria monocytogenes is positively regulated by the Agr system[J]. PLoS One, 2014, 9(4): e95385.
doi: 10.1371/journal.pone.0095385 URL |
| [11] | Lee YJ, Wang C. Links between S-adenosylmethionine and Agr-based quorum sensing for biofilm development in Listeria monocytogenes EGD-E[J]. MicrobiologyOpen, 2020, 9(5): e1015. |
| [12] | Merchel Piovesan Pereira B, Tagkopoulos I. Benzalkonium chlorides: uses, regulatory status, and microbial resistance[J]. Appl Environ Microbiol, 2019, 85(13): e00377-e00319. |
| [13] |
Barber OW, Hartmann EM. Benzalkonium chloride: a systematic review of its environmental entry through wastewater treatment, potential impact, and mitigation strategies[J]. Crit Rev Environ Sci Technol, 2022, 52(15): 2691-2719.
doi: 10.1080/10643389.2021.1889284 URL |
| [14] | Kampf G. Biocidal agents used for disinfection can enhance antibiotic resistance in gram-negative species[J]. Antibiotics(Basel), 2018, 7(4): 110. |
| [15] |
Karygianni L, Ren Z, Koo H, et al. Biofilm matrixome: extracellular components in structured microbial communities[J]. Trends Microbiol, 2020, 28(8): 668-681.
doi: S0966-842X(20)30087-1 pmid: 32663461 |
| [16] |
Mazaheri T, Cervantes-Huamán BRH, Bermúdez-Capdevila M, et al. Listeria monocytogenes biofilms in the food industry: is the current hygiene program sufficient to combat the persistence of the pathogen?[J]. Microorganisms, 2021, 9(1): 181.
doi: 10.3390/microorganisms9010181 URL |
| [17] | 田翠芳, 张昭寰, 陶倩, 等. 抗生物被膜材料在食品微生物安全领域的应用研究进展[J]. 食品科学, 2022, 43(11): 265-272. |
| Tian CF, Zhang ZH, Tao Q, et al. Progress in the application of anti-biofilm materials in the field of food microbial safety[J]. Food Sci, 2022, 43(11): 265-272. | |
| [18] |
da Silva DAL, de Melo Tavares R, Camargo AC, et al. Biofilm growth by Listeria monocytogenes on stainless steel and expression of biofilm-related genes under stressing conditions[J]. World J Microbiol Biotechnol, 2021, 37(7): 119.
doi: 10.1007/s11274-021-03092-5 URL |
| [19] |
Janež N, Škrlj B, Sterniša M, et al. The role of the Listeria monocytogenes surfactome in biofilm formation[J]. Microb Biotechnol, 2021, 14(4): 1269-1281.
doi: 10.1111/1751-7915.13847 pmid: 34106516 |
| [20] | Henly EL, Dowling JAR, Maingay JB, et al. Biocide exposure induces changes in susceptibility, pathogenicity, and biofilm formation in uropathogenic Escherichia coli[J]. Antimicrob Agents Chemother, 2019, 63(3): e01892-e01818. |
| [21] |
O’May C, Ciobanu A, Lam H, et al. Tannin derived materials can block swarming motility and enhance biofilm formation in Pseudomonas aeruginosa[J]. Biofouling, 2012, 28(10): 1063-1076.
doi: 10.1080/08927014.2012.725130 URL |
| [1] | 杜冬冬, 钱晶, 李思琪, 刘雯菲, 魏向利, 刘长勇, 罗瑞峰, 康立超. 单核细胞增生李斯特菌LMXJ15全基因组测序及分析[J]. 生物技术通报, 2023, 39(7): 298-306. |
| [2] | 陈勇, 李亚鑫, 王亚瑄, 梁露洁, 冯思源, 田国宝. MCR-1介导多黏菌素耐药性的分子机制研究进展[J]. 生物技术通报, 2023, 39(6): 102-108. |
| [3] | 李海利, 郎利敏, 张青娴, 游一, 朱文豪, 王治方, 张立宪, 王克领. 同时产碳青霉烯酶NDM-1和NDM-5的猪源大肠埃希氏菌的鉴定及耐药性研究[J]. 生物技术通报, 2022, 38(9): 106-115. |
| [4] | 文畅, 刘晨, 卢诗韵, 许忠兵, 艾超凡, 廖汉鹏, 周顺桂. 一株新的多重耐药福氏志贺菌噬菌体生物学特性及基因组分析[J]. 生物技术通报, 2022, 38(9): 127-135. |
| [5] | 李霁虹, 荆玉玲, 马桂珍, 郭荣君, 李世东. 无色杆菌77的基因组构成及其趋化和耐药特性[J]. 生物技术通报, 2022, 38(9): 136-146. |
| [6] | 石成龙, 汪锡武, 李安琪, 钱森和, 王洲, 赵世光, 刘艳, 薛正莲. ε-聚赖氨酸对阪崎克罗诺杆菌细胞结构与生物被膜形成的影响[J]. 生物技术通报, 2022, 38(9): 147-157. |
| [7] | 鲁兆祥, 王夕冉, 连新磊, 廖晓萍, 刘雅红, 孙坚. 基于功能宏基因组学挖掘抗生素耐药基因研究进展[J]. 生物技术通报, 2022, 38(9): 17-27. |
| [8] | 胡功政, 崔小蝶, 翟亚军, 贺丹丹. 细菌黏菌素耐药性及其逆转机制研究进展[J]. 生物技术通报, 2022, 38(9): 28-34. |
| [9] | 刘艺云, 邓利敏, 岳慧颖, 岳超, 刘健华. 质粒接合转移及其抑制剂的研究进展[J]. 生物技术通报, 2022, 38(9): 35-46. |
| [10] | 刘成程, 胡小芳, 冯友军. 细菌耐药:生化机制与应对策略[J]. 生物技术通报, 2022, 38(9): 4-16. |
| [11] | 赵艳坤, 刘慧敏, 孟璐, 王成, 王加启, 郑楠. 大肠埃希菌异质性耐药的研究进展[J]. 生物技术通报, 2022, 38(9): 59-71. |
| [12] | 刘晓黎, 童真艺, 赵亮, 尹丽, 刘晨光. 非抗生素类活性物质抗幽门螺杆菌研究进展[J]. 生物技术通报, 2022, 38(9): 96-105. |
| [13] | 陈福暖, 黄瑜, 蔡佳, 王忠良, 简纪常, 王蓓. ABC转运蛋白结构及其在细菌致病性中的研究进展[J]. 生物技术通报, 2022, 38(6): 43-52. |
| [14] | 赵海晴, 李耘, 梁严内, 刘哲, 任亚林, 李金娟. 联合用药对嗜水气单胞菌耐药性影响研究进展[J]. 生物技术通报, 2022, 38(6): 53-65. |
| [15] | 朱浩, 张严伟, 刘润, 梁艳, 杨奕, 徐天乐, 杨章平. 抗生素佐剂与抗生素联用的抑菌作用研究进展[J]. 生物技术通报, 2022, 38(6): 66-73. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||