








生物技术通报 ›› 2023, Vol. 39 ›› Issue (1): 157-165.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0560
林蓉1( ), 郑月萍2(
), 郑月萍2( ), 徐雪珍2, 李丹丹2, 郑志富1,2
), 徐雪珍2, 李丹丹2, 郑志富1,2
收稿日期:2022-05-09
出版日期:2023-01-26
发布日期:2023-02-02
作者简介:林蓉,女,硕士研究生,研究方向:植物基因工程与种质创新;E-mail: 基金资助:
LIN Rong1( ), ZHENG Yue-ping2(
), ZHENG Yue-ping2( ), XU Xue-zhen2, LI Dan-dan2, ZHENG Zhi-fu1,2
), XU Xue-zhen2, LI Dan-dan2, ZHENG Zhi-fu1,2
Received:2022-05-09
Published:2023-01-26
Online:2023-02-02
摘要:
植物激素乙烯在多种生理生化过程中发挥重要作用,但其在特定组织器官中的合成机制尚不完全清楚。拟南芥中存在12个功能未知的ACC氧化酶类似蛋白(ACO-like homolog,ACOL),运用基因定点编辑技术构建了ACOL8的功能丧失型突变体,发现该基因的突变削弱了经典的乙烯“三重反应”。与野生型相比,突变体黄化幼苗下胚轴及主根的长度显著增加,这与突变体对外源ACC的敏感性下降现象一致。同时还发现ACOL8基因的表达受乙烯信号的正反馈调控,EIN3过表达增强其表达水平,而etr1-3的突变则产生相反效应。再者,在正常条件下,ACOL8基因的突变并未影响拟南芥的生长;但在盐胁迫条件下,突变体的根冠比显著下降,这说明该基因参与植物的盐胁迫响应。综上,这些结果说明ACOL8可能具有ACC氧化酶的功能,参与乙烯的合成与响应。
林蓉, 郑月萍, 徐雪珍, 李丹丹, 郑志富. 拟南芥ACOL8基因在乙烯合成与响应中的功能分析[J]. 生物技术通报, 2023, 39(1): 157-165.
LIN Rong, ZHENG Yue-ping, XU Xue-zhen, LI Dan-dan, ZHENG Zhi-fu. Functional Analysis of ACOL8 Gene in the Ethylene Synthesis and Response in Arabidopsis thaliana[J]. Biotechnology Bulletin, 2023, 39(1): 157-165.
| 靶基因 Target gene | sgRNA序列 sgRNA sequence(5'-3') | 位置 Location |
|---|---|---|
| ACOL8 | TCTTTCGAGGAGACTATGACAGG | 外显子(1) |
| ACOL8 | GCACGTCGAGCGGGATCCCGTGG | 外显子(1) |
表1 用于ACOL8基因编辑的靶序列
Table 1 Target sequences for ACOL8 gene editing
| 靶基因 Target gene | sgRNA序列 sgRNA sequence(5'-3') | 位置 Location |
|---|---|---|
| ACOL8 | TCTTTCGAGGAGACTATGACAGG | 外显子(1) |
| ACOL8 | GCACGTCGAGCGGGATCCCGTGG | 外显子(1) |
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 用途 Utility |
|---|---|---|
| ZYP209-BsF | ATATATggtctcGATTGCTTTCGA- GGAGACTATGACGTT | 构建sgRNA表达盒 |
| ZYP210-F0 | TGCTTTCGAGGAGACTATGAC- GTTTTAGAGCTAGAAATAGC | 构建sgRNA表达盒 |
| ZYP211-R0 | AACCGGGATCCCGCTCGACGT- GCAATCTCTTAGTCGACTCTAC | 构建sgRNA表达盒 |
| ZYP212-BsR | ATTATTggtctcGAAACCGGGAT- CCCGCTCGACGTGC | 构建sgRNA表达盒 |
| ZYP237-FP | ATTCCTTTGATGCCGTGATAGT | 鉴定筛选,测序 |
| ZYP238-RP | TAGCTGGAACCTCTTTGATTCC | 鉴定筛选 |
| ZYP239-FP | ATCGATCTGAACGGAGGAGTAG | 鉴定筛选 |
| ZYP240-RP | TTTCGAGTGTGATCACGAGAGT | 鉴定筛选,测序 |
表2 引物序列
Table 2 Primer sequences
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 用途 Utility |
|---|---|---|
| ZYP209-BsF | ATATATggtctcGATTGCTTTCGA- GGAGACTATGACGTT | 构建sgRNA表达盒 |
| ZYP210-F0 | TGCTTTCGAGGAGACTATGAC- GTTTTAGAGCTAGAAATAGC | 构建sgRNA表达盒 |
| ZYP211-R0 | AACCGGGATCCCGCTCGACGT- GCAATCTCTTAGTCGACTCTAC | 构建sgRNA表达盒 |
| ZYP212-BsR | ATTATTggtctcGAAACCGGGAT- CCCGCTCGACGTGC | 构建sgRNA表达盒 |
| ZYP237-FP | ATTCCTTTGATGCCGTGATAGT | 鉴定筛选,测序 |
| ZYP238-RP | TAGCTGGAACCTCTTTGATTCC | 鉴定筛选 |
| ZYP239-FP | ATCGATCTGAACGGAGGAGTAG | 鉴定筛选 |
| ZYP240-RP | TTTCGAGTGTGATCACGAGAGT | 鉴定筛选,测序 |
| 引物名称 Primer name | 基因 Gene | 用途 Purpose | 引物序列 Primer sequence(5'-3') |
|---|---|---|---|
| ZZ200 qPCR-FP | ACTIN | 内参基因 | GTCGTACAACCGGTATTGTGCT |
| ZZ201 qPCR-RP | TGTCTCTTACAATTTCCCGCTCT | ||
| ZYP197 qPCR-FP | ACOL8 | 目标基因 | GGGGCTCTTGTCGTTAACCT |
| ZYP198 qPCR-RP | TCCATATACTCGATGGCTCTCC |
表3 实时荧光定量PCR引物序列
Table 3 Sequences of the primers used in real-time fluore-scent quantitative PCR
| 引物名称 Primer name | 基因 Gene | 用途 Purpose | 引物序列 Primer sequence(5'-3') |
|---|---|---|---|
| ZZ200 qPCR-FP | ACTIN | 内参基因 | GTCGTACAACCGGTATTGTGCT |
| ZZ201 qPCR-RP | TGTCTCTTACAATTTCCCGCTCT | ||
| ZYP197 qPCR-FP | ACOL8 | 目标基因 | GGGGCTCTTGTCGTTAACCT |
| ZYP198 qPCR-RP | TCCATATACTCGATGGCTCTCC |
| 基因 Gene | 基因ID Gene ID | 编码蛋白 Encoded protein | 氨基酸 Amino acid/aa |
|---|---|---|---|
| ACO1 | AT2G19590 | ACO1 | 310 |
| ACO2 | AT1G62380 | ACO2 | 320 |
| ACO3 | AT1G12010 | ACO3 | 320 |
| ACO4 | AT1G05010 | ACO4 | 323 |
| ACO5 | AT1G77330 | ACO5 | 307 |
| ACOL1 | AT1G06620 | ACO-like homolog 1 | 365 |
| ACOL2 | AT1G06640 | ACO-like homolog 2 | 369 |
| ACOL3 | AT1G06650 | ACO-like homolog 3 | 369 |
| ACOL4 | AT1G03400 | ACO-like homolog 4 | 351 |
| ACOL5 | AT1G03410 | ACO-like homolog 5 | 398 |
| ACOL6 | AT1G04350 | ACO-like homolog 6 | 360 |
| ACOL7 | AT1G04380 | ACO-like homolog 7 | 345 |
| ACOL8 | AT3G61400 | ACO-like homolog 8 | 370 |
| ACOL9 | AT5G43440 | ACO-like homolog 9 | 365 |
| ACOL10 | AT5G43450 | ACO-like homolog 10 | 362 |
| ACOL11 | AT5G59530 | ACO-like homolog 11 | 364 |
| ACOL12 | AT5G59540 | ACO-like homolog 12 | 366 |
表4 编码拟南芥ACC氧化酶及其类似蛋白的基因特性
Table 4 Characteristics of genes encoding Arabidopsis ACC oxidases and ACO-like homologs
| 基因 Gene | 基因ID Gene ID | 编码蛋白 Encoded protein | 氨基酸 Amino acid/aa |
|---|---|---|---|
| ACO1 | AT2G19590 | ACO1 | 310 |
| ACO2 | AT1G62380 | ACO2 | 320 |
| ACO3 | AT1G12010 | ACO3 | 320 |
| ACO4 | AT1G05010 | ACO4 | 323 |
| ACO5 | AT1G77330 | ACO5 | 307 |
| ACOL1 | AT1G06620 | ACO-like homolog 1 | 365 |
| ACOL2 | AT1G06640 | ACO-like homolog 2 | 369 |
| ACOL3 | AT1G06650 | ACO-like homolog 3 | 369 |
| ACOL4 | AT1G03400 | ACO-like homolog 4 | 351 |
| ACOL5 | AT1G03410 | ACO-like homolog 5 | 398 |
| ACOL6 | AT1G04350 | ACO-like homolog 6 | 360 |
| ACOL7 | AT1G04380 | ACO-like homolog 7 | 345 |
| ACOL8 | AT3G61400 | ACO-like homolog 8 | 370 |
| ACOL9 | AT5G43440 | ACO-like homolog 9 | 365 |
| ACOL10 | AT5G43450 | ACO-like homolog 10 | 362 |
| ACOL11 | AT5G59530 | ACO-like homolog 11 | 364 |
| ACOL12 | AT5G59540 | ACO-like homolog 12 | 366 |
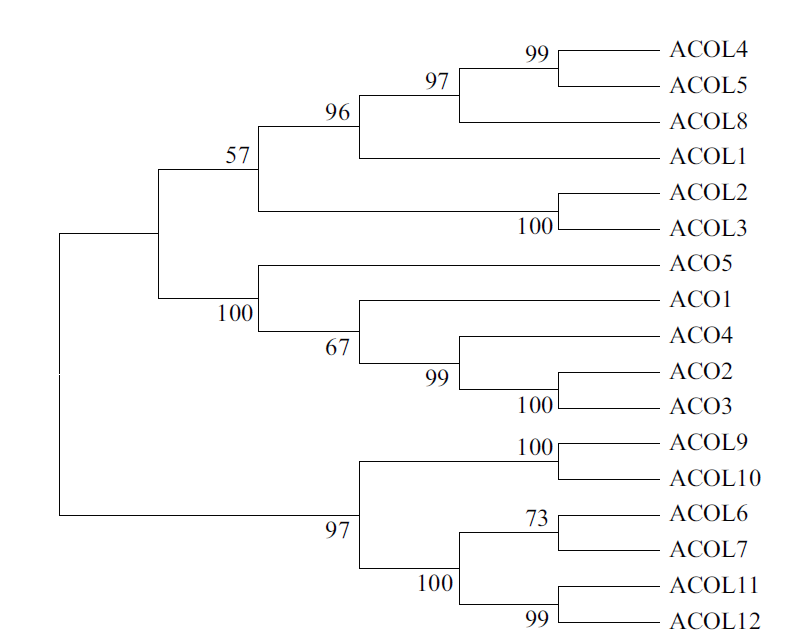
图2 ACC氧化酶家族及其类似蛋白的系统树图分析 MEGA7根据氨基酸序列比对的结果将序列分群,归为同一群的蛋白具有比较亲近的关系
Fig. 2 A dendrogram analysis of members of the ACC oxidase family and ACO-like homologs The MEGA7 software groups the sequences according to the results of amino acid sequence alignment,and the proteins classified into the same group have a relatively close relationship

图3 ACC氧化酶家族及ACOL8的氨基酸序列比对 相同颜色的氨基酸位点具有同源性,黑色=100%,红色>75%,绿色>50%;sgRNA指基因编辑位点对应的氨基酸序列
Fig. 3 Amino acid sequences alignment of the ACC oxidase family members and ACOL8 Amino acid sites with the same color indicate homology,black=100%,red>75%,green>50%;sgRNA refers to the amino acid sequence corresponding to the gene editing site
| 株系Line | 突变体编号Mutant code | 突变类型Mutation type | 突变位点Mutation site | 突变特点Mutation characteristics |
|---|---|---|---|---|
| acol8-1 | A10/WT-14-5-38 | 插入 | 308-309:5 bp插入CTGGA | 移码突变 |
| acol8-2 | A10/WT-230-17 | 缺失 | 66-308:243 bp缺失 | 大片段缺失 |
| acol8-3 | A10/WT-254-19 | 插入 | 65-66:1 bp插入T 308-309:1 bp插入T | 提前终止 |
表5 三个acol8突变体的突变位点
Table 5 Mutation sites in the three acol8 mutants
| 株系Line | 突变体编号Mutant code | 突变类型Mutation type | 突变位点Mutation site | 突变特点Mutation characteristics |
|---|---|---|---|---|
| acol8-1 | A10/WT-14-5-38 | 插入 | 308-309:5 bp插入CTGGA | 移码突变 |
| acol8-2 | A10/WT-230-17 | 缺失 | 66-308:243 bp缺失 | 大片段缺失 |
| acol8-3 | A10/WT-254-19 | 插入 | 65-66:1 bp插入T 308-309:1 bp插入T | 提前终止 |
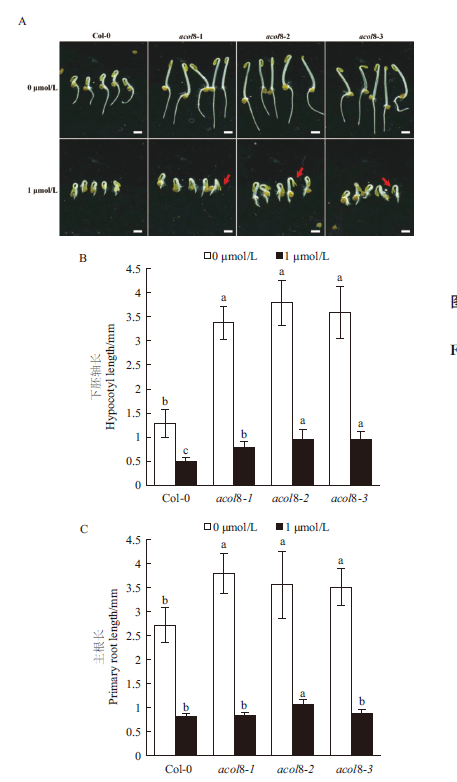
图5 acol8突变体与野生型拟南芥黄化幼苗的“三重反应”比较 A:acol8突变体与野生型拟南芥黄化幼苗在不同浓度ACC处理下的表型,标尺= 2 mm;B:acol8突变体与野生型拟南芥黄化幼苗在不同浓度ACC处理下的下胚轴长;C:acol8突变体与野生型拟南芥黄化幼苗在不同浓度ACC处理下的主根长。不同字母表示存在0.05水平上的显著性差异,下同
Fig. 5 Comparison of the “triple responses” of etiolated seedlings of wild type Arabidopsis and acol8 mutant A:The phenotype of etiolated seedlings of acol8 single mutant and wild type Arabidopsis treated with different concentrations of ACC,Bar = 2 mm. B:Length of hypocotyl of etiolated seedlings of acol8 single mutants and wild-type Arabidopsis treated with different concentrations of ACC. C:Length of primary root of etiolated seedlings of acol8 single mutants and wild type Arabidopsis. Different letters indicate significant differences at the 0.05 level,the same below
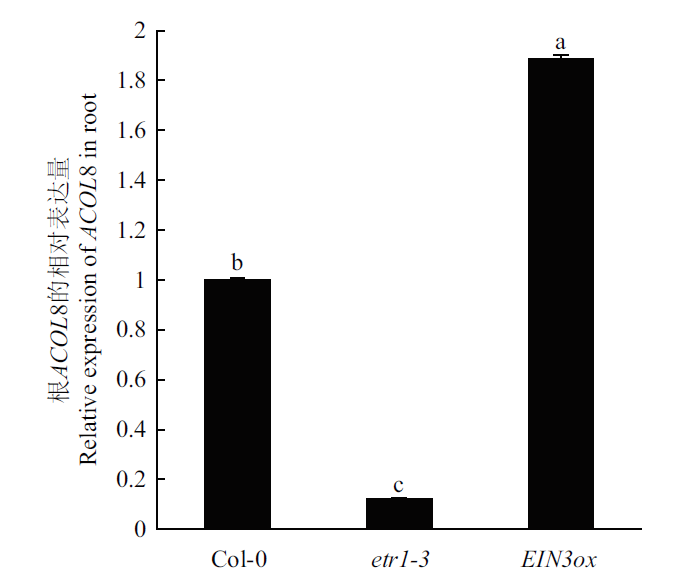
图6 etr1-3和 EIN3ox与野生型拟南芥的ACOL8基因相对表达量比较
Fig. 6 Comparison of the relative expressions of ACOL8 gene in the roots of wild type Arabidopsis and etr1-3 and EIN3ox line
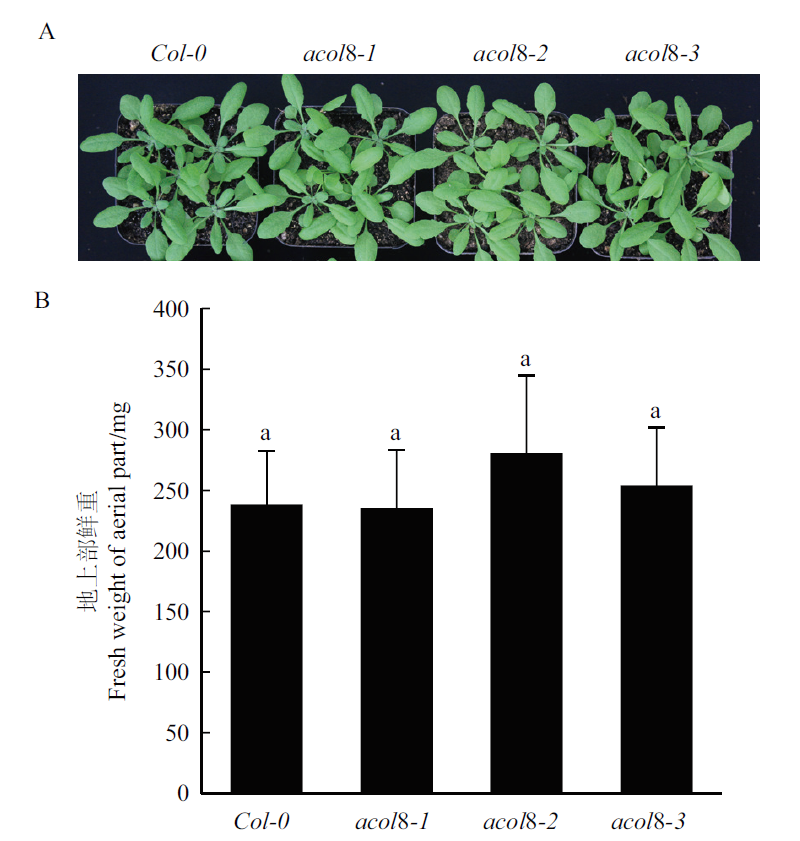
图7 三个acol8突变体与野生型拟南芥的表型比较 A:acol8突变体与野生型拟南芥的地上部表型,标尺=1 cm;B:acol8突变体与野生型拟南芥的地上部鲜重。
Fig. 7 Comparison of the phenotypes of wild type Arabid-opsis and three acol8 mutants A:The aboveground phenotype of wild type Arabidopsis and acol8 single mutant,Bar=1 cm. B:The fresh weight of aboveground biomass of wild type Arabidopsis and acol8 single mutants
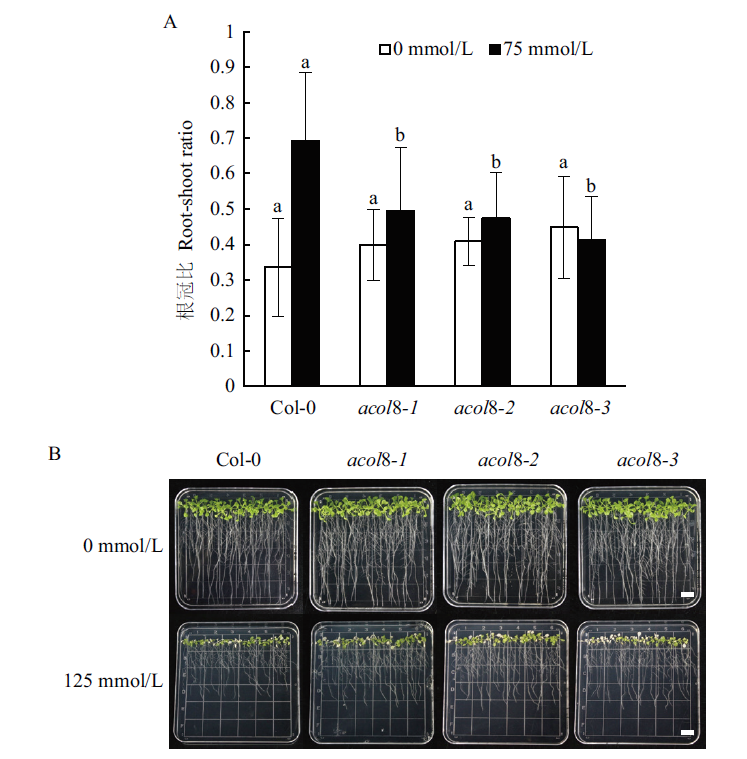
图8 acol8突变体与野生型拟南芥的耐盐性比较 acol8突变体与野生型拟南芥在不同浓度NaCl处理下的根冠比(A)和表型比较(B),标尺=1 cm
Fig. 8 Comparison of salt tolerances of wild type Arabido-psis and acol8 mutants Comparison of the root-shoot ratio (A)and the phenotype (B)of wild type Arabi-dopsis with that of acol8 mutant under different concentrations of NaCl,Bar=1 cm
| [1] |
Ahammed GJ, Gantait S, Mitra M, et al. Role of ethylene crosstalk in seed germination and early seedling development:a review[J]. Plant Physiol Biochem, 2020, 151:124-131.
doi: 10.1016/j.plaphy.2020.03.016 URL |
| [2] |
Nascimento VL, Pereira AM, Pereira AS, et al. Physiological and metabolic bases of increased growth in the tomato ethylene-insensitive mutant Never ripe:extending ethylene signaling functions[J]. Plant Cell Rep, 2021, 40(8): 1377-1393.
doi: 10.1007/s00299-020-02623-y pmid: 33074436 |
| [3] |
Tripathi SK, Tuteja N. Integrated signaling in flower senescence[J]. Plant Signal Behav, 2007, 2(6): 437-445.
doi: 10.4161/psb.2.6.4991 URL |
| [4] |
Dubois M, van den Broeck L, Inzé D. The pivotal role of ethylene in plant growth[J]. Trends Plant Sci, 2018, 23(4): 311-323.
doi: S1360-1385(18)30015-3 pmid: 29428350 |
| [5] | Tao JJ, Chen HW, Ma B, et al. The role of ethylene in plants under Sal Inity stress[J]. Front Plant Sci, 2015, 6:1059. |
| [6] |
Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance[J]. Trends Plant Sci, 2015, 20(4): 219-229.
doi: 10.1016/j.tplants.2015.02.001 pmid: 25731753 |
| [7] |
Yu YB, Adams DO, Yang SF. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis[J]. Arch Biochem Biophys, 1979, 198(1): 280-286.
pmid: 507845 |
| [8] |
Adams DO, Yang SF. Ethylene biosynthesis:identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene[J]. Proc Natl Acad Sci USA, 1979, 76(1): 170-174.
doi: 10.1073/pnas.76.1.170 pmid: 16592605 |
| [9] | Park CH, Roh J, Youn JH, et al. Arabidopsis ACC oxidase 1 coordinated by multiple signals mediates ethylene biosynthesis and is involved in root development[J]. Mol Cells, 2018, 41(10): 923-932. |
| [10] |
Ning Q, Jian YN, Du YF, et al. An ethylene biosynthesis enzyme controls quantitative variation in maize ear length and kernel yield[J]. Nat Commun, 2021, 12(1): 5832.
doi: 10.1038/s41467-021-26123-z pmid: 34611160 |
| [11] |
Chersicola M, Kladnik A, Žnidarič MT, et al. 1-aminocyclopropane-1-carboxylate oxidase induction in tomato flower pedicel phloem and abscission related processes are differentially sensitive to ethylene[J]. Front Plant Sci, 2017, 8:464.
doi: 10.3389/fpls.2017.00464 pmid: 28408916 |
| [12] |
Penarrubia L, Aguilar M, Margossian L, et al. An antisense gene stimulates ethylene hormone production during tomato fruit ripening[J]. Plant Cell, 1992, 4(6): 681-687.
doi: 10.2307/3869526 URL |
| [13] |
Lincoln JE, Fischer RL. Diverse mechanisms for the regulation of ethylene-inducible gene expression[J]. Mol Gen Genet, 1988, 212(1): 71-75.
doi: 10.1007/BF00322446 URL |
| [14] |
Wang ZP, Xing HL, Dong L, et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation[J]. Genome Biol, 2015, 16(1): 144.
doi: 10.1186/s13059-015-0715-0 URL |
| [15] | 朱丽颖, 郑月萍, 徐雪珍, 等. 一种准确、简便测定CRISPR/Cas9基因编辑效率的方法[J]. 江苏农业学报, 2020, 36(2): 299-305. |
| Zhu LY, Zheng YP, Xu XZ, et al. A convenient and accurate method for determining the efficiency of CRISPR/Cas9-based gene editing[J]. Jiangsu J Agric Sci, 2020, 36(2): 299-305. | |
| [16] | Giovannoni JJ. Genetic regulation of fruit development and ripening[J]. Plant Cell, 2004, 16(Suppl):S170-S180. |
| [17] |
Yin XR, Allan AC, Chen KS, et al. Kiwifruit EIL and ERF genes involved in regulating fruit ripening[J]. Plant Physiol, 2010, 153(3): 1280-1292.
doi: 10.1104/pp.110.157081 URL |
| [18] |
Riyazuddin R, Verma R, Singh K, et al. Ethylene:a master regulator of Sal Inity stress tolerance in plants[J]. Biomolecules, 2020, 10(6): 959.
doi: 10.3390/biom10060959 URL |
| [19] |
Jiang CF, Belfield EJ, Cao Y, et al. An Arabidopsis soil-Sal Inity-tolerance mutation confers ethylene-mediated enhancement of sodium/potassium homeostasis[J]. Plant Cell, 2013, 25(9): 3535-3552.
doi: 10.1105/tpc.113.115659 URL |
| [20] |
Lockhart J. Salt of the earth:ethylene promotes salt tolerance by enhancing Na/K homeostasis[J]. Plant Cell, 2013, 25(9): 3150.
doi: 10.1105/tpc.113.250911 URL |
| [1] | 韩志阳, 贾子苗, 梁秋菊, 王轲, 唐华丽, 叶兴国, 张双喜. 二套小麦-簇毛麦染色体附加系苗期耐盐性及籽粒硒和叶酸的含量[J]. 生物技术通报, 2023, 39(8): 185-193. |
| [2] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [3] | 李帜奇, 袁月, 苗荣庆, 庞秋颖, 张爱琴. 盐胁迫盐芥和拟南芥褪黑素含量及合成相关基因表达模式分析[J]. 生物技术通报, 2023, 39(5): 142-151. |
| [4] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [5] | 陈奕博, 杨万明, 岳爱琴, 王利祥, 杜维俊, 王敏. 基于SLAF标记的大豆遗传图谱构建及苗期耐盐性QTL定位[J]. 生物技术通报, 2023, 39(2): 70-79. |
| [6] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| [7] | 阮航, 多浩源, 范文艳, 吕清晗, 姜述君, 朱生伟. AtERF49在拟南芥应答盐碱胁迫中的作用[J]. 生物技术通报, 2023, 39(1): 150-156. |
| [8] | 陈宏艳, 李小二, 李忠光. 糖信号及其在植物响应逆境胁迫中的作用[J]. 生物技术通报, 2022, 38(7): 80-89. |
| [9] | 高聪, 萧楚健, 鲁帅, 王苏蓉, 袁卉华, 曹云英. 氧化石墨烯对拟南芥生长的促进作用[J]. 生物技术通报, 2022, 38(6): 120-128. |
| [10] | 徐红云, 张明意. GRAS转录因子AtSCL4负调控拟南芥应答渗透胁迫[J]. 生物技术通报, 2022, 38(6): 129-135. |
| [11] | 古盼, 齐学影, 李莉, 张曦, 单晓昳. AtRGS1胞吞动态调控G蛋白参与拟南芥生长发育和抗性反应[J]. 生物技术通报, 2022, 38(6): 34-42. |
| [12] | 石广成, 杨万明, 杜维俊, 王敏. 大豆耐盐种质的筛选及其耐盐生理特性分析[J]. 生物技术通报, 2022, 38(4): 174-183. |
| [13] | 周娟, 阎晋东, 李新梅, 刘雪晴, 赵强, 赵小英. 拟南芥F-box蛋白FKF1与转录因子FUL互作调控开花研究[J]. 生物技术通报, 2022, 38(3): 1-8. |
| [14] | 杨佳慧, 孙玉萍, 陆雅宁, 刘欢, 卢存福, 陈玉珍. 拟南芥AtTERT对大肠杆菌非生物胁迫抗性的影响[J]. 生物技术通报, 2022, 38(2): 1-9. |
| [15] | 李兵娟, 郑璐, 沈仁芳, 兰平. 拟南芥RPP1A参与幼苗生长的蛋白质组学分析[J]. 生物技术通报, 2022, 38(2): 10-20. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||