








生物技术通报 ›› 2023, Vol. 39 ›› Issue (5): 142-151.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0975
收稿日期:2022-08-10
出版日期:2023-05-26
发布日期:2023-06-08
通讯作者:
张爱琴,女,博士,副教授,研究方向:植物逆境生理与分子生物学;E-mail: aiqinaegean@nefu.edu.cn作者简介:李帜奇,男,硕士研究生,研究方向:植物逆境生物学;E-mail: lzq1321059957@163.com
基金资助:
LI Zhi-qi( ), YUAN Yue, MIAO Rong-qing, PANG Qiu-ying, ZHANG Ai-qin(
), YUAN Yue, MIAO Rong-qing, PANG Qiu-ying, ZHANG Ai-qin( )
)
Received:2022-08-10
Published:2023-05-26
Online:2023-06-08
摘要:
盐生植物盐芥(Eutrema salsugineum)耐盐适应性强且具备模式植物特征,是研究植物逆境适应机理的理想材料。作为一种多功能的激素信号分子,褪黑素在盐芥耐盐性中的作用仍不清楚。本研究以盐芥为主要材料,以拟南芥做对比,主要通过褪黑素酶联免疫以及实时荧光定量PCR分析,比较了二者在不同组织中褪黑素的积累和在响应盐胁迫过程中褪黑素合成、相关基因的表达模式以及外源褪黑素处理对其盐应答表型的影响。结果显示,两种植物的褪黑素合成均在幼叶中最高,盐芥本底褪黑素合成水平显著高于拟南芥,且盐胁迫诱导了两种植物中的褪黑素含量,但不同于盐芥,拟南芥在处理7 d后褪黑素合成明显下降。通过序列比对发现在不同植物中,盐芥和拟南芥褪黑素合成相关基因的亲缘关系较近。盐应答表达分析显示,盐芥SNAT1、ASMT和COMT在盐处理3 d表达上调,而拟南芥中的相关基因在处理1 d和3 d后受盐诱导,7 d后拟南芥中表达下降而盐芥中则无明显变化,表明两种植物相关基因响应盐信号的表达变化存在差异。此外,外源褪黑素处理明显缓解了两种植物在盐逆境下的胁迫表型。综上,褪黑素有效贡献于盐芥抗盐性,参与调节盐芥和拟南芥的耐盐适应过程,但二者对褪黑素合成的诱导模式不同。
李帜奇, 袁月, 苗荣庆, 庞秋颖, 张爱琴. 盐胁迫盐芥和拟南芥褪黑素含量及合成相关基因表达模式分析[J]. 生物技术通报, 2023, 39(5): 142-151.
LI Zhi-qi, YUAN Yue, MIAO Rong-qing, PANG Qiu-ying, ZHANG Ai-qin. Melatonin Contents in Eutrema salsugineum and Arabidopsis thaliana Under Salt Stress, and Expression Pattern Analysis of Synthesis Related Genes[J]. Biotechnology Bulletin, 2023, 39(5): 142-151.
| 基因名称 Gene name | 引物方向 Primer direction | 引物序列 Primer sequence(5'-3') |
|---|---|---|
| AtACTIN | Forward | CATCAGGAAGGACTTGTACGG |
| Reverse | GATGGACCTGACTCGTCATAC | |
| AtSNAT1 | Forward | CGTCTATTAGCCCTCCTCC |
| Reverse | GCCATCCCACCTTATCACA | |
| AtSNAT2 | Forward | GGCACAATCTCCACTCCTC |
| Reverse | TCAACCACAACATCCCATA | |
| AtASMT | Forward | GGTCAACGGTTCAACTCCA |
| Reverse | ATCGCCCTCAACATTCTCA | |
| AtCOMT | Forward | ATGCTCCTTCTCATCCTGG |
| Reverse | CACGCAATGTTCGTCACTC | |
| EsACTIN | Forward | GCACAATCCAAAAGAGGTATTCTCACCT |
| Reverse | GGAGCCTCGGTAAGAAGAACAGGG | |
| EsSNAT1 | Forward | AAGAATAACAATACGACCCA |
| Reverse | ACATCAATCTCACCACCAG | |
| EsASMT | Forward | ATGTTACCGAAGTTGCG |
| Reverse | CGTTCTTTGCCTGTGCT | |
| EsCOMT | Forward | CGGCTTACTCCGTCCTCAC |
| Reverse | TAGCACCAATGCCACCACC |
表1 RT-qPCR分析所用引物序列
Table 1 Primer sequences used for RT-qPCR analysis
| 基因名称 Gene name | 引物方向 Primer direction | 引物序列 Primer sequence(5'-3') |
|---|---|---|
| AtACTIN | Forward | CATCAGGAAGGACTTGTACGG |
| Reverse | GATGGACCTGACTCGTCATAC | |
| AtSNAT1 | Forward | CGTCTATTAGCCCTCCTCC |
| Reverse | GCCATCCCACCTTATCACA | |
| AtSNAT2 | Forward | GGCACAATCTCCACTCCTC |
| Reverse | TCAACCACAACATCCCATA | |
| AtASMT | Forward | GGTCAACGGTTCAACTCCA |
| Reverse | ATCGCCCTCAACATTCTCA | |
| AtCOMT | Forward | ATGCTCCTTCTCATCCTGG |
| Reverse | CACGCAATGTTCGTCACTC | |
| EsACTIN | Forward | GCACAATCCAAAAGAGGTATTCTCACCT |
| Reverse | GGAGCCTCGGTAAGAAGAACAGGG | |
| EsSNAT1 | Forward | AAGAATAACAATACGACCCA |
| Reverse | ACATCAATCTCACCACCAG | |
| EsASMT | Forward | ATGTTACCGAAGTTGCG |
| Reverse | CGTTCTTTGCCTGTGCT | |
| EsCOMT | Forward | CGGCTTACTCCGTCCTCAC |
| Reverse | TAGCACCAATGCCACCACC |
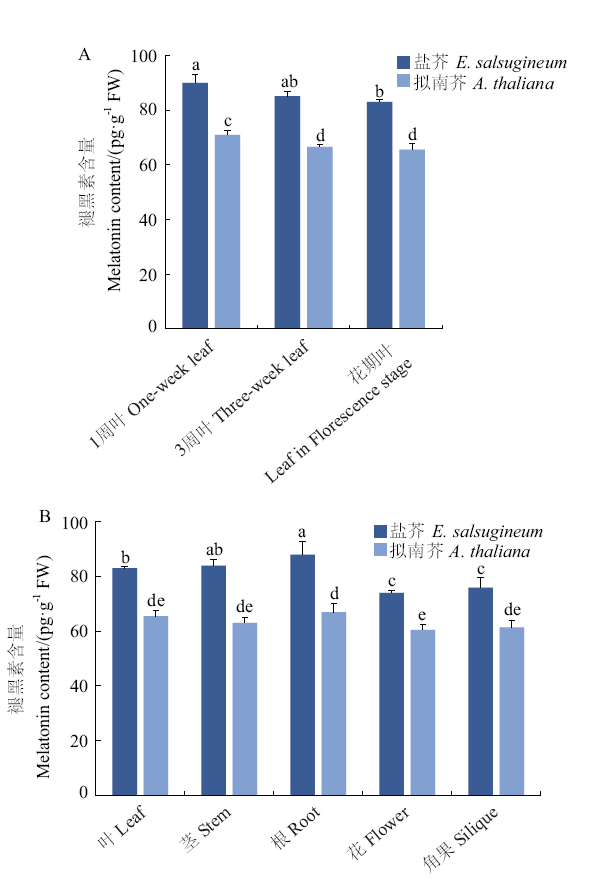
图1 盐芥和拟南芥不同组织中褪黑素的含量 A:营养生长时期;B:生殖生长时期。不同字母表示差异显著性(P < 0.05),下同
Fig. 1 Melatonin contents in different tissues of E. salsug-ineum and A. thaliana A: Vegetative growth stage. B: Reproductive growth stage. Different letters indicate significant difference(P < 0.05). The same below

图3 不同植物褪黑素合成酶氨基酸序列的多重比对 A:SNAT1;B:SNAT2;C:ASMT;D:COMT。黑色框表示蛋白酶中保守功能位点或结构域,*表示酚类底物结合位点。At :拟南芥;Es :盐芥;Vv :葡萄;Sl :番茄;Os :水稻;Zm :玉米。下同
Fig. 3 Multiple alignment of amino acid sequences of melatonin synthases in different plants Black boxes indicate conserved functional sites or domains in proteases, and * indicates the binding sites of phenolic substrate. At: Arabidopsis thaliana; Es: Eutrema salsugineum; Vv: Vitis vinifera L.; Sl: Solanum lycopersicum L.; Os: Oryza sativa L.; Zm: Zea mays L. The same below
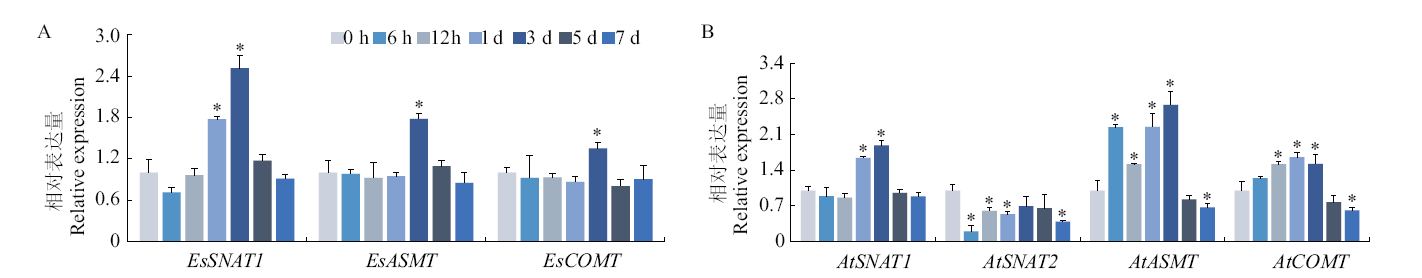
图5 褪黑素合成基因响应盐胁迫的表达模式 A:盐芥;B:拟南芥。*表示未处理与盐处理各时间点的差异显著性(P < 0.05)
Fig. 5 Expression pattern of melatonin synthesis genes in response to salt stress A: E. salsugineum. B: A. thaliana. * indicates significant difference between untreated and salt treated plants at different time point(P < 0.05)

图6 外源褪黑素处理下盐芥和拟南芥的盐应答表型 A:盐芥;B:拟南芥。C:未处理;MT:褪黑素处理;S:盐处理;S+MT:盐处理和褪黑素处理
Fig. 6 Salt-responsive phenotypes of E. salsugineum and A. thaliana treated with exogenous melatonin A: E. salsugineum. B: A. thaliana. C: Control. MT: Melatonin treatment. S: Salt treatment. S+MT: Salt and melatonin treatment
| [1] |
Morton MJL, Awlia M, Al-Tamimi N, et al. Salt stress under the scalpel - dissecting the genetics of salt tolerance[J]. Plant J, 2019, 97(1): 148-163.
doi: 10.1111/tpj.2019.97.issue-1 URL |
| [2] |
Gong ZZ, Xiong LM, Shi HZ, et al. Plant abiotic stress response and nutrient use efficiency[J]. Sci China Life Sci, 2020, 63(5): 635-674.
doi: 10.1007/s11427-020-1683-x pmid: 32246404 |
| [3] |
Parihar P, Singh S, Singh R, et al. Effect of salinity stress on plants and its tolerance strategies: a review[J]. Environ Sci Pollut Res Int, 2015, 22(6): 4056-4075.
doi: 10.1007/s11356-014-3739-1 URL |
| [4] |
Apse MP, Blumwald E. Engineering salt tolerance in plants[J]. Curr Opin Biotechnol, 2002, 13(2): 146-150.
doi: 10.1016/S0958-1669(02)00298-7 URL |
| [5] |
Zhu JK. Abiotic stress signaling and responses in plants[J]. Cell, 2016, 167(2): 313-324.
doi: 10.1016/j.cell.2016.08.029 URL |
| [6] |
Park HJ, Kim WY, Yun DJ. A new insight of salt stress signaling in plant[J]. Mol Cells, 2016, 39(6): 447-459.
doi: 10.14348/molcells.2016.0083 pmid: 27239814 |
| [7] |
Kanwar MK, Yu JQ, Zhou J. Phytomelatonin: Recent advances and future prospects[J]. J Pineal Res, 2018, 65(4): e12526.
doi: 10.1111/jpi.2018.65.issue-4 URL |
| [8] | Lerner AB, Case JD, Takahashi Y, et al. Isolation of melatonin, the pineal gland factor that lightens melanocytes[J]. J Am Chem Soc, 1958, 80(10): 2587. |
| [9] |
Dubbels R, Reiter RJ, Klenke E, et al. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry[J]. J Pineal Res, 1995, 18(1): 28-31.
doi: 10.1111/j.1600-079x.1995.tb00136.x pmid: 7776176 |
| [10] |
Hattori A, Migitaka H, Iigo M, et al. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates[J]. Biochem Mol Biol Int, 1995, 35(3): 627-634.
pmid: 7773197 |
| [11] |
Lee HY, Byeon Y, Lee K, et al. Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations[J]. J Pineal Res, 2014, 57(4): 418-426.
doi: 10.1111/jpi.12181 URL |
| [12] | 宋雪飞. 褪黑素调控番茄幼苗耐盐性的浓度效应及其生理机制的研究[D]. 扬州: 扬州大学, 2018. |
| Song XF. Study on the concentration effect of melatonin on salt tolerance of tomato seedlings and its physiological mechanism[D]. Yangzhou: Yangzhou University, 2018. | |
| [13] | 乔沛, 殷菲胧, 王雨萱, 等. 外源褪黑素处理对采后荔枝褐变及活性氧代谢的影响[J]. 食品工业科技, 2021, 42(6): 282-287. |
| Qiao P, Yin FL, Wang YX, et al. Effects of exogenous melatonin on browning and active oxygen metabolism of postharvest Litchi[J]. Sci Technol Food Ind, 2021, 42(6): 282-287. | |
| [14] |
Sun CL, Liu LJ, Wang LX, et al. Melatonin: a master regulator of plant development and stress responses[J]. J Integr Plant Biol, 2021, 63(1): 126-145.
doi: 10.1111/jipb.12993 |
| [15] |
Zhan HS, Nie XJ, Zhang T, et al. Melatonin: a small molecule but important for salt stress tolerance in plants[J]. Int J Mol Sci, 2019, 20(3): 709.
doi: 10.3390/ijms20030709 URL |
| [16] |
Li JP, Liu J, Zhu TT, et al. The role of melatonin in salt stress responses[J]. Int J Mol Sci, 2019, 20(7): 1735.
doi: 10.3390/ijms20071735 URL |
| [17] |
Gong QQ, Li PH, Ma SS, et al. Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana[J]. Plant J, 2005, 44(5): 826-839.
doi: 10.1111/tpj.2005.44.issue-5 URL |
| [18] |
Amtmann A. Learning from evolution: Thellungiella generates new knowledge on essential and critical components of abiotic stress tolerance in plants[J]. Mol Plant, 2009, 2(1): 3-12.
doi: 10.1093/mp/ssn094 pmid: 19529830 |
| [19] |
Inan G, Zhang Q, Li PH, et al. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles[J]. Plant Physiol, 2004, 135(3): 1718-1737.
doi: 10.1104/pp.104.041723 URL |
| [20] |
Pelagio-Flores R, Muñoz-Parra E, Ortiz-Castro R, et al. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling[J]. J Pineal Res, 2012, 53(3): 279-288.
doi: 10.1111/j.1600-079X.2012.00996.x pmid: 22507071 |
| [21] | 张悦新. 褪黑素对棉花耐盐性的影响及相关基因GhSNAT34的功能研究[D]. 郑州: 郑州大学, 2021. |
| Zhang YX. Study on effect of melatonin on salt tolerance of cotton and function of GhSNAT34 gene[D]. Zhengzhou: Zhengzhou University, 2021. | |
| [22] |
Li JH, Yang YQ, Sun K, et al. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant(Camellia sinensis(L.) O. Kuntze)[J]. Molecules, 2019, 24(9): 1826.
doi: 10.3390/molecules24091826 URL |
| [23] | 吴艳迪. 葡萄褪黑素含量变化及其合成基因SNAT原核表达分析[D]. 北京: 中国农业科学院, 2018. |
| Wu YD. Changes of melatonin content and prokaryotic expression analysis of SNAT gene in grapevine[D]. Beijing: Chinese Academy of Agricultural Sciences, 2018. | |
| [24] |
Arnao MB, Hernández-Ruiz J. Chemical stress by different agents affects the melatonin content of barley roots[J]. J Pineal Res, 2009, 46(3): 295-299.
doi: 10.1111/j.1600-079X.2008.00660.x pmid: 19196434 |
| [25] |
Byeon Y, Lee HY, Lee K, et al. Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT[J]. J Pineal Res, 2014, 56(1): 107-114.
doi: 10.1111/jpi.12103 pmid: 24134674 |
| [26] |
Liu DD, Sun XS, Liu L, et al. Overexpression of the melatonin synthesis-related gene SlCOMT1 improves the resistance of tomato to salt stress[J]. Molecules, 2019, 24(8): 1514.
doi: 10.3390/molecules24081514 URL |
| [27] |
Chen YE, Mao JJ, Sun LQ, et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity[J]. Physiol Plant, 2018, 164(3): 349-363.
doi: 10.1111/ppl.2018.164.issue-3 URL |
| [28] |
Yan FY, Zhang JY, Li WW, et al. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings[J]. Plant Physiol Biochem, 2021, 163: 367-375.
doi: 10.1016/j.plaphy.2021.03.058 URL |
| [29] |
Zhang ZH, Liu LT, Li HY, et al. Exogenous melatonin promotes the salt tolerance by removing active oxygen and maintaining ion balance in wheat(Triticum aestivum L.)[J]. Front Plant Sci, 2022, 12: 787062.
doi: 10.3389/fpls.2021.787062 URL |
| [30] |
Xu LL, Xiang GQ, Sun QH, et al. Melatonin enhances salt tolerance by promoting MYB108A-mediated ethylene biosynthesis in grapevines[J]. Hortic Res, 2019, 6: 114.
doi: 10.1038/s41438-019-0197-4 |
| [1] | 杨志晓, 侯骞, 刘国权, 卢志刚, 曹毅, 芶剑渝, 王轶, 林英超. 不同抗性烟草品系Rubisco及其活化酶对赤星病胁迫的响应[J]. 生物技术通报, 2023, 39(9): 202-212. |
| [2] | 康凌云, 韩露露, 韩德平, 陈建胜, 甘瀚凌, 邢凯, 马友记, 崔凯. 褪黑素缓解空肠黏膜上皮细胞氧化损伤的效果研究[J]. 生物技术通报, 2023, 39(9): 291-299. |
| [3] | 韩志阳, 贾子苗, 梁秋菊, 王轲, 唐华丽, 叶兴国, 张双喜. 二套小麦-簇毛麦染色体附加系苗期耐盐性及籽粒硒和叶酸的含量[J]. 生物技术通报, 2023, 39(8): 185-193. |
| [4] | 魏茜雅, 秦中维, 梁腊梅, 林欣琪, 李映志. 褪黑素种子引发处理提高朝天椒耐盐性的作用机制[J]. 生物技术通报, 2023, 39(7): 160-172. |
| [5] | 李宇, 李素贞, 陈茹梅, 卢海强. 植物bHLH转录因子调控铁稳态的研究进展[J]. 生物技术通报, 2023, 39(7): 26-36. |
| [6] | 刘奎, 李兴芬, 杨沛欣, 仲昭晨, 曹一博, 张凌云. 青杄转录共激活因子PwMBF1c的功能研究与验证[J]. 生物技术通报, 2023, 39(5): 205-216. |
| [7] | 赖瑞联, 冯新, 高敏霞, 路喻丹, 刘晓驰, 吴如健, 陈义挺. 猕猴桃过氧化氢酶基因家族全基因组鉴定与表达分析[J]. 生物技术通报, 2023, 39(4): 136-147. |
| [8] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [9] | 陈强, 邹明康, 宋家敏, 张冲, 吴隆坤. 甜瓜LBD基因家族的鉴定和果实发育进程中的表达分析[J]. 生物技术通报, 2023, 39(3): 176-183. |
| [10] | 崔吉洁, 蔡文波, 庄庆辉, 高爱平, 黄建峰, 陈亚辉, 宋志忠. 杧果Fe-S簇装配基因MiISU1的生物学功能[J]. 生物技术通报, 2023, 39(2): 139-146. |
| [11] | 姚晓文, 梁晓, 陈青, 伍春玲, 刘迎, 刘小强, 税军, 乔阳, 毛奕茗, 陈银华, 张银东. 二斑叶螨抗性木薯木质素合成途径基因表达特性研究[J]. 生物技术通报, 2023, 39(2): 161-171. |
| [12] | 李彦霞, 王晋鹏, 冯芬, 包斌武, 董益闻, 王兴平, 罗仍卓么. 大肠杆菌型奶牛乳房炎对产奶性状相关基因表达的影响[J]. 生物技术通报, 2023, 39(2): 274-282. |
| [13] | 陈奕博, 杨万明, 岳爱琴, 王利祥, 杜维俊, 王敏. 基于SLAF标记的大豆遗传图谱构建及苗期耐盐性QTL定位[J]. 生物技术通报, 2023, 39(2): 70-79. |
| [14] | 冯策婷, 江律, 刘鑫颖, 罗乐, 潘会堂, 张启翔, 于超. 单叶蔷薇NAC基因家族鉴定及干旱胁迫响应分析[J]. 生物技术通报, 2023, 39(11): 283-296. |
| [15] | 鄢梦雨, 韦晓薇, 曹婧, 兰海燕. 异子蓬SabHLH169基因的克隆及抗旱功能分析[J]. 生物技术通报, 2023, 39(11): 328-339. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||