








生物技术通报 ›› 2024, Vol. 40 ›› Issue (5): 280-289.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1100
收稿日期:2023-11-22
出版日期:2024-05-26
发布日期:2024-06-13
通讯作者:
王忠华,男,博士,教授,研究方向:植物生理与分子生物学;E-mail: wang1972@zwu.edu.cn作者简介:潘萍萍,女,硕士研究生,研究方向:植物生理与分子生物学;E-mail: panmou110@163.com
基金资助:
PAN Ping-ping( ), XU Zhi-hao, ZHANG Yi-wen, LI Qing, WANG Zhong-hua(
), XU Zhi-hao, ZHANG Yi-wen, LI Qing, WANG Zhong-hua( )
)
Received:2023-11-22
Published:2024-05-26
Online:2024-06-13
摘要:
【目的】探究查尔酮合酶(chalcone synthase, PcCHS)基因在多花黄精类黄酮合成中的作用,为后续解析PcCHS功能以及多花黄精新品种选育提供可靠的理论依据。【方法】以多花黄精为cDNA模板,克隆多花黄精PcCHS基因的编码序列,对该基因进行生物信息学分析。通过构建PcCHS的原核表达载体,纯化目的重组蛋白,验证该酶的体外表达活性。利用瞬时超表达体系探究该基因过表达后总黄酮的含量变化。利用Gateway技术构建亚细胞定位载体35S::PcCHS-GFP,通过本氏烟草表达系统确定目的蛋白亚细胞定位情况。【结果】PcCHS基因的开放阅读框为1 251 bp,理论分子量为44.63 kD,等电点为5.89,属于亲水蛋白,与石刁柏(Asparagus officinalis)CHS亲缘关系较近。原核表达实验表明,pET28a-PcCHS经IPTG(异丙基-β-D-硫代半乳糖苷)诱导表达可溶性重组蛋白,Western-blot显示大小约为45 kD,与预期大小一致,且纯化的目的蛋白具有一定的酶活性,能催化对香豆酰辅酶A和丙二酰辅酶A转化为柚皮素查尔酮。此外,PcCHS瞬时超表达中,PcCHS组的表达量显著高于空载K组,总黄酮含量也显著高于空载K组,最高可达1.83倍。亚细胞定位结果显示,该基因在细胞膜和细胞核中发挥作用。【结论】PcCHS基因原核表达的酶具有体外酶活性,其亚细胞定位于细胞膜和细胞核,且瞬时超表达能够显著提高多花黄精叶片总黄酮含量。
潘萍萍, 徐志浩, 张怡雯, 李青, 王忠华. 多花黄精查尔酮合酶PcCHS的原核表达、亚细胞定位及表达分析[J]. 生物技术通报, 2024, 40(5): 280-289.
PAN Ping-ping, XU Zhi-hao, ZHANG Yi-wen, LI Qing, WANG Zhong-hua. Prokaryotic Expression, Subcellular Localization and Expression Analysis of PcCHS Gene from Polygonatum cyrtonema Hua[J]. Biotechnology Bulletin, 2024, 40(5): 280-289.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 用途 Usage |
|---|---|---|
| PcCHS-F | ATGGGTTCCATTCCGGAGATG | 基因克隆 |
| PcCHS-R | TCACGGACAGCGGAGGAC | |
| 28-CHS-F | AATGGGTCGCGGATCCATGGGTTCCATTCCGGAGATGC | 重组蛋白制备 |
| 28-CHS-R | CCGCAAGCTTGTCGACCGGACAGCGGAGGACGACAGTCTCC | |
| T7 | TAATACGACTCACTATAGGG | 菌落PCR |
| T7t | GCTAGTTATTGCTCAGCGG | |
| D-CHS-F | GGGGACAATGTTGTACAAAAAAGCAGGCTGCATGGGTTCCATTCCGGAGATG | 入门载体构建 |
| D-CHS-R | GGGGACCACTTTGTACAAGAAAGCTGGGTCTCACGGACAGCGGAGGAC | |
| 35s-CHS-F | CGGTACCCGGGGATCCATGGGTTCCATTCCGGAGATGC | 过表达载体构建 |
| 35s-CHS-R | GGGCGAATTGGTCGACCGGACAGCGGAGGACGACAGTCTCC | |
| qPCR-CHS | CAAGGTCACAAACAGCGAGC | qPCR |
| qPCR-CHS | TATGCCGCAATGGATGGGTT | |
| UBQ-10-F | GGACCCAGAAGTACGCAATG | qPCR(内参) |
| UBQ-10-R | AATTACCAGGGATACAGCACC |
表1 实验所用引物
Table 1 Primers used in the experiment
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 用途 Usage |
|---|---|---|
| PcCHS-F | ATGGGTTCCATTCCGGAGATG | 基因克隆 |
| PcCHS-R | TCACGGACAGCGGAGGAC | |
| 28-CHS-F | AATGGGTCGCGGATCCATGGGTTCCATTCCGGAGATGC | 重组蛋白制备 |
| 28-CHS-R | CCGCAAGCTTGTCGACCGGACAGCGGAGGACGACAGTCTCC | |
| T7 | TAATACGACTCACTATAGGG | 菌落PCR |
| T7t | GCTAGTTATTGCTCAGCGG | |
| D-CHS-F | GGGGACAATGTTGTACAAAAAAGCAGGCTGCATGGGTTCCATTCCGGAGATG | 入门载体构建 |
| D-CHS-R | GGGGACCACTTTGTACAAGAAAGCTGGGTCTCACGGACAGCGGAGGAC | |
| 35s-CHS-F | CGGTACCCGGGGATCCATGGGTTCCATTCCGGAGATGC | 过表达载体构建 |
| 35s-CHS-R | GGGCGAATTGGTCGACCGGACAGCGGAGGACGACAGTCTCC | |
| qPCR-CHS | CAAGGTCACAAACAGCGAGC | qPCR |
| qPCR-CHS | TATGCCGCAATGGATGGGTT | |
| UBQ-10-F | GGACCCAGAAGTACGCAATG | qPCR(内参) |
| UBQ-10-R | AATTACCAGGGATACAGCACC |

图4 菌落PCR(A)、原核载体酶切验证(B)和过表达载体酶切验证(C)
Fig. 4 Colony PCR(A), prokaryotic vector digestion verification(B)and overexpressed vector digestion verification(C)

图5 不同浓度IPTG诱导蛋白、蛋白纯化情况及Western-blot鉴定 A:不同浓度IPTG诱导(1、2为0 mmol/L IPTG诱导的沉淀和上清,3、4为0.5 mmol/L IPTG诱导的沉淀和上清,5、6为1 mmol/L IPTG的沉淀和上清);B:目的蛋白纯化(1为0.5 mmol/L IPTG诱导的沉淀,2为0.5 mmol/L IPTG诱导的上清,3、4为穿透液,5-7为杂质洗脱液,8-14为50 mmol/L洗脱液洗脱的蛋白);C:重组蛋白PcCHS表达的Western-blot鉴定
Fig. 5 Protein induced by IPTG at different concentrations, protein purification and Western-blot identification A: Induced by different concentrations of IPTG(1 and 2 were 0 IPTG-induced precipitation and supernatant; 3 and 4 were 0.5 mmol/L IPTG-induced precipitation and supernatant; 5 and 6 were 1 mmol/L IPTG precipitating and supernatant). B: Objective for protein purification(1 was precipitated by 0.5 mmol/L IPTG, 2 was supernatant induced by 0.5 mmol/L IPTG, 3 and 4 were penetrating fluid, 5-7 were impurity eluents, 8-14 were proteins eluted by 50 mmol/L eluent). C: Expression of recombinant protein PcCHS identified by Western-blot
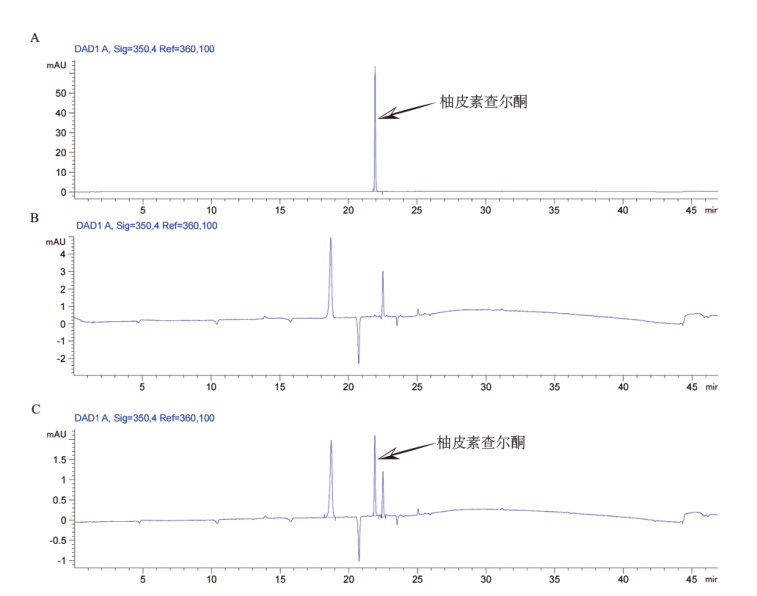
图6 体外酶活分析 A:350 nm下的柚皮素查尔酮标品峰图;B:对照组峰图,对香豆酰辅酶A、丙二酰辅酶A和100℃ 10 min纯化的PcCHS酶;C:实验组峰图,对香豆酰辅酶A、丙二酰辅酶A和纯化的PcCHS酶
Fig. 6 Enzyme activity analysis in vitro A: Naringin chalcone standard peak at 350 nm. B: Control group peak, para-coumaryl CoA, malonyl CoA and product enzyme at 100℃ for 10 min. C: Experimental group peak, para-coumaryl CoA, malonyl CoA and product enzyme
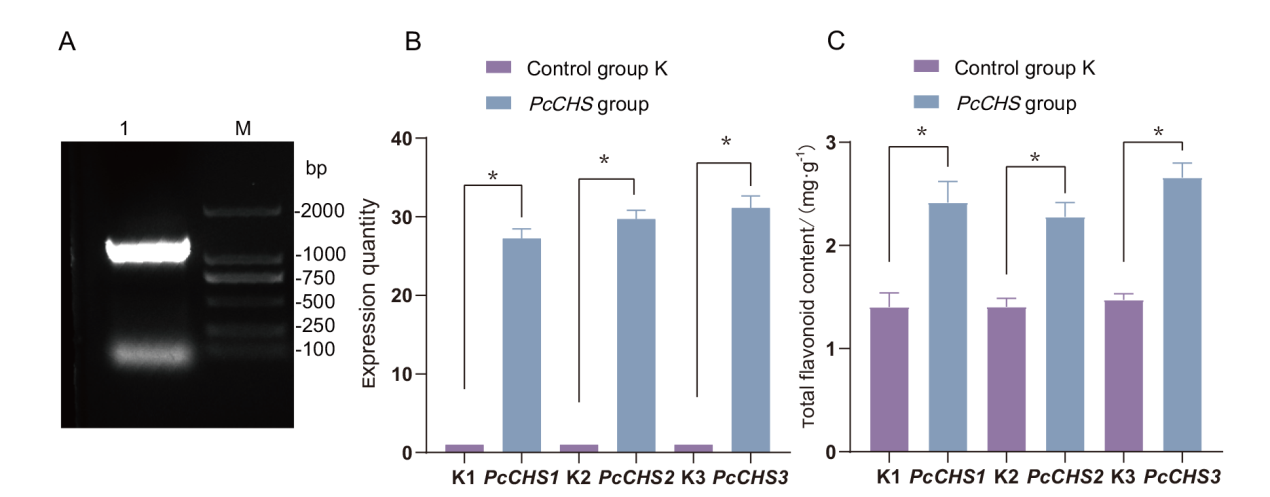
图7 瞬时超表达的DNA验证、PcCHS表达量和总黄酮含量 A:DNA验证;B:PcCHS瞬时超表达的表达量;C:PcCHS瞬时超表达的总黄酮含量;*表示在P<0.05水平差异显著
Fig. 7 Transient overexpressions of DNA validation, PcCHS expression amount and total flavone content A: DNA verification. B: The amount of transient overexpression of PcCHS. C: Transient overexpressed total flavonoid content of PcCHS. * indicates significant difference at P<0.05 level
| [1] | 章鹏飞, 张虹, 张小波, 等. 多花黄精生态适宜性区划研究[J]. 中国中药杂志, 2020, 45(13): 3073-3078. |
| Zhang PF, Zhang H, Zhang XB, et al. Ecology suitability study of Polygonatum cyrtonema[J]. China J Chin Mater Med, 2020, 45(13): 3073-3078. | |
| [2] | 徐宇琳, 王元忠, 杨美权, 等. 黄精的本草考证及民族用法[J]. 中国实验方剂学杂志, 2021, 27(17): 237-250. |
| Xu YL, Wang YZ, Yang MQ, et al. Herbal textual research on polygonati rhizoma and ethnic usage[J]. China Ind Econ, 2021, 27(17): 237-250. | |
| [3] | 王育红, 张晓宇, 钱志伟. 响应面优化低共熔溶剂提取黄精黄酮工艺及其生物活性研究[J]. 中国食品添加剂, 2023, 34(4): 116-123. |
| Wang YH, Zhang XY, Qian ZW. Optimization of extraction of flavonoid from Polygonatum kingianum with deep eutectic solvent by response surface method and its bioactivity[J]. China Food Addit, 2023, 34(4): 116-123. | |
| [4] | 郭凯丽, 刘继平, 赵重博, 等. 酶辅助超声法提取陕产黄精总黄酮及其抗氧化、抗A549细胞增殖活性研究[J]. 天然产物研究与开发, 2022, 34(4): 630-638. |
| Guo KL, Liu JP, Zhao CB, et al. Enzymatic-ultrasonic assisted extraction of total flavonoids from Shaanxi Polygonatum sibiricum and in vitro evaluation of their anti-oxidant and anti-A549 proliferation activities[J]. Nat Prod Res Dev, 2022, 34(4): 630-638. | |
| [5] | 陶爱恩, 张晓灿, 杜泽飞, 等. 黄精属植物中黄酮类化合物及其药理活性研究进展[J]. 中草药, 2018, 49(9): 2163-2171. |
| Tao AE, Zhang XC, Du ZF, et al. Research progress on flavonoids in plants of Polygonatum Mill. and their pharmacological activities[J]. Chin Tradit Herb Drugs, 2018, 49(9): 2163-2171. | |
| [6] | Peng F, Yin HY, Du B, et al. Anti-fatigue activity of purified flavonoids prepared from chestnut(Castanea mollissima)flower[J]. J Funct Foods, 2021, 79: 104365. |
| [7] | Shen WD, Li XY, Deng YY, et al. Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling[J]. Int J Biol Macromol, 2021, 175: 235-241. |
| [8] | Huang ZZ, Du X, Ma CD, et al. Identification of antitumor active constituents in Polygonatum sibiricum flower by UPLC-Q-TOF-MSE and network pharmacology[J]. ACS Omega, 2020, 5(46): 29755-29764. |
| [9] | Zhang HL, Hao FL, Yao ZF, et al. Efficient extraction of flavonoids from Polygonatum sibiricum using a deep eutectic solvent as a green extraction solvent[J]. Microchem J, 2022, 175: 107168. |
| [10] | 陈忠海. 青藏高原水毛茛查尔酮合成酶(CHS)基因家族鉴定及表达载体构建[D]. 拉萨: 西藏大学, 2022. |
| Chen ZH. Identification of Chalcone synthase(CHS)gene family of Ranunculus ternatus in Qinghai-Tibet Plateau and construction of expression vector[D]. Lasa: Tibet University, 2022. | |
| [11] | Liu WX, Feng Y, Yu SH, et al. The flavonoid biosynthesis network in plants[J]. Int J Mol Sci, 2021, 22(23): 12824. |
| [12] | 王文静. 马铃薯CHS基因家族鉴定及功能解析[D]. 合肥: 安徽农业大学, 2023. |
| Wang WJ. Identification and functional analysis of CHS gene family in potato[D]. Hefei: Anhui Agricultural University, 2023. | |
| [13] | 黎霜. 利用樱桃查尔酮合酶CpCHS1基因创制烟草抗旱种质[D]. 贵阳: 贵州大学, 2022. |
| Li S. Using cherry chalcone synthase CpCHS1 gene to create drought-resistant tobacco germplasm[D]. Guiyang: Guizhou University, 2022. | |
| [14] | 王会娟. 南极苔藓类黄酮合成2——酮戊二酸依赖型双加氧酶的生物功能研究[D]. 济南: 山东大学, 2022. |
| Wang HJ. Study on biological function of 2-ketoglutarate-dependent dioxygenase for flavonoid synthesis in Antarctic moss[D]. Jinan: Shandong University, 2022. | |
| [15] |
郭三保, 宋美玲, 李灵心, 等. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-1130 |
| Guo SB, Song ML, Li LX, et al. Cloning and analysis of Chalcone synthase gene and its promoter from Euphorbia maculata[J]. Biotechnol Bull, 2023, 39(4): 148-156. | |
| [16] | 汪王, 吉乃喆, 崔娇鹏, 等. 月季新型PKSIII基因的生物信息学分析及原核表达[J]. 西北农林科技大学学报: 自然科学版, 2022, 50(12): 97-107. |
| Wang W, Ji NZ, Cui JP, et al. Bioinformatics analysis and prokaryotic expression of novel PKS III genes in Rosa chinensis[J]. J Northwest A F Univ Nat Sci Ed, 2022, 50(12): 97-107. | |
| [17] | 何金娇, 张万方, 侯玥如, 等. 茄子查尔酮合酶基因的克隆与生物信息学分析[J]. 北方园艺, 2023(19): 16-21. |
| He JJ, Zhang WF, Hou YR, et al. Cloning and bioinformatics analysis of Chalcone synthase gene in eggplant[J]. North Hortic, 2023(19): 16-21. | |
| [18] | 翟佳丽, 坝玉蓉, 甘雪, 等. 番茄SlMYB86基因的原核表达及缺氮胁迫下的表达分析[J]. 西北植物学报, 2022, 42(1): 13-20. |
| Zhai JL, Ba YR, Gan X, et al. Prokaryotic expression and expression analysis of tomato SlMYB86 gene under nitrogen deficiency[J]. Acta Bot Boreali Occidentalia Sin, 2022, 42(1): 13-20. | |
| [19] |
刘玥, 李月庆, 孟祥宇, 等. 大花君子兰查尔酮合酶基因CmCHS的克隆及其功能验证[J]. 园艺学报, 2021, 48(10): 1847-1858.
doi: 10.16420/j.issn.0513-353x.2021-0090 |
| Liu Y, Li YQ, Meng XY, et al. Cloning and functional characterization of Chalcone synthase genes(CmCHS)from Clivia mini-ata[J]. Acta Hortic Sin, 2021, 48(10): 1847-1858. | |
| [20] | 李志凌. 红花FT基因的克隆及参与成花和类黄酮合成的初步研究[D]. 长春: 吉林农业大学, 2023. |
| Li ZL. Cloning of FT gene in Safflower and its involvement in flower formation and flavonoid synthesis[D]. Changchun: Jilin Agricultural University, 2023. | |
| [21] |
安飞飞, 齐剑雄, 陈松笔, 等. 木薯查尔酮合酶MeCHS基因家族特征与表达分析[J]. 热带作物学报, 2023, 44(12): 2384-2391.
doi: 10.3969/j.issn.1000-2561.2023.12.003 |
| An FF, Qi JX, Chen SB, et al. Characteristics and expression analysis of Chalcone synthase MeCHS family in cassava[J]. Chin J Trop Crops, 2023, 44(12): 2384-2391. | |
| [22] | 肖晓燕, 朱成磊, 杨克彬, 等. 低温促进毛竹叶片类黄酮合成及相关基因的表达模式分析[J]. 热带亚热带植物学报, 2024, 32(1):101-110. |
| Xiao XY, Zhu CL, Yang KB, et al. Effect of low temperature on flavonoid synthesis and expression pattern of related genes in leaves of Bamboo[J]. Journal of Tropical and Subtropical Botany, 2024, 32(1):101-110. | |
| [23] | 成永娟, 张明月, 曹雪璟, 等. 葡萄CHS基因家族鉴定与表达分析[J]. 果树学报, 2023, 40(5): 861-874. |
| Cheng YJ, Zhang MY, Cao XJ, et al. Identification and expression analysis of CHS gene family in grape[J]. J Fruit Sci, 2023, 40(5): 861-874. | |
| [24] | 刘慧, 张衡, 叶丽红, 等. DchCHS1过表达载体构建及其在中国石竹离体花苞中瞬时表达分析[J]. 北方园艺, 2023(20): 47-53. |
| Liu H, Zhang H, Ye LH, et al. Construction of overexpression vector for DchCHS1 and its transient expression in detached floral buds of Dianthus chinensis[J]. North Hortic, 2023(20): 47-53. | |
| [25] | 王蒙蒙, 刘雨然, 杨慧莹, 等. LHCGR胞外结构域蛋白的生信分析、原核表达及纯化[J]. 石河子大学学报:自然科学版, 2024, 42(2):150-158. |
| Wang MM, Liu YR, Yang HY, et al. Biogenic analysis, prokaryotic expression and purification of extracellular domain protein of LHCGR[J]. J Shihezi Univ Nat Sci Edi, 2024, 42(2):150-158. | |
| [26] | 罗在柒, 陆俊, 田凡, 等. 植物类型III聚酮合酶基因工程的研究进展[J]. 贵州林业科技, 2021, 49(4): 60-64. |
| Luo ZQ, Lu J, Tian F, et al. Research progress on plant type III polyketide synthase gene engineering[J]. Guizhou For Sci Technol, 2021, 49(4): 60-64. | |
| [27] | 朱雪雯, 米要磊, 孟祥霄, 等. 汉麻聚酮合酶基因家族成员鉴定与表达分析[J]. 中草药, 2023, 54(3): 886-897. |
| Zhu XW, Mi YL, Meng XX, et al. Identification and expression analysis of polyketide synthase gene family in Cannabis sativa[J]. Chin Tradit Herb Drugs, 2023, 54(3): 886-897. | |
| [28] | 邱海玲, 王方明, 米芯雨, 等. 管花肉苁蓉花中查耳酮合酶的基因克隆、功能鉴定与表达分析[J]. 中草药, 2023, 54(23): 7797-7805. |
| Qiu HL, Wang FM, Mi XY, et al. Cloning, functional identification and expression analysis of chalcone synthase from Cistanche tubulosa[J]. Chin Tradit Herb Drugs, 2023, 54(23): 7797-7805. | |
| [29] | 何春艳. 草地早熟禾花发育相关基因PpCHS1与PpSPL4的克隆和功能分析[D]. 北京: 北京林业大学, 2018. |
| He CY. Cloning and functional analysis of PpCHS1 and PpSPL4 genes associated flower development in Kentucky bluegrass[D]. Beijing: Beijing Forestry University, 2018. | |
| [30] | Awasthi P, Mahajan V, Jamwal VL, et al. Cloning and expression analysis of chalcone synthase gene from Coleus forskohlii[J]. J Genet, 2016, 95(3): 647-657. |
| [31] | Liu JH, Wang RG, Li GJ, et al. Cloning and prokaryotic expression of WRKY48 from Caragana intermedia[J]. Open Life Sci, 2022, 17(1): 131-138. |
| [32] | 余永亮, 鲁丹丹, 谭政委, 等. 不同品种忍冬ANR基因克隆、表达模式及原核表达分析[J]. 药学学报: 2023, 58(11): 3449-3460. |
| Yu YL, Lu DD, Tan ZW, et al. Cloning and expression analysis of ANR genes from different species of Lonicera japonica Thunb[J]. Acta Pharm Sin, 2023, 58(11): 3449-3460. | |
| [33] | Liu ML, Yu HW, Li J, et al. Cloning, expression, and functional analysis of the full-length cDNA of acetyl-CoA C-acetyltransferase(AACT)genes related to terpenoid synthesis in Platycodon grandiflorus[J]. Protein Pept Lett, 2022, 29(12): 1061-1071. |
| [34] | Wang HL, Wang W, Zhan JC, et al. The accumulation and localization of chalcone synthase in grapevine(Vitis vinifera L.)[J]. Plant Physiol Biochem, 2016, 106: 165-176. |
| [35] | Sun W, Meng XY, Liang LJ, et al. Molecular and biochemical analysis of chalcone synthase from Freesia hybrid in flavonoid biosynthetic pathway[J]. PLoS One, 2015, 10(3): e0119054. |
| [36] | 张衡. 农杆菌介导的中国石竹基因瞬时过表达体系建立及验证[D]. 呼和浩特: 内蒙古农业大学, 2023. |
| Zhang H, Establishment and verification of transient overexpression system of Chinese Caryophylla gene mediated by Dianthus chinensis[D]. Hohhot: Inner Mongolia Agricultural University, 2023. | |
| [37] | 吴榕, 田再民, 郑国华, 等. 白三叶CHS基因的过表达提高烟草类黄酮的含量[J]. 草业科学, 2020, 37(2): 305-313. |
| Wu R, Tian ZM, Zheng GH, et al. Overexpression of CHS genes from Trifolium repens increases tobacco flavonoid content[J]. Pratacultural Sci, 2020, 37(2): 305-313. |
| [1] | 张娜, 刘梦楠, 屈展帆, 崔祎平, 倪嘉瑶, 王华忠. 小麦烯醇化酶基因ENO2的可变翻译分析和原核表达[J]. 生物技术通报, 2024, 40(5): 112-119. |
| [2] | 张震, 李清, 徐菁, 陈凯园, 张春芝, 祝光涛. 马铃薯线粒体靶向表达载体的构建与应用[J]. 生物技术通报, 2024, 40(5): 66-73. |
| [3] | 钟匀, 林春, 刘正杰, 董陈文华, 毛自朝, 李兴玉. 芦笋皂苷合成相关糖基转移酶基因克隆及原核表达分析[J]. 生物技术通报, 2024, 40(4): 255-263. |
| [4] | 杨艳, 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振, 张建成. ‘红满堂’苹果MbbZIP43基因的克隆与功能研究[J]. 生物技术通报, 2024, 40(2): 146-159. |
| [5] | 朱毅, 柳唐镜, 宫国义, 张洁, 王晋芳, 张海英. 西瓜ClPP2C3克隆及表达分析[J]. 生物技术通报, 2024, 40(1): 243-249. |
| [6] | 谢宏, 周丽莹, 李舒文, 王梦迪, 艾晔, 晁跃辉. 蒺藜苜蓿MtCIM基因结构和功能分析[J]. 生物技术通报, 2024, 40(1): 262-269. |
| [7] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [8] | 刘保财, 陈菁瑛, 张武君, 黄颖桢, 赵云青, 刘剑超, 危智诚. 多花黄精种子微根茎基因表达特征分析[J]. 生物技术通报, 2023, 39(8): 220-233. |
| [9] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [10] | 梅欢, 李玥, 刘可蒙, 刘吉华. 小檗碱桥酶高效原核表达及生物合成l-SLR的研究[J]. 生物技术通报, 2023, 39(7): 277-287. |
| [11] | 滕梦鑫, 徐亚, 何静, 汪奇, 乔飞, 李敬阳, 李新国. 香蕉MaMC6的克隆及原核表达分析[J]. 生物技术通报, 2023, 39(12): 179-186. |
| [12] | 尚怡彤, 闫欢欢, 王丽红, 田学琴, 薛萍红, 罗涛, 胡志宏. 米曲霉磷酸甲羟戊酸激酶功能研究[J]. 生物技术通报, 2023, 39(12): 311-319. |
| [13] | 杨旭妍, 赵爽, 马天意, 白玉, 王玉书. 三个甘蓝WRKY基因的克隆及其对非生物胁迫的表达[J]. 生物技术通报, 2023, 39(11): 261-269. |
| [14] | 郭志浩, 金泽鑫, 刘琦, 高利. 小麦矮腥黑粉菌效应蛋白g11335的生物信息学分析、亚细胞定位及毒性验证[J]. 生物技术通报, 2022, 38(8): 110-117. |
| [15] | 索青青, 吴楠, 杨慧, 李莉, 王锡锋. 水稻咖啡酰辅酶A-O-甲基转移酶基因的原核表达、抗体制备和应用[J]. 生物技术通报, 2022, 38(8): 135-141. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||