








生物技术通报 ›› 2025, Vol. 41 ›› Issue (3): 51-61.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0898
陈海敏1,2( ), 孙菲2, 袁源2, 吴佳雯1,2, 江红2(
), 孙菲2, 袁源2, 吴佳雯1,2, 江红2( ), 周剑1,2(
), 周剑1,2( )
)
收稿日期:2024-09-16
出版日期:2025-03-26
发布日期:2025-03-20
通讯作者:
周剑,男,硕士,研究员,研究方向 :微生物药物学;E-mail: zjian503@163.com作者简介:陈海敏,女,硕士研究生,研究方向 :微生物药物学;E-mail: c363784591@163.com
基金资助:
CHEN Hai-min1,2( ), SUN Fei2, YUAN Yuan2, WU Jia-wen1,2, JIANG Hong2(
), SUN Fei2, YUAN Yuan2, WU Jia-wen1,2, JIANG Hong2( ), ZHOU Jian1,2(
), ZHOU Jian1,2( )
)
Received:2024-09-16
Published:2025-03-26
Online:2025-03-20
摘要:
目的 巴弗洛霉素是由灰褐色链霉菌(Streptomyces griseobrunneus)发酵产生的一类大环内酯化合物,对多种植物真菌具有拮抗作用,其代表性化合物为巴弗洛霉素A1(Baf A1)。为了进一步提升链霉菌(Streptomyces sp.) FIM-B0711合成Baf A1的发酵产量,降低生产成本,以其为出发菌株选育Baf A1高产菌株,并对其发酵工艺进行优化。 方法 采用紫外诱变选育高产突变株,单因素试验、最陡爬坡试验和响应面法进行摇瓶发酵工艺优化。 结果 紫外诱变菌株UV-21的Baf A1产量达到397.99 mg/L,较原始菌株提高了23.60%。单因素试验表明,该菌株发酵的最佳碳源、氮源和无机盐分别为麦芽糊精、大豆蛋白胨和碳酸钙,最佳培养条件为pH 7.0、接种量3%(体积分数)、装液量30 mL/250 mL、发酵培养时间96 h。最陡爬坡试验表明,在麦芽糊精45 g/L、大豆蛋白胨15 g/L、碳酸钙0.5 g/L时相对效价达到最高,为147.18%。响应面优化试验表明,该菌株的最佳发酵工艺:麦芽糊精45.98 g/L、大豆蛋白胨14.96 g/L、碳酸钙0.48 g/L、缬氨酸3 g/L,初始pH 7.0、接种量3%(体积分数)、装液量30 mL/250 mL、发酵培养时间96 h。优化后高产菌株UV-21摇瓶发酵产量为627.58 mg/L,较初始工艺提高了57.69%。 结论 基于紫外诱变及培养基的响应面优化试验获得1株Baf A1产量为627.58 mg/L的高产菌株,较原始菌株FIM-B0711产量提高了57.69%。
陈海敏, 孙菲, 袁源, 吴佳雯, 江红, 周剑. 紫外诱变选育巴弗洛霉素A1高产菌株及其培养基优化[J]. 生物技术通报, 2025, 41(3): 51-61.
CHEN Hai-min, SUN Fei, YUAN Yuan, WU Jia-wen, JIANG Hong, ZHOU Jian. The High-yield Bafilomycin A1 Strain Obtained from UV Mutagenesis and Its Medium Optimization[J]. Biotechnology Bulletin, 2025, 41(3): 51-61.
水平 Level | A:麦芽糊精 Maltodextrin/(g·L-1) | B:大豆蛋白胨 Soybean peptone/(g·L-1) | C:碳酸钙 CaCO3/(g·L-1) |
|---|---|---|---|
| -1 | 35 | 10 | 0.3 |
| 0 | 45 | 15 | 0.5 |
| 1 | 55 | 20 | 0.7 |
表1 Box-Behnken试验因素与水平
Table 1 Box-Behnken test factors and levels
水平 Level | A:麦芽糊精 Maltodextrin/(g·L-1) | B:大豆蛋白胨 Soybean peptone/(g·L-1) | C:碳酸钙 CaCO3/(g·L-1) |
|---|---|---|---|
| -1 | 35 | 10 | 0.3 |
| 0 | 45 | 15 | 0.5 |
| 1 | 55 | 20 | 0.7 |
菌株编号 Strain No. | 菌浓 PMV/% | 相对效价 Relative titer/% |
|---|---|---|
| FIM-B0711 | 21.02 | 100.00 |
| UV-21 | 29.62 | 123.60 |
| UV-34 | 23.78 | 120.19 |
表2 UV诱变菌株摇瓶复筛结果
Table 2 Shake flask re-screening results with UV mutagenic strains
菌株编号 Strain No. | 菌浓 PMV/% | 相对效价 Relative titer/% |
|---|---|---|
| FIM-B0711 | 21.02 | 100.00 |
| UV-21 | 29.62 | 123.60 |
| UV-34 | 23.78 | 120.19 |
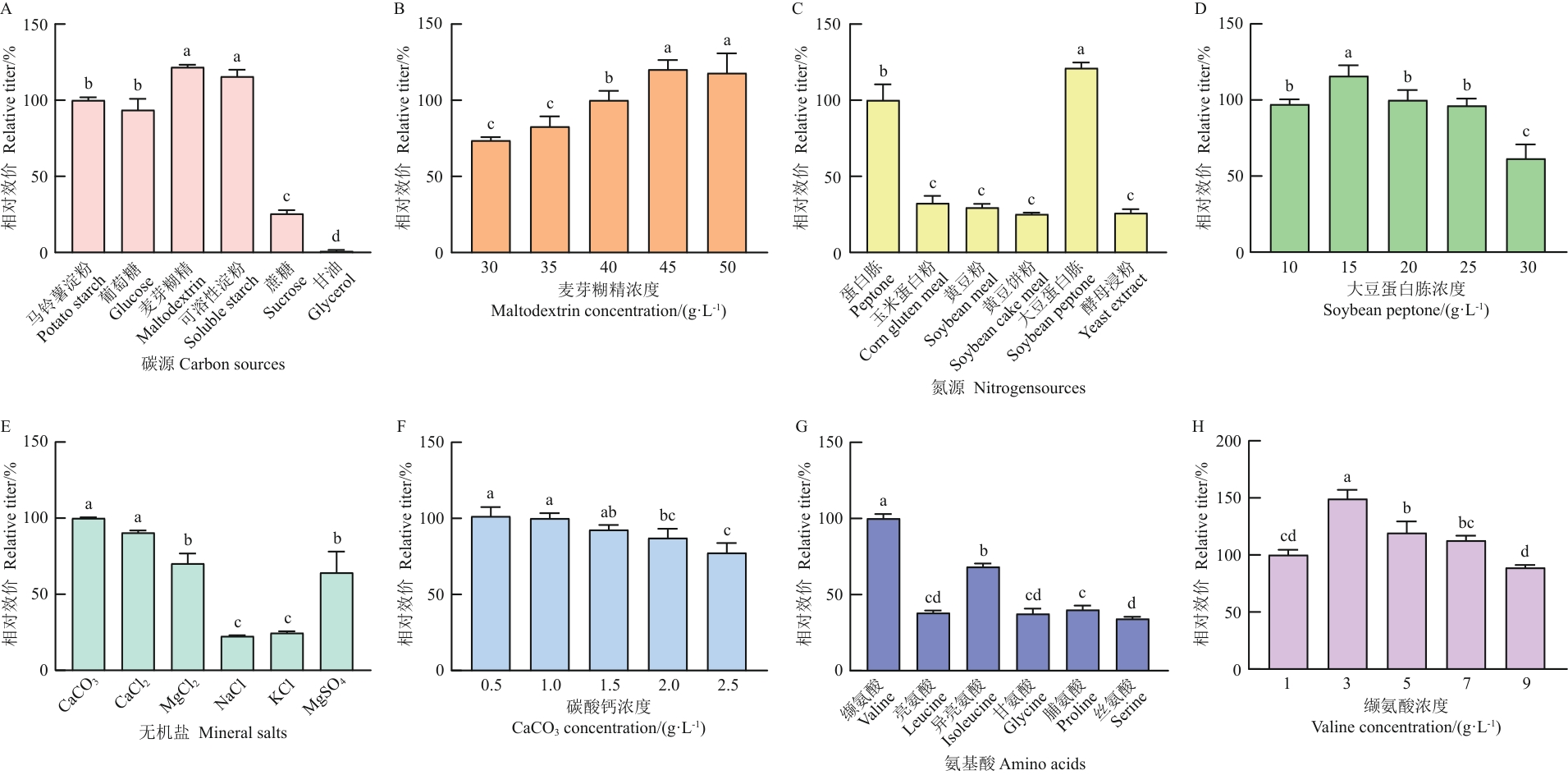
图5 不同培养基组分和浓度对Baf A1相对效价的影响不同小写字母表示差异显著(P<0.05),下同
Fig. 5 Effects of different media components and concentrations on the relative titers of Baf A1Different lowercase letters indicate significant difference at 0.05 level. The same below
分组 Group | 麦芽糊精 Maltodextrin/(g·L-1) | 大豆蛋白胨 Soybean peptone/(g·L-1) | 碳酸钙 CaCO3/(g·L-1) | 相对效价 Relative titer/% |
|---|---|---|---|---|
| 1 | 25 | 5 | 0.1 | 93.79 |
| 2 | 35 | 10 | 0.3 | 131.41 |
| 3 | 45 | 15 | 0.5 | 147.18 |
| 4 | 55 | 20 | 0.7 | 122.96 |
| 5 | 65 | 25 | 0.9 | 119.62 |
表3 最陡爬坡试验设计及结果
Table 3 Design and results of the steepest ascent test
分组 Group | 麦芽糊精 Maltodextrin/(g·L-1) | 大豆蛋白胨 Soybean peptone/(g·L-1) | 碳酸钙 CaCO3/(g·L-1) | 相对效价 Relative titer/% |
|---|---|---|---|---|
| 1 | 25 | 5 | 0.1 | 93.79 |
| 2 | 35 | 10 | 0.3 | 131.41 |
| 3 | 45 | 15 | 0.5 | 147.18 |
| 4 | 55 | 20 | 0.7 | 122.96 |
| 5 | 65 | 25 | 0.9 | 119.62 |
分组 Group | A:麦芽糊精 Maltodextrin/(g·L-1) | B:大豆蛋白胨 Soybean peptone/(g·L-1) | C:碳酸钙 CaCO3/(g·L-1) | Y:相对效价 Relative titer/% |
|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 96.78 |
| 2 | 0 | 0 | 0 | 154.74 |
| 3 | -1 | -1 | 0 | 77.81 |
| 4 | 1 | 0 | -1 | 97.22 |
| 5 | 0 | 0 | 0 | 156.93 |
| 6 | 0 | 0 | 0 | 153.96 |
| 7 | 0 | 1 | 1 | 63.76 |
| 8 | 0 | 0 | 0 | 162.08 |
| 9 | -1 | 1 | 0 | 71.85 |
| 10 | 1 | -1 | 0 | 94.26 |
| 11 | 1 | 0 | 1 | 88.37 |
| 12 | -1 | 0 | -1 | 91.74 |
| 13 | 0 | 1 | -1 | 96.08 |
| 14 | 0 | -1 | -1 | 85.91 |
| 15 | -1 | 0 | 1 | 84.93 |
| 16 | 0 | -1 | 1 | 82.55 |
| 17 | 0 | 0 | 0 | 162.35 |
表4 响应面实验设计及结果
Table 4 Response surface design and test results
分组 Group | A:麦芽糊精 Maltodextrin/(g·L-1) | B:大豆蛋白胨 Soybean peptone/(g·L-1) | C:碳酸钙 CaCO3/(g·L-1) | Y:相对效价 Relative titer/% |
|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 96.78 |
| 2 | 0 | 0 | 0 | 154.74 |
| 3 | -1 | -1 | 0 | 77.81 |
| 4 | 1 | 0 | -1 | 97.22 |
| 5 | 0 | 0 | 0 | 156.93 |
| 6 | 0 | 0 | 0 | 153.96 |
| 7 | 0 | 1 | 1 | 63.76 |
| 8 | 0 | 0 | 0 | 162.08 |
| 9 | -1 | 1 | 0 | 71.85 |
| 10 | 1 | -1 | 0 | 94.26 |
| 11 | 1 | 0 | 1 | 88.37 |
| 12 | -1 | 0 | -1 | 91.74 |
| 13 | 0 | 1 | -1 | 96.08 |
| 14 | 0 | -1 | -1 | 85.91 |
| 15 | -1 | 0 | 1 | 84.93 |
| 16 | 0 | -1 | 1 | 82.55 |
| 17 | 0 | 0 | 0 | 162.35 |
来源 Source | 平方和 Sum of squares | 自由度 Df | 均方 Mean square | F | P |
|---|---|---|---|---|---|
| 模型 Model | 19 374.19 | 9 | 2 152.69 | 60.56 | <0.000 1 |
| A-麦芽糊精 | 316.26 | 1 | 316.26 | 8.90 | 0.020 4 |
| B-大豆蛋白胨 | 18.18 | 1 | 18.18 | 0.511 5 | 0.497 7 |
| C-碳酸钙 | 329.47 | 1 | 329.47 | 9.27 | 0.018 7 |
| AB | 17.98 | 1 | 17.98 | 0.505 8 | 0.500 0 |
| AC | 1.04 | 1 | 1.04 | 0.029 3 | 0.869 0 |
| BC | 209.67 | 1 | 209.67 | 5.90 | 0.045 5 |
| A² | 4 358.46 | 1 | 4 358.46 | 122.61 | < 0.000 1 |
| B² | 6 962.19 | 1 | 6 962.19 | 195.86 | < 0.000 1 |
| C² | 5 238.82 | 1 | 5 238.82 | 147.38 | < 0.000 1 |
| 失拟 Lack of fit | 185.16 | 3 | 61.72 | 3.88 | 0.111 7 |
| 残差误差 Residual error | 248.82 | 7 | 35.55 | R2=0.987 3 | |
| 纯误差 Pure error | 63.66 | 4 | 15.92 | Adj R2=0.971 0 | |
| 合计 Total | 19 623.01 | 16 |
表5 回归方程方差分析和显著性误差
Table 5 Variance analysis and significance error of regression equation
来源 Source | 平方和 Sum of squares | 自由度 Df | 均方 Mean square | F | P |
|---|---|---|---|---|---|
| 模型 Model | 19 374.19 | 9 | 2 152.69 | 60.56 | <0.000 1 |
| A-麦芽糊精 | 316.26 | 1 | 316.26 | 8.90 | 0.020 4 |
| B-大豆蛋白胨 | 18.18 | 1 | 18.18 | 0.511 5 | 0.497 7 |
| C-碳酸钙 | 329.47 | 1 | 329.47 | 9.27 | 0.018 7 |
| AB | 17.98 | 1 | 17.98 | 0.505 8 | 0.500 0 |
| AC | 1.04 | 1 | 1.04 | 0.029 3 | 0.869 0 |
| BC | 209.67 | 1 | 209.67 | 5.90 | 0.045 5 |
| A² | 4 358.46 | 1 | 4 358.46 | 122.61 | < 0.000 1 |
| B² | 6 962.19 | 1 | 6 962.19 | 195.86 | < 0.000 1 |
| C² | 5 238.82 | 1 | 5 238.82 | 147.38 | < 0.000 1 |
| 失拟 Lack of fit | 185.16 | 3 | 61.72 | 3.88 | 0.111 7 |
| 残差误差 Residual error | 248.82 | 7 | 35.55 | R2=0.987 3 | |
| 纯误差 Pure error | 63.66 | 4 | 15.92 | Adj R2=0.971 0 | |
| 合计 Total | 19 623.01 | 16 |
| 1 | Intra B, Euanorasetr J, Nihira T, et al. Characterization of a gamma-butyrolactone synthetase gene homologue (stcA) involved in bafilomycin production and aerial mycelium formation in Streptomyces sp. SBI034 [J]. Appl Microbiol Biotechnol, 2016, 100(6): 2749-2760. |
| 2 | Xie X, Lu SS, Pan XY, et al. Antiviral bafilomycins from a feces-inhabiting Streptomyces sp [J]. J Nat Prod, 2021, 84(2): 537-543. |
| 3 | Shacka JJ, Klocke BJ, Roth KA. Autophagy, bafilomycin and cell death: the "a-B-cs" of plecomacrolide-induced neuroprotection [J]. Autophagy, 2006, 2(3): 228-230. |
| 4 | 黄议莹, 李喆, 潘信利, 等. 产体外抗鼻咽癌物质红树林土壤细菌筛选及其活性成分分析 [J]. 广西科学, 2022, 29(5): 846-853. |
| Huang YY, Li Z, Pan XL, et al. Screening of mangrove soil bacteria producing in vitro anti-nasopharyngeal carcinoma substance and analysis of its active components [J]. Guangxi Sci, 2022, 29(5): 846-853. | |
| 5 | 陈锋龙, 陈金龙, 张弋. 巴佛洛霉素A1抑制胶质瘤迁移、侵袭及血管生成拟态的生物机制研究 [J]. 立体定向和功能性神经外科杂志, 2022, 35(2): 75-80. |
| Chen FL, Chen JL, Zhang Y. Biological mechanism of bafilomycin A1 inhibiting glioma migration, invasion and vasculogenic-mimicry [J]. Chin J Stereotact Funct Neurosurg, 2022, 35(2): 75-80. | |
| 6 | 魏秉洁, 张翠玲, 尚超, 等. 巴弗洛霉素A1体外广谱抗冠状病毒作用研究 [J]. 中国病原生物学杂志, 2023, 18(3): 260-264, 270. |
| Wei BJ, Zhang CL, Shang C, et al. Broad-spectrum antiviral effect of bafilomycin A1 on coronavirus [J]. J Pathog Biol, 2023, 18(3): 260-264, 270. | |
| 7 | Xu J, Cheng T, Feng HT, et al. Structure and function of V-ATPases in osteoclasts: potential therapeutic targets for the treatment of osteolysis [J]. Histol Histopathol, 2007, 22(4): 443-454. |
| 8 | 杨巍民, 斯聪聪, 杨星, 等. 海洋放线菌Y-0117农用活性代谢产物的研究 [J]. 化学与生物工程, 2013, 30(1): 24-27. |
| Yang WM, Si CC, Yang X, et al. Study of agro-active metabolites of marine actinomycete Y-0117 [J]. Chem Bioeng, 2013, 30(1): 24-27. | |
| 9 | Kretschmer A, Dorgerloh M, Deeg M, et al. The structures of novel insecticidal macrolides: bafilomycins D and E, and oxohygrolidin [J]. Agricultural and Biological Chemistry, 1985, 49(8): 2509-2511. |
| 10 | Moon SS, Hwang WH, Chung YR, et al. New cytotoxic bafilomycin C1-amide produced by Kitasatospora cheerisanensis [J]. J Antibiot, 2003, 56(10): 856-861. |
| 11 | 魏刚, 苏超, 张道敬, 等. 海洋放线菌Y12-26代谢产物中bafilomycins分离纯化及结构鉴定 [J]. 中国抗生素杂志, 2011, 36(8): 571-575. |
| Wei G, Su C, Zhang DJ, et al. Isolation, purification and structure identification of secondary metabolites produced by marine actinomycete Y12-26 [J]. Chin J Antibiot, 2011, 36(8): 571-575. | |
| 12 | Lee DW, Ng BG, Kim BS. Increased valinomycin production in mutants of Streptomyces sp. M10 defective in bafilomycin biosynthesis and branched-chain α-keto acid dehydrogenase complex expression [J]. J Ind Microbiol Biotechnol, 2015, 42(11): 1507-1517. |
| 13 | 李盛英, 李众, 张伟, 等. 一种巴弗洛霉素高产工程菌及其构建和应用: CN111378610B [P]. 2022-06-14. |
| Li SY, Li Z, Zhang W, et al. Construction and application of a high-yield bafilomycin engineering strain: CN111378610B [P]. 2022-06-14. | |
| 14 | 周剑, 方志锴, 孙菲, 等. 一株链霉菌的鉴定及其产bafilomycin A1的发酵工艺研究 [J]. 食品与发酵工业, 2019, 45(6): 30-35. |
| Zhou J, Fang ZK, Sun F, et al. Identification of a Streptomyces isolate and fermentation process of bafilomycin A1 production [J]. Food Ferment Ind, 2019, 45(6): 30-35. | |
| 15 | 周剑, 孙菲, 方志锴, 等. 小分子前体物对巴弗洛霉素A1生物合成的影响 [J]. 生物技术通报, 2019, 35(6): 125-130. |
| Zhou J, Sun F, Fang ZK, et al. Effects of small molecule precursors on bafilomycin A1 biosynthesis [J]. Biotechnol Bull, 2019, 35(6): 125-130. | |
| 16 | Nisamedtinov I, Kevvai K, Orumets K, et al. Metabolic changes underlying the higher accumulation of glutathione in Saccharomyces cerevisiae mutants [J]. Appl Microbiol Biotechnol, 2011, 89(4): 1029-1037. |
| 17 | 卢承蓉, 叶美芝, 上官文丹, 等. 高产胞外多糖乳酸菌的诱变育种及其益生特性 [J]. 食品与发酵工业, 2020, 46(12): 14-20. |
| Lu CR, Ye MZ, Shangguan WD, et al. Mutation breeding for high-yield exopolysaccharide lactic acid bacteria and evaluation of its probiotic properties [J]. Food Ferment Ind, 2020, 46(12): 14-20. | |
| 18 | Xu L, Yuan N, Liu H, et al. Bafilomycin A1 targets patient-derived CD34+CD19+ leukemia stem cells [J]. Haematologica, 2020, 105(1): e17-e21. |
| 19 | Xu ZJ, Liu RY, Ke HY, et al. ATP6V1D drives hepatocellular carcinoma stemness and progression via both lysosome acidification-dependent and-independent mechanisms [J]. Autophagy, 2024: 1-17. |
| 20 | Sabino C, Basic M, Bender D, et al. Bafilomycin A1 and U18666A efficiently impair ZIKV infection [J]. Viruses, 2019, 11(6): 524. |
| 21 | de Lima Júnior AA, de Sousa EC, de Oliveira THB, et al. Genus Streptomyces: Recent advances for biotechnological purposes [J]. Biotechnol Appl Biochem, 2023, 70(4): 1504-1517. |
| 22 | Lee N, Hwang S, Lee Y, et al. Synthetic biology tools for novel secondary metabolite discovery in Streptomyces [J]. J Microbiol Biotechnol, 2019, 29(5): 667-686. |
| 23 | Yang ZJ, He JQ, Wei X, et al. Exploration and genome mining of natural products from marine Streptomyces [J]. Appl Microbiol Biotechnol, 2020, 104(1): 67-76. |
| 24 | Schwarz J, Hubmann G, Rosenthal K, et al. Triaging of culture conditions for enhanced secondary metabolite diversity from different bacteria [J]. Biomolecules, 2021, 11(2): 193. |
| 25 | 梅柏杨, 李宇诗, 孙国刚, 等. 暹罗芽孢杆菌N2对茄立枯丝核菌发酵培养基及其发酵条件的优化[J/OL].吉林农业大学学报, 2024. . |
| Mei BY, Li YS, Sun GG, et al. Optimization of fermentation medium and fermentation conditions of Bacillus siamensis N2 for Solanicum [J]. J Jilin Agric Univ, 2024. . | |
| 26 | Mondal S, Rai VR. Molecular profiling of endophytic Streptomyces cavourensis MH16 inhabiting Millingtonia hortensis Linn. and influence of different culture media on biosynthesis of antimicrobial metabolites [J]. Naturwissenschaften, 2019, 106(9/10): 51. |
| 27 | Wu K, Ding LJ, Zhu P, et al. Application of the response surface methodology to optimize the fermentation parameters for enhanced docosahexaenoic acid (DHA) production by Thraustochytrium sp. ATCC 26185 [J]. Molecules, 2018, 23(4): 974. |
| 28 | 宋健, 张海剑, 丰硕, 等. 对韭菜迟眼蕈蚊高活性的苏云金芽胞杆菌JQD117发酵培养基及摇瓶发酵条件优化 [J]. 中国生物防治学报, 2022, 38(2): 333-341. |
| Song J, Zhang HJ, Feng S, et al. Optimization of fermentation culture medium and flask fermentation conditions for Bacillus thuringiensis strain JQD117 with high toxicity against Bradysia odoriphaga [J]. Chin J Biol Contr, 2022, 38(2): 333-341. | |
| 29 | Abdel-Mageed HM, Barakat AZ, Bassuiny RI, et al. Biotechnology approach using watermelon rind for optimization of α-amylase enzyme production from Trichoderma virens using response surface methodology under solid-state fermentation [J]. Folia Microbiol, 2022, 67(2): 253-264. |
| 30 | 王大红, 张颖, 郑迎莹, 等. L-缬氨酸对Streptomyces natalensis HW-2合成纳他霉素的影响 [J]. 精细化工, 2019, 36(4): 708-714. |
| Wang DH, Zhang Y, Zheng YY, et al. Effect of L-valine on biosynthesis of natamycin by Streptomyces natalensis HW-2 [J]. Fine Chem, 2019, 36(4): 708-714. |
| [1] | 杨鹭, 袁源, 方志锴, 林如, 江红, 周剑. 一株链霉菌的鉴定及其产格尔德霉素的发酵工艺研究[J]. 生物技术通报, 2024, 40(6): 299-309. |
| [2] | 程亚楠, 张文聪, 周圆, 孙雪, 李玉, 李庆刚. 乳酸乳球菌生产2'-岩藻糖基乳糖的途径构建及发酵培养基优化[J]. 生物技术通报, 2023, 39(9): 84-96. |
| [3] | 车永梅, 刘广超, 郭艳苹, 叶青, 赵方贵, 刘新. 一种耐盐复合菌剂的制备和促生作用研究[J]. 生物技术通报, 2023, 39(11): 217-225. |
| [4] | 唐碧瑶, 付学鹏. 链霉菌Streptomyces sp. FXP04全基因组测序分析[J]. 生物技术通报, 2023, 39(10): 268-280. |
| [5] | 张瑶心, 王亮节, 郑文, 徐汉琴, 郑恋, 钟静. 产几丁质酶的无色杆菌ZWW8的发酵产酶及酶学性质研究[J]. 生物技术通报, 2021, 37(4): 96-106. |
| [6] | 任思羽, 程新宽, 张宇辉, 庄建文, 马龙. 两种新型L-苏氨酸醛缩酶的鉴定及活性检测方法[J]. 生物技术通报, 2021, 37(3): 233-240. |
| [7] | 高振峰, 赵佳. 微白黄链霉菌G-1发酵液抗真菌特性研究和发酵条件优化[J]. 生物技术通报, 2021, 37(3): 53-64. |
| [8] | 吴蓉, 曹佳睿, 曹君, 刘飞翔, 杨猛, 苏二正. 南极假丝酵母脂肪酶B基因在大肠杆菌中的表达和发酵优化[J]. 生物技术通报, 2021, 37(2): 138-148. |
| [9] | 张美君, 吴庆, 尹翠, 王妮, 马晓庆, 马晓霞, 曹云娥. 尖镰孢黄瓜专化型枯萎病菌拮抗菌的筛选、鉴定及培养条件优化[J]. 生物技术通报, 2020, 36(9): 125-136. |
| [10] | 李静舒, 赵佳. 生防细菌ML-3抗菌蛋白发酵条件的优化及其防治应用[J]. 生物技术通报, 2020, 36(6): 83-92. |
| [11] | 宋道平, 康妮, 张佩佩, 宗工理, 王世立, 张堃钰, 曹广祥, 付加芳. 转录水平分析转速对克拉维酸发酵产量的影响机制[J]. 生物技术通报, 2020, 36(6): 150-156. |
| [12] | 田文佳, 窦桂铭, 王莎, 孙靖雅, 马玉超. 利用CRISPR/Cas9系统建立内生链霉菌SAT1的基因簇敲除体系[J]. 生物技术通报, 2019, 35(6): 1-8. |
| [13] | 周剑, 孙菲, 方志锴, 江红. 小分子前体物对巴弗洛霉素A1生物合成的影响[J]. 生物技术通报, 2019, 35(6): 125-130. |
| [14] | 杨勇, 章帅文, 张勇, 刘群, 李昆太. 黄麻链霉菌AUH-1发酵液的抗菌活性及其稳定性研究[J]. 生物技术通报, 2019, 35(2): 80-84. |
| [15] | 周恒, 姜芸, 许叶祥, 钱生辉, 缪莉. 一株海洋微小链霉菌群体感应抑制活性及培养条件的研究[J]. 生物技术通报, 2019, 35(10): 137-143. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||