








生物技术通报 ›› 2025, Vol. 41 ›› Issue (12): 254-266.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0469
曾梁秦1,2( ), 董陈文华1, 林春1, 刘正杰1, 毛自朝1(
), 董陈文华1, 林春1, 刘正杰1, 毛自朝1( )
)
收稿日期:2025-05-08
出版日期:2025-12-26
发布日期:2026-01-06
通讯作者:
毛自朝,男,博士,教授,研究方向 :植物基因功能;E-mail: zmao@ynau.edu.cn作者简介:曾梁秦,女,博士,副教授,研究方向 :植物基因功能;E-mail: zengliangqin2007@163.com
基金资助:
ZENG Liang-qin1,2( ), DONG Chen-wen-hua1, LIN Chun1, LIU Zheng-jie1, MAO Zi-chao1(
), DONG Chen-wen-hua1, LIN Chun1, LIU Zheng-jie1, MAO Zi-chao1( )
)
Received:2025-05-08
Published:2025-12-26
Online:2026-01-06
摘要:
目的 甾醇14α-去甲基化酶(sterol 14α-demethylase, CYP51)是植物甾醇生物合成途径中的关键酶,在植物应对非生物胁迫中发挥重要作用。从天门冬属全基因组范围内鉴定CYP51基因家族成员,分析其分子特征及验证大理天门冬AtaCYP51G2基因在非生物胁迫的响应功能,为理解天门冬属植物的抗逆分子机制提供依据。 方法 利用生物信息学方法,鉴定了天门冬属大理天门冬、芦笋和文竹的CYP51基因家族成员,分析其理化性质、染色体定位、基因复制、系统进化、基因结构和启动子顺式作用元件等。通过构建AtaCYP51G2转基因拟南芥株系,在干旱、盐和渗透胁迫条件下,对其进行表型观察和生理生化指标测定。利用实时荧光定量PCR检测转基因株系中基因的表达水平及胁迫处理下AtaCYP51G2的表达变化。 结果 共鉴定出天门冬属植物4个CYP51基因家族成员。启动子分析显示存在激素和胁迫响应元件,在AtaCYP51G2中富集。亚细胞定位实验表明AtaCYP51G2定位于内膜系统。过表达AtaCYP51G2增强了转基因拟南芥对干旱和渗透胁迫的耐受性,对盐胁迫下的氧化损伤也有一定的缓解作用。 结论 天门冬属CYP51基因家族成员数量少,在基因结构和蛋白序列上高度保守。过表达AtaCYP51G2增强了转基因拟南芥对干旱、盐和渗透胁迫的耐受性,为改良天门冬属植物的抗逆性提供了理论基础和潜在基因资源。
曾梁秦, 董陈文华, 林春, 刘正杰, 毛自朝. 天门冬属CYP51基因家族鉴定及在非生物胁迫中的响应[J]. 生物技术通报, 2025, 41(12): 254-266.
ZENG Liang-qin, DONG Chen-wen-hua, LIN Chun, LIU Zheng-jie, MAO Zi-chao. Identification of the AsparagusCYP51 Gene Family and the Response to Abiotic Stress[J]. Biotechnology Bulletin, 2025, 41(12): 254-266.
| 引物编号 Primer number | 引物序列 Primer sequence(5'-3') | 目的 Purpose |
|---|---|---|
| AtaCYP51G2-1F | ATGGATTTAACAGAGAACAAGTTCTTG | 基因克隆 |
| AtaCYP51G2-1R | TTATTCAACCGAAAGCTTCCTCC | 基因克隆 |
| AtaCYP51G2-2F | TGGTCGACGTACTAGATGGATTTAACAGAGAACAAGTTCTTGAGCA | 载体构建 |
| AtaCYP51G2-2R | GCTCACCATCACTAGTTCAACCGAAAGCTTCCTCCTCTTGT | 载体构建 |
| AtaCYP51G2-qF | TCCCGTAAATGCTCAGGTCG | RT-qPCR |
| AtaCYP51G2-qR | GTTGAGGTGATCGAGCTGGT | RT-qPCR |
| ACTIN-qF | TGACTACGAGCAGGAGATGGAA | RT-qPCR |
| ACTIN-qR | AAACGAGGGCTGGAACAAGA | RT-qPCR |
表1 本研究所用引物
Table 1 Primers used in this study
| 引物编号 Primer number | 引物序列 Primer sequence(5'-3') | 目的 Purpose |
|---|---|---|
| AtaCYP51G2-1F | ATGGATTTAACAGAGAACAAGTTCTTG | 基因克隆 |
| AtaCYP51G2-1R | TTATTCAACCGAAAGCTTCCTCC | 基因克隆 |
| AtaCYP51G2-2F | TGGTCGACGTACTAGATGGATTTAACAGAGAACAAGTTCTTGAGCA | 载体构建 |
| AtaCYP51G2-2R | GCTCACCATCACTAGTTCAACCGAAAGCTTCCTCCTCTTGT | 载体构建 |
| AtaCYP51G2-qF | TCCCGTAAATGCTCAGGTCG | RT-qPCR |
| AtaCYP51G2-qR | GTTGAGGTGATCGAGCTGGT | RT-qPCR |
| ACTIN-qF | TGACTACGAGCAGGAGATGGAA | RT-qPCR |
| ACTIN-qR | AAACGAGGGCTGGAACAAGA | RT-qPCR |
物种 Species | 基因名称 Gene name | 基因编号 Gene ID | 蛋白长度 Protein length (aa) | 分子量 Molecular weight (kD) | 等电点 Isoelectric point | 脂肪指数 Aliphatic index | 平均亲水性 Grand average of hydropathicity | 不稳定指数 Instability index | 亚细胞定位预测 Predict subcellular localization |
|---|---|---|---|---|---|---|---|---|---|
大理天门冬 A. taliensis | AtaCYP51G1 | Ata0G001960.1 | 488 | 55.4 | 8.7 | 93.3 | -0.116 | 35.7 | 质膜 Plasma membrane |
| AtaCYP51G2 | Ata09G008170.1 | 488 | 55.4 | 8.69 | 94.1 | -0.101 | 34.05 | 内膜 Inner membrane | |
芦笋 A. officinalis | AoCYP51G1 | AsparagusV1_Unassigned.240 | 510 | 58.0 | 8.94 | 93.84 | -0.106 | 34.85 | 质膜 Plasma membrane |
文竹 A. setaceus | AsCYP51G1 | Ase07G1939-RA | 488 | 55.5 | 8.55 | 91.68 | -0.14 | 36.22 | 质膜 Plasma membrane |
表2 天门冬属植物CYP51基因家族成员的理化性质
Table 2 Physicochemical characteristics of CYP51 gene family in Asparagus species
物种 Species | 基因名称 Gene name | 基因编号 Gene ID | 蛋白长度 Protein length (aa) | 分子量 Molecular weight (kD) | 等电点 Isoelectric point | 脂肪指数 Aliphatic index | 平均亲水性 Grand average of hydropathicity | 不稳定指数 Instability index | 亚细胞定位预测 Predict subcellular localization |
|---|---|---|---|---|---|---|---|---|---|
大理天门冬 A. taliensis | AtaCYP51G1 | Ata0G001960.1 | 488 | 55.4 | 8.7 | 93.3 | -0.116 | 35.7 | 质膜 Plasma membrane |
| AtaCYP51G2 | Ata09G008170.1 | 488 | 55.4 | 8.69 | 94.1 | -0.101 | 34.05 | 内膜 Inner membrane | |
芦笋 A. officinalis | AoCYP51G1 | AsparagusV1_Unassigned.240 | 510 | 58.0 | 8.94 | 93.84 | -0.106 | 34.85 | 质膜 Plasma membrane |
文竹 A. setaceus | AsCYP51G1 | Ase07G1939-RA | 488 | 55.5 | 8.55 | 91.68 | -0.14 | 36.22 | 质膜 Plasma membrane |
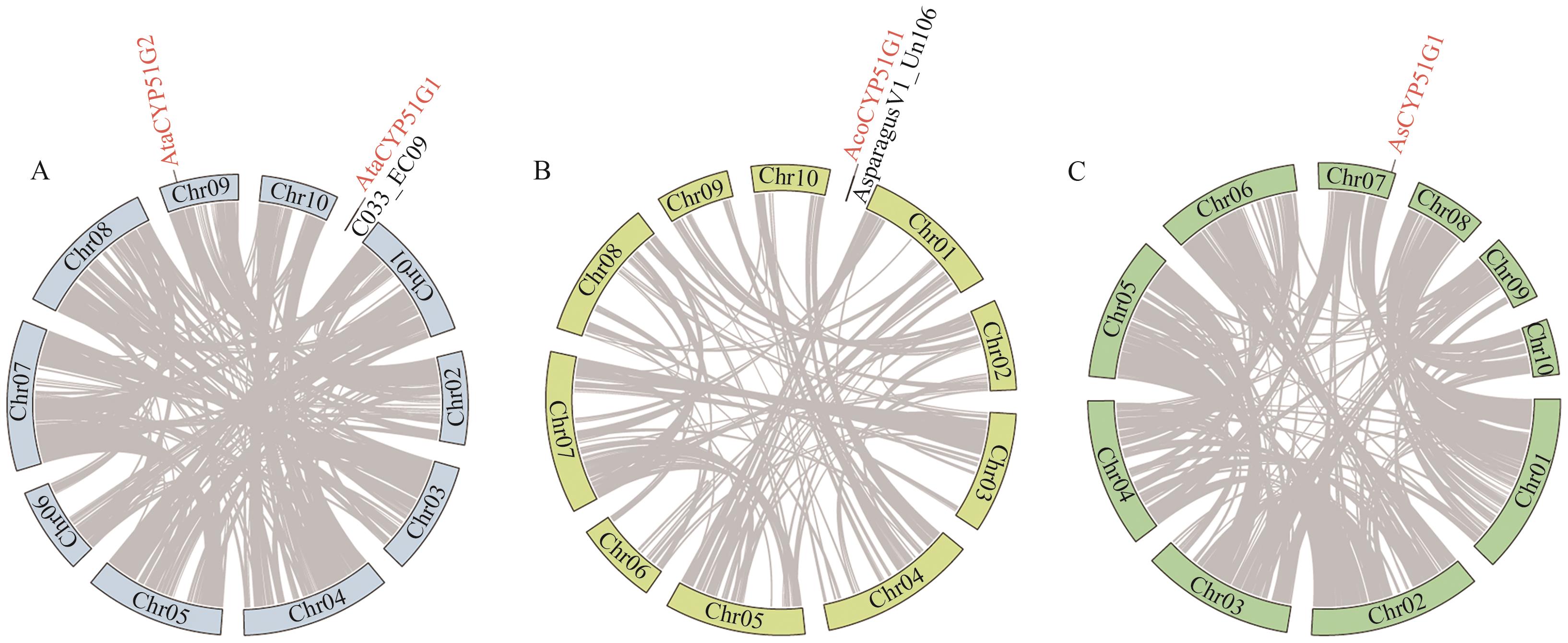
图1 天门冬属CYP51基因家族的染色体定位A-C分别是大理天门冬、芦笋、文竹的CYP51基因家族的染色体定位;标注为红色的是天门冬属CYP51基因;灰色线条是种内共线基因对
Fig. 1 Chromosomal localization of the CYP51 gene family in Asparagus speciesA-C indicate the chromosomal localization of the CYP51 gene family in A. taliensis, A. officinalis, and A. setaceus, respectively. The CYP51 genes in Asparagus species are marked in red. Gray lines indicate intraspecific collinear gene pairs
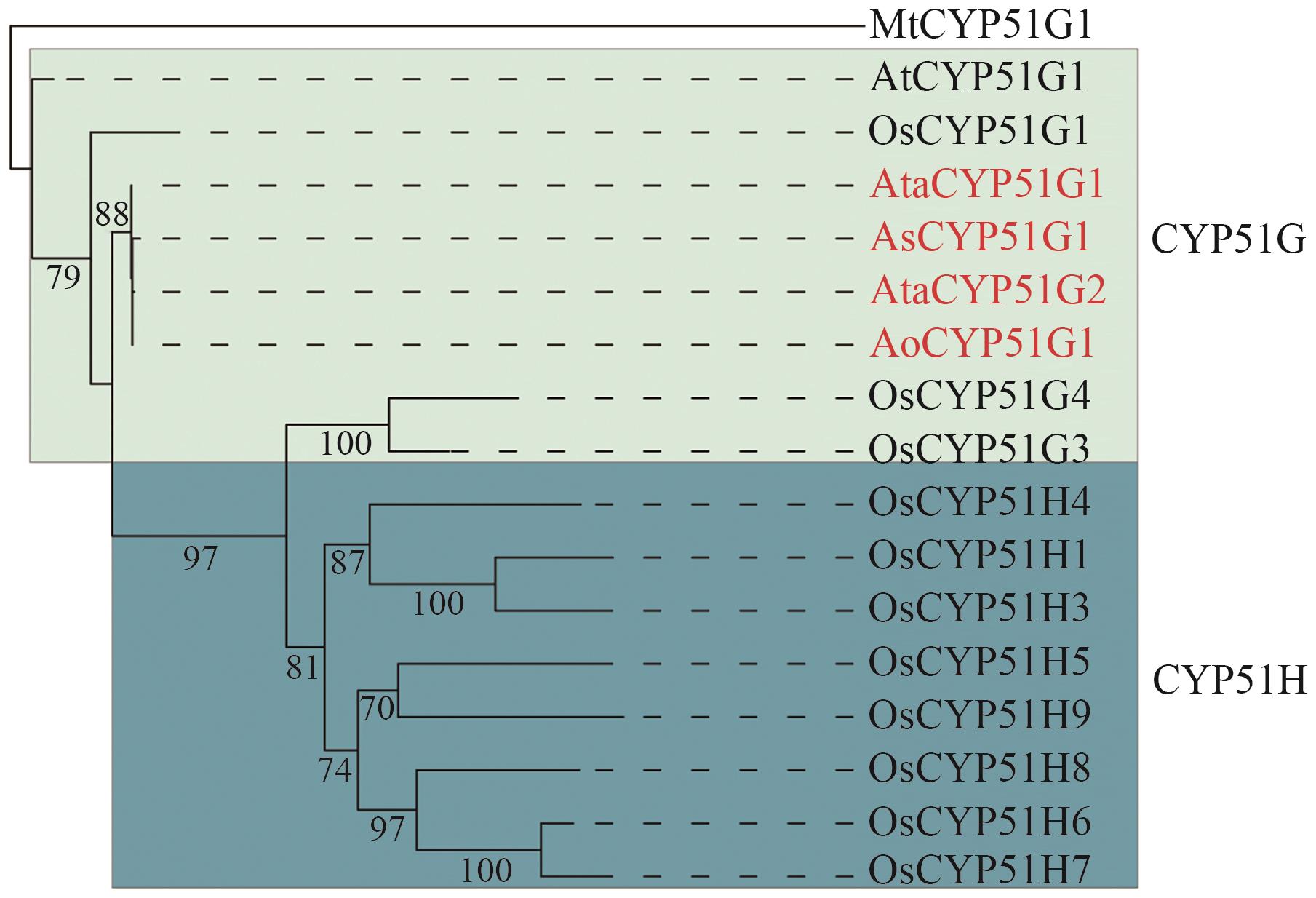
图2 CYP51蛋白系统进化树红色为天门冬属CYP51基因家族成员。Ata:大理天门冬;Ao:芦笋;As:文竹;At:拟南芥;Os:水稻;Mt:结核杆菌
Fig. 2 Phylogenetic tree of CYP51 proteinsThe CYP51 genes in Asparagus species are marked in red. Ata: A. taliensis; Ao: A. officinalis; As: A. setaceus; At: A. thaliana; Os: O. sativa; Mt: Mycobacterium tuberculosis
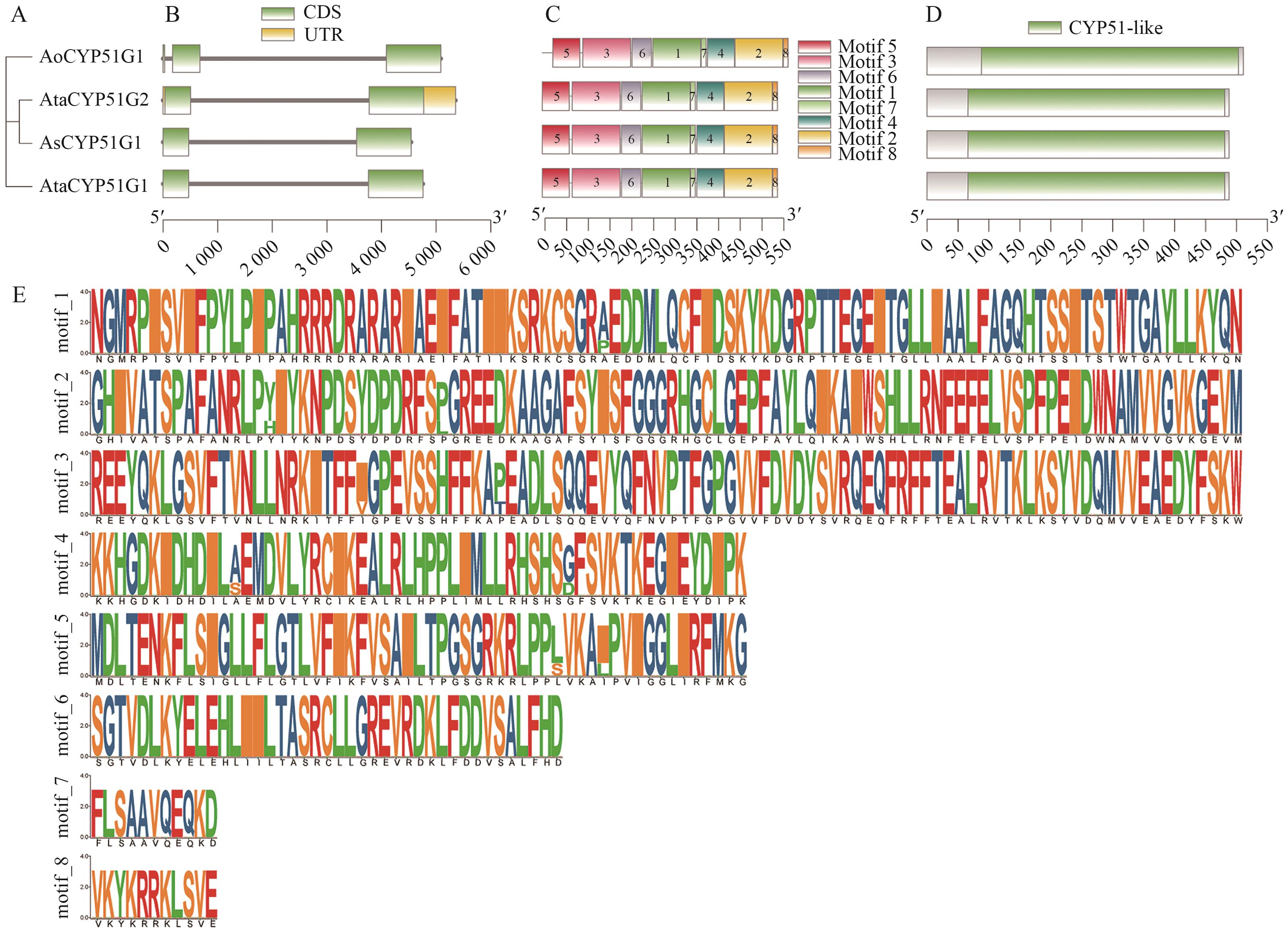
图3 天门冬属植物CYP51基因家族基因结构、保守基序和保守结构域分析A:天门冬属CYP51蛋白系统进化树;B:基因结构;C:保守基序;D:保守结构域;E:保守基序标识
Fig. 3 Gene structure, conserved motifs, and conserved domain analysis of the CYP51 gene family in Asparagus speciesA: Phylogenetic tree of Asparagus CYP51 protein. B: Gene structure. C: Conserved motifs. D: Conserved domains. E: Conserved motif annotations
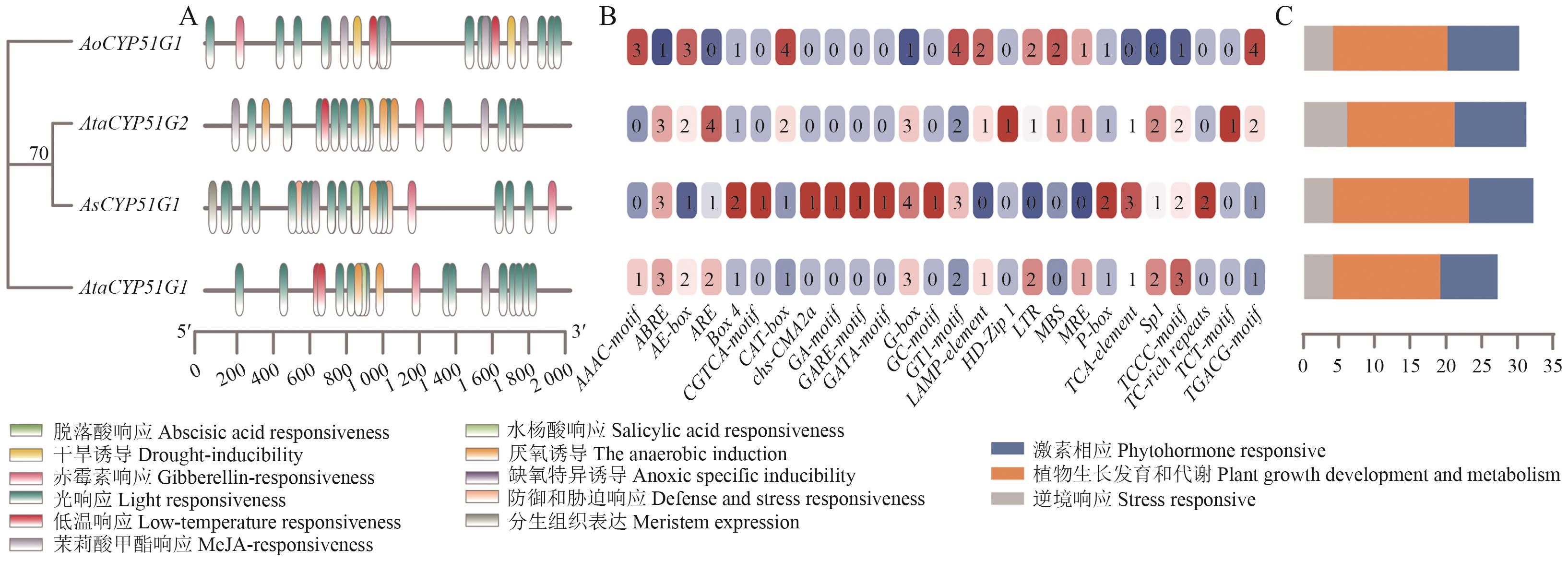
图4 天门冬属植物CYP51基因家族顺式作用元件分析A:顺式作用元件分布;B:顺式作用元件数量;C:顺式作用元件类型及数量
Fig. 4 Cis-acting element analysis of the CYP51 gene family in Asparagus speciesA: Distribution of cis-acting elements. B: Number of cis-acting elements. C: Types and quantities of cis-acting elements
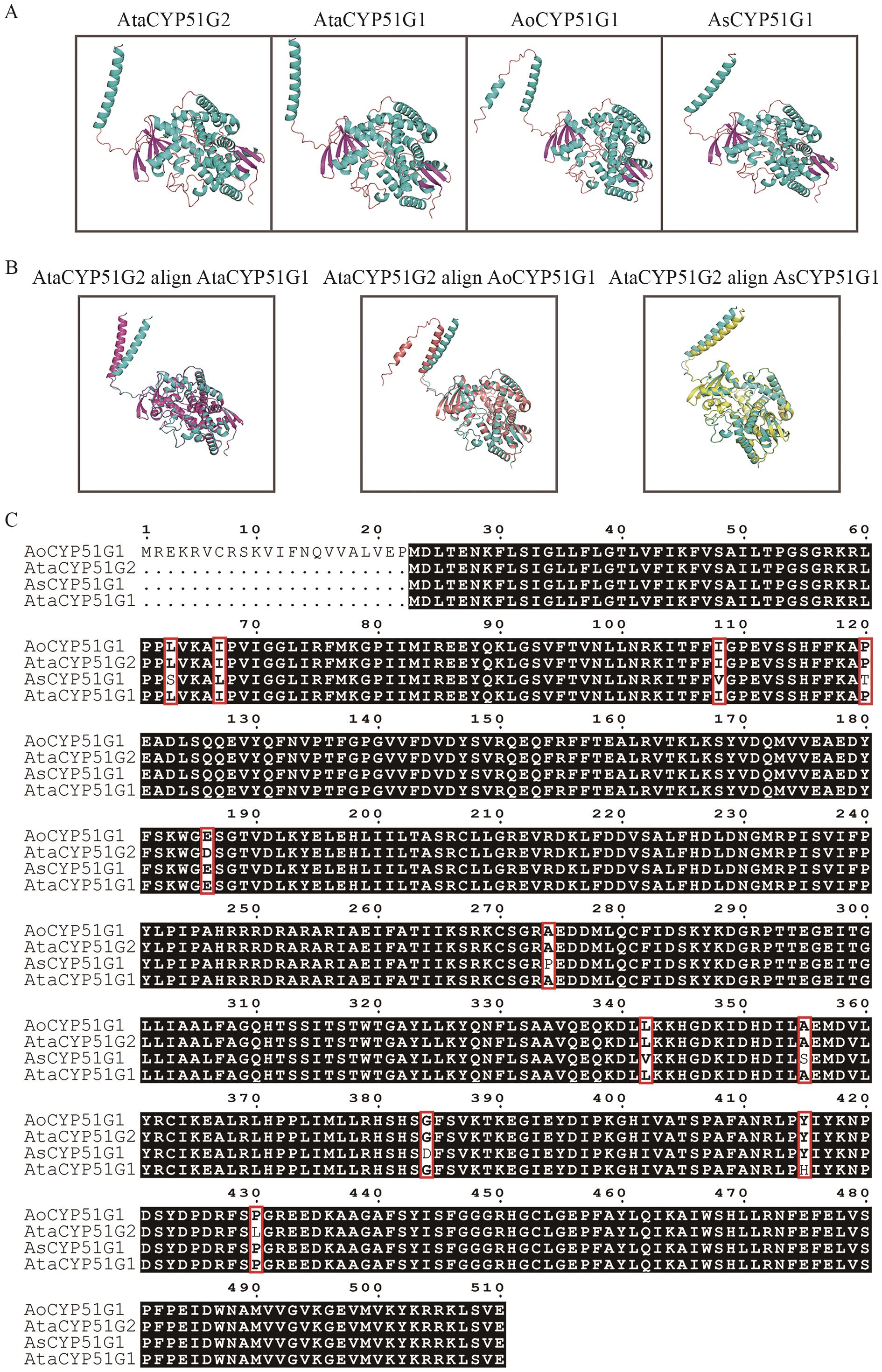
图5 天门冬属植物CYP51蛋白的一级结构多序列比对、三级结构预测及比对A:蛋白三级结构预测;B:蛋白三级结构比对;C:蛋白一级结构多序列比对
Fig. 5 Primary sequence alignment, tertiary structure prediction and alignment of CYP51 proteins in Asparagus speciesA: Predicting tertiary structure of proteins. B: Alignment of tertiary structures. C: Multiple-sequence alignment of primary structures
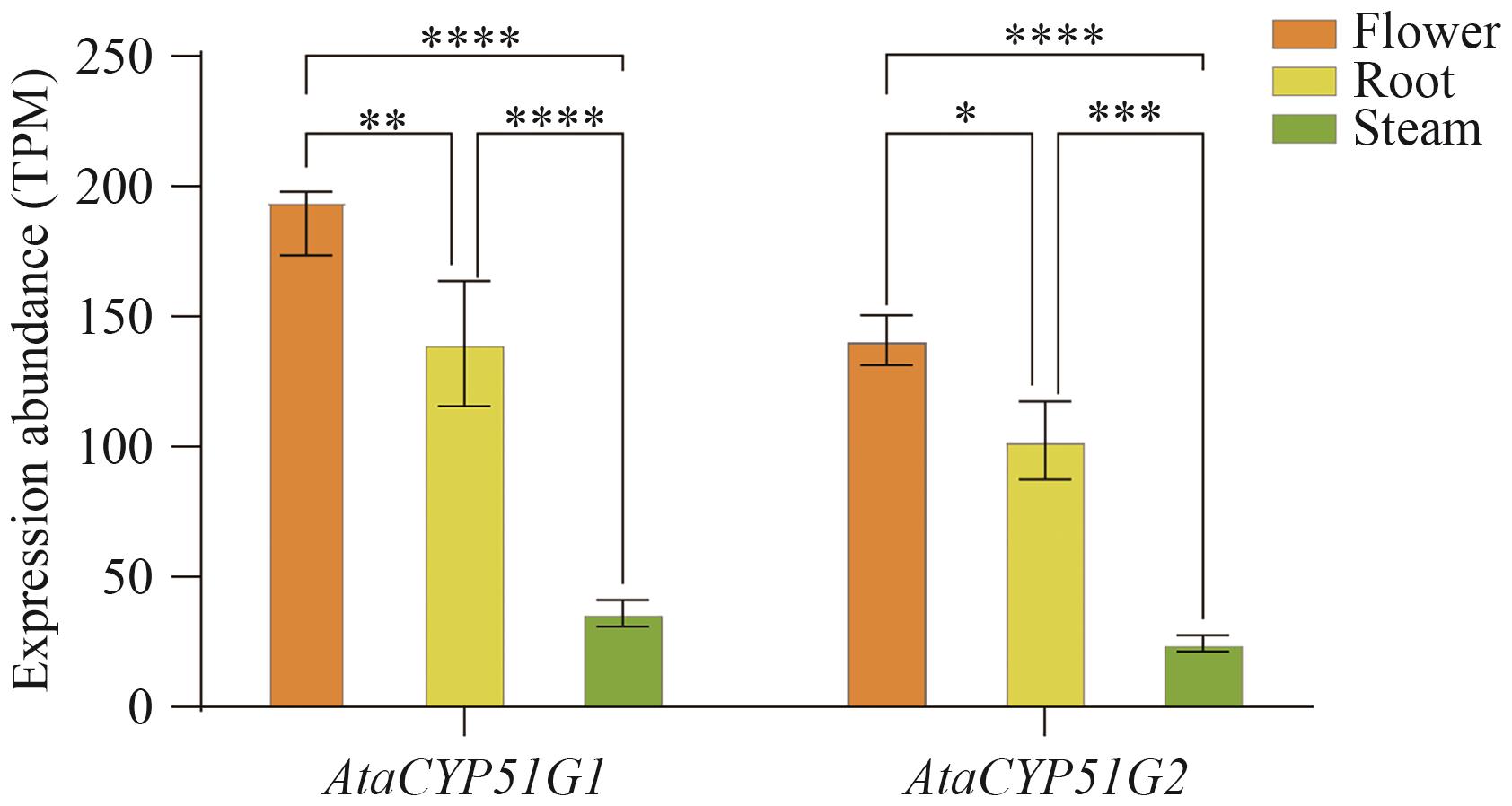
图6 大理天门冬不同组织中CYP51基因的相对表达量*P<0.05, **P<0.01, ***P<0.01, ****P<0.001; the same below
Fig. 6 Gene expressions of CYP51 genes in different tissues of A. taliensis
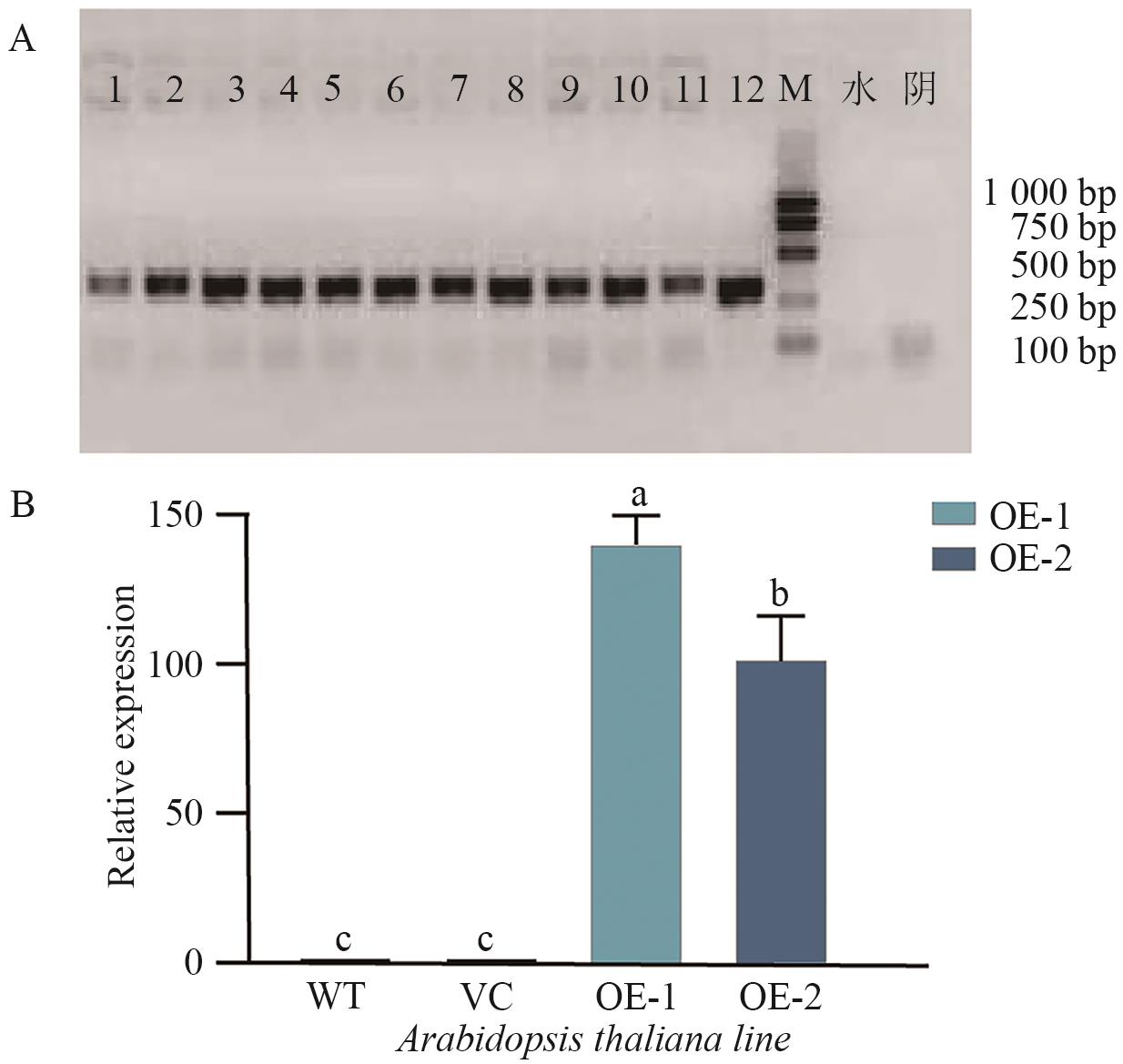
图7 AtaCYP51G2转基因拟南芥株系的鉴定A:T2代株系的基因组PCR检测。泳道1-6:OE-1株系;泳道7-12:OE-2株系。B:AtaCYP51G2基因在野生型(WT)、空载体对照(VC)、转基因株系(OE-1、OE-2)中的相对表达量。数据表示平均值±标准差(n=3),不同字母表示显著差异(P<0.05)
Fig. 7 Identification of AtaCYP51G2 transgenic Arabidopsis linesA: Genomic PCR analysis of T2 plants. Lane 1-6: line OE-1; lane 7-12: line OE-2. B: Relative expression of AtaCYP51G2 in wild type (WT), vector control (VC), and transgenic lines (OE-1 and OE-2), determined by qRT-PCR. Data are presented as mean±SD (n=3). Different letters indicate statistically significant differences (P<0.05)
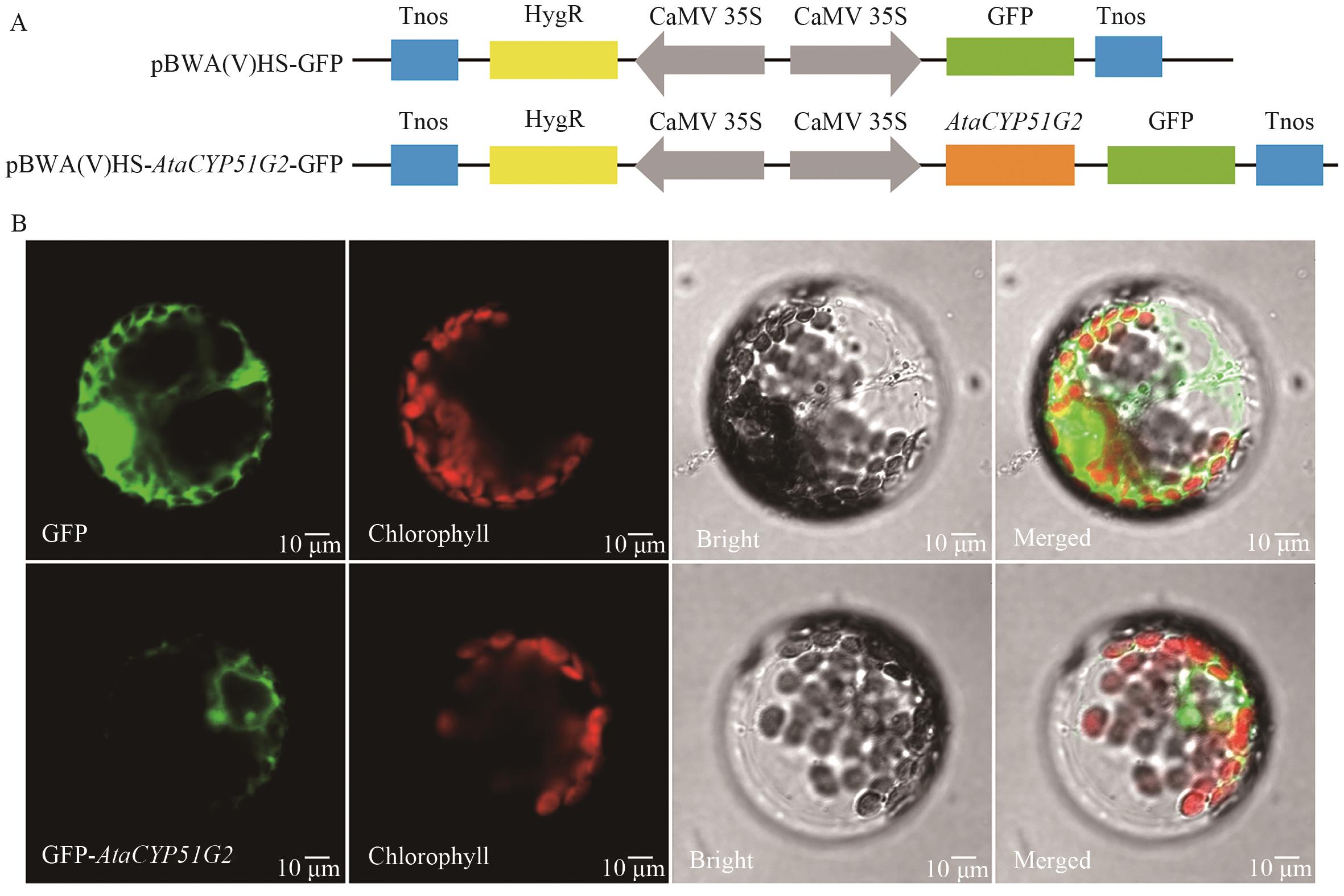
图8 AtaCYP51G2蛋白亚细胞定位A:表达载体构建;B:在烟草原生质体中的亚细胞定位
Fig. 8 Subcellular localization of AtaCYP51G2 proteinA: Construction of the expression vector. B: Subcellular localization in tobacco protoplasts
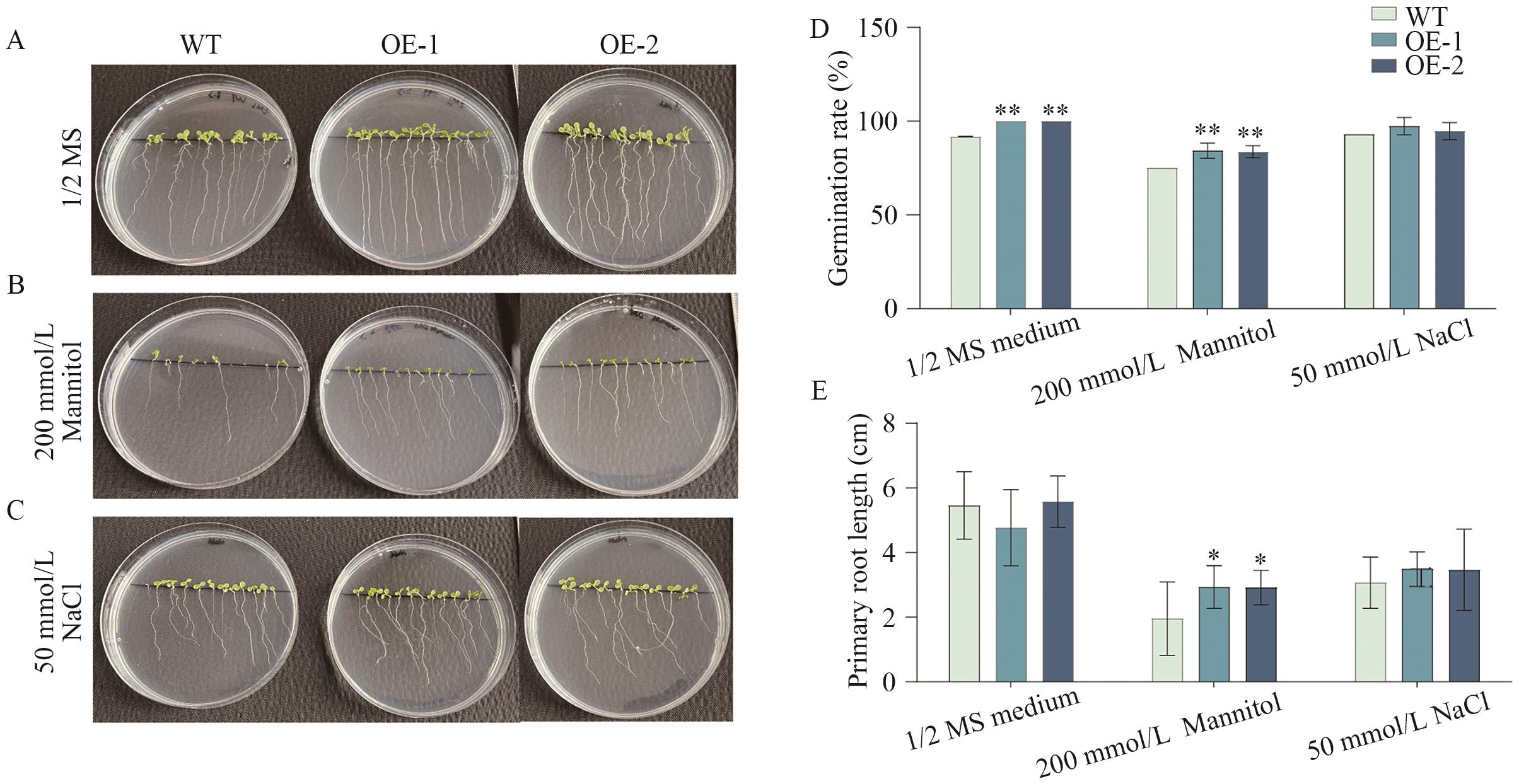
图9 盐胁迫和渗透胁下转基因拟南芥种子萌发和根长的测定A-C:固体培养基中拟南芥表型观察;D:拟南芥种子萌发率测定;E:拟南芥主根长测定
Fig. 9 Evaluation of seed germination and root length in transgenic Arabidopsis under salt and osmotic stressA-C: Phenotypic observation of Arabidopsis grown on solid medium. D: Measurement of Arabidopsis seed germination rate. E: Measurement of Arabidopsis primary root length
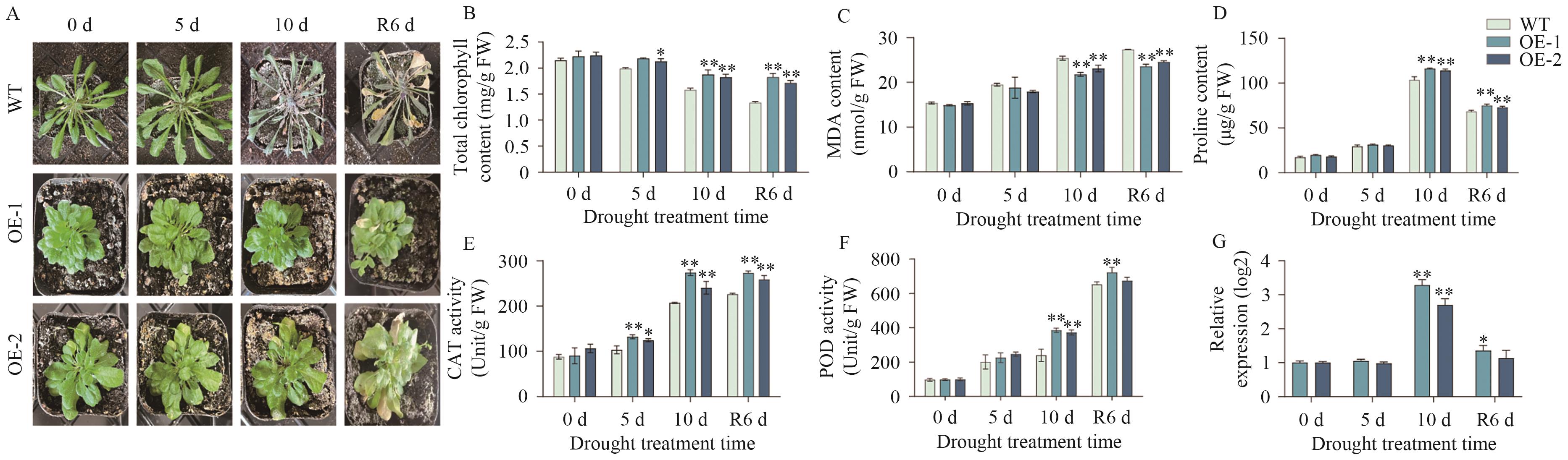
图10 过表达AtaCYP51G2拟南芥在干旱胁迫下的表型及生理生化指标测定A:干旱胁迫下WT及OE拟南芥株系的表型;B-F:分别是干旱胁迫下WT及OE拟南芥的生理生化指标:总叶绿素、丙二醛、脯氨酸含量及过氧化氢酶活性、过氧化物酶活性;下同;G:相对基因表达水平
Fig. 10 Phenotype and physiological and biochemical measurements of AtaCYP51G2-overexpressing Arabidopsis under drought stressA: Phenotype of wild-type (WT) and AtaCYP51G2-overexpressing (OE) Arabidopsis lines under drought stress; B-F: the physiological and biochemical indicators of WT and OE Arabidopsis plants under drought stress, respectively: total chlorophyll content, malondialdehyde content, proline content, catalase activity, and peroxidase activity. The same below. G: Relative gene expressions
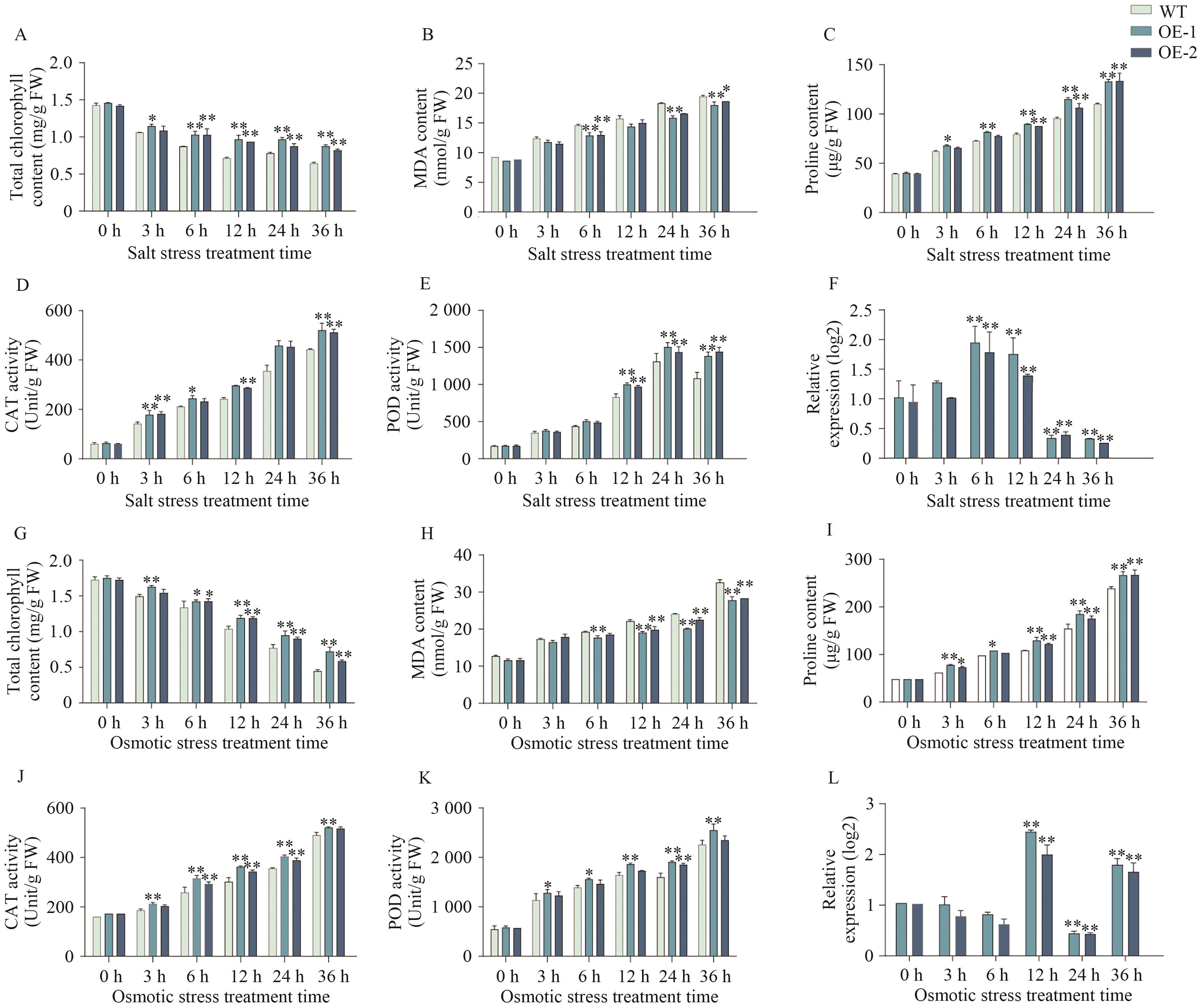
图11 过表达AtaCYP51G2拟南芥在盐胁迫和渗透胁迫下的生理生化指标测定A-F:盐胁迫下过表达AtaCYP51G2的生理生化指标值和基因相对表达量测定值;G-L:渗透胁迫下对应的生理生化指标值和基因相对表达量测定值
Fig. 11 Physiological measurements of AtaCYP51G2-overexpressing Arabidopsis under salt and osmotic stressA-F: Physiological and biochemical parameters and the relative transcript levels of AtaCYP51G2 overexpressing lines under salt stress, whereas panel G-L display the corresponding data under osmotic stress
| [1] | Zhu JK. Salt and drought stress signal transduction in plants [J]. Annu Rev Plant Biol, 2002, 53: 247-273. |
| [2] | Hartmann MA. Plant sterols and the membrane environment [J]. Trends Plant Sci, 1998, 3(5): 170-175. |
| [3] | Lepesheva GI, Waterman MR. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological Kingdoms [J]. Biochim Biophys Acta BBA Gen Subj, 2007, 1770(3): 467-477. |
| [4] | Du YL, Fu XZ, Chu YY, et al. Biosynthesis and the roles of plant sterols in development and stress responses [J]. Int J Mol Sci, 2022, 23(4): 2332. |
| [5] | Jiao ZL, Yin LJ, Zhang QM, et al. The putative obtusifoliol 14α-demethylase OsCYP51H3 affects multiple aspects of rice growth and development [J]. Physiol Plant, 2022, 174(5): e13764. |
| [6] | Xia KF, Ou XJ, Tang HD, et al. Rice microRNA Osa-miR1848 targets the obtusifoliol 14α-demethylase gene OsCYP51G3 and mediates the biosynthesis of phytosterols and brassinosteroids during development and in response to stress [J]. New Phytol, 2015, 208(3): 790-802. |
| [7] | 唐万虎, 张祖新, 邹锡玲, 等. 玉米耐渍功能基因组分析及相关基因Sicyp51的鉴定与克隆 [J]. 中国科学C辑: 生命科学, 2005, 35(1): 29-36. |
| Tang WH, Zhang ZX, Zou XL, et al. Genome analysis of waterlogging tolerance function in maize and identification and cloning of related gene Sicyp51 [J]. Sci China Ser C, 2005, 35(1): 29-36. | |
| [8] | 温晶媛, 李颖, 丁声颂, 等. 中国百合科天门冬属九种药用植物的药理作用筛选 [J]. 上海医科大学学报, 1993, 20(2): 107-111. |
| Wen JY, Li Y, Ding SS, et al. Pharmacological screening of 9 medicinal plants of the genus Asparagus (Liliaceae) in China [J]. Fudan Univ J Med Sci, 1993, 20(2): 107-111. | |
| [9] | Zeng LQ, Brown SE, Wu H, et al. Comprehensive genome-wide analysis of the HMGR gene family of Asparagus taliensis and functional validation of AtaHMGR10 under different abiotic stresses [J]. Front Plant Sci, 2025, 16: 1455592. |
| [10] | Zhang XR, Henriques R, Lin SS, et al. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method [J]. Nat Protoc, 2006, 1(2): 641-646. |
| [11] | Paquette SM, Jensen K, Bak S. A web-based resource for the Arabidopsis P450, cytochromes b5, NADPH-cytochrome P450 reductases, and family 1 glycosyltransferases (http://www. P450. kvl. dk) [J]. Phytochemistry, 2009, 70(17-18): 1940-1947. |
| [12] | Malhotra K, Franke J. Cytochrome P450 monooxygenase-mediated tailoring of triterpenoids and steroids in plants [J]. Beilstein J Org Chem, 2022, 18: 1289-1310. |
| [13] | McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools [J]. Nucleic Acids Res, 2004, 32(Web Server issue): W20-W25. |
| [14] | Chen CJ, Wu Y, Li JW, et al. TBtools-II: a “one for all, all for one” bioinformatics platform for biological big-data mining [J]. Mol Plant, 2023, 16(11): 1733-1742. |
| [15] | Lu SN, Wang JY, Chitsaz F, et al. CDD/SPARCLE: the conserved domain database in 2020 [J]. Nucleic Acids Res, 2020, 48(D1): D265-D268. |
| [16] | Yu CS, Chen YC, Lu CH, et al. Prediction of protein subcellular localization [J]. Proteins, 2006, 64(3): 643-651. |
| [17] | Wang YP, Li JP, Paterson AH. MCScanX-transposed: detecting transposed gene duplications based on multiple colinearity scans [J]. Bioinformatics, 2013, 29(11): 1458-1460. |
| [18] | Inagaki YS, Etherington G, Geisler K, et al. Investigation of the potential for triterpene synthesis in rice through genome mining and metabolic engineering [J]. New Phytol, 2011, 191(2): 432-448. |
| [19] | Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput [J]. Nucleic Acids Res, 2004, 32(5): 1792-1797. |
| [20] | Bailey TL, Johnson J, Grant CE, et al. The MEME suite [J]. Nucleic Acids Res, 2015, 43(w1): W39-W49. |
| [21] | Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server [J]. Nucleic Acids Res, 2014, 42(Web Server issue): W320-W324. |
| [22] | Callaway E. AI protein-prediction tool AlphaFold3 is now more open [J]. Nature, 2024, 635(8039): 531-532. |
| [23] | Abramson J, Adler J, Dunger J, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3 [J]. Nature, 2024, 630(8016): 493-500. |
| [24] | Bowie JU, Lüthy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure [J]. Science, 1991, 253(5016): 164-170. |
| [25] | Lüthy R, Bowie JU, Eisenberg D. Assessment of protein models with three-dimensional profiles [J]. Nature, 1992, 356(6364): 83-85. |
| [26] | Colovos C, Yeates TO. Verification of protein structures: patterns of nonbonded atomic interactions [J]. Protein Sci, 1993, 2(9): 1511-1519. |
| [27] | Pontius J, Richelle J, Wodak SJ. Deviations from standard atomic volumes as a quality measure for protein crystal structures [J]. J Mol Biol, 1996, 264(1): 121-136. |
| [28] | DeLano WL. Pymol: An open-source molecular graphics tool [J]. CCP4 Newsl Protein Crystallogr, 2002, 40(1): 82-92. |
| [29] | Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements [J]. Nat Methods, 2015, 12(4): 357-360. |
| [30] | Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features [J]. Bioinformatics, 2014, 30(7): 923-930. |
| [31] | Zhang W, Li HX, Li QH, et al. Genome-wide identification, comparative analysis and functional roles in flavonoid biosynthesis of cytochrome P450 superfamily in pear (Pyrus spp.) [J]. BMC Genom Data, 2023, 24(1): 58. |
| [32] | 杨娇艳, 廖明军, 杨劭. 甾醇14α-去甲基化酶(CYP51)的研究进展 [J]. 生物工程学报, 2008, 24(10): 1681-1688. |
| Yang JY, Liao MJ, Yang S. Advances in sterol 14α-demethylase (CYP51) [J]. Chin J Biotechnol, 2008, 24(10): 1681-1688. | |
| [33] | Li SF, Wang J, Dong R, et al. Chromosome-level genome assembly, annotation and evolutionary analysis of the ornamental plant Asparagus setaceus [J]. Hortic Res, 2020, 7(1): 48. |
| [34] | Shang LG, He WC, Wang TY, et al. A complete assembly of the rice Nipponbare reference genome [J]. Mol Plant, 2023, 16(8): 1232-1236. |
| [35] | Kim HB, Schaller H, Goh CH, et al. Arabidopsis cyp51 mutant shows postembryonic seedling lethality associated with lack of membrane integrity [J]. Plant Physiol, 2005, 138(4): 2033-2047. |
| [1] | 程婷婷, 刘俊, 王利丽, 练从龙, 魏文君, 郭辉, 吴尧琳, 杨晶凡, 兰金旭, 陈随清. 杜仲查尔酮异构酶基因家族全基因组鉴定及其表达模式分析[J]. 生物技术通报, 2025, 41(9): 242-255. |
| [2] | 李珊, 马登辉, 马红义, 姚文孔, 尹晓. 葡萄SKP1基因家族鉴定与表达分析[J]. 生物技术通报, 2025, 41(9): 147-158. |
| [3] | 黄国栋, 邓宇星, 程宏伟, 但焱南, 周会汶, 吴兰花. 大豆ZIP基因家族鉴定及响应铝胁迫的表达分析[J]. 生物技术通报, 2025, 41(9): 71-81. |
| [4] | 化文平, 刘菲, 浩佳欣, 陈尘. 丹参ADH基因家族的鉴定与表达模式分析[J]. 生物技术通报, 2025, 41(8): 211-219. |
| [5] | 腊贵晓, 赵玉龙, 代丹丹, 余永亮, 郭红霞, 史贵霞, 贾慧, 杨铁钢. 红花质膜H+-ATPase基因家族成员鉴定及响应低氮低磷胁迫的表达分析[J]. 生物技术通报, 2025, 41(8): 220-233. |
| [6] | 黄诗宇, 田姗姗, 杨天为, 高曼熔, 张尚文. 赤苍藤WRI1基因家族的全基因组鉴定及表达模式分析[J]. 生物技术通报, 2025, 41(8): 242-254. |
| [7] | 程雪, 付颖, 柴晓娇, 王红艳, 邓欣. 谷子LHC基因家族鉴定及非生物胁迫表达分析[J]. 生物技术通报, 2025, 41(8): 102-114. |
| [8] | 任睿斌, 司二静, 万广有, 汪军成, 姚立蓉, 张宏, 马小乐, 李葆春, 王化俊, 孟亚雄. 大麦条纹病菌GH17基因家族的鉴定及表达分析[J]. 生物技术通报, 2025, 41(8): 146-154. |
| [9] | 翟莹, 计俊杰, 陈炯辛, 于海伟, 李珊珊, 赵艳, 马天意. 异源过表达大豆GmNF-YB24提高转基因烟草抗旱性[J]. 生物技术通报, 2025, 41(8): 137-145. |
| [10] | 韩燚, 侯昌林, 唐露, 孙璐, 谢晓东, 梁晨, 陈小强. 大麦HvERECTA基因的克隆及功能分析[J]. 生物技术通报, 2025, 41(7): 106-116. |
| [11] | 李霞, 张泽伟, 刘泽军, 王楠, 郭江波, 辛翠花, 张彤, 简磊. 马铃薯转录因子StMYB96的克隆及功能研究[J]. 生物技术通报, 2025, 41(7): 181-192. |
| [12] | 龚钰涵, 陈兰, 尚方慧子, 郝灵颖, 刘硕谦. 茶树TRB基因家族鉴定及表达模式分析[J]. 生物技术通报, 2025, 41(7): 214-225. |
| [13] | 魏雨佳, 李岩, 康语涵, 弓晓楠, 杜敏, 涂岚, 石鹏, 于子涵, 孙彦, 张昆. 白颖苔草CrMYB4基因的克隆和表达分析[J]. 生物技术通报, 2025, 41(7): 248-260. |
| [14] | 张泽, 杨秀丽, 宁东贤. 花生4CL基因家族鉴定及对干旱与盐胁迫响应分析[J]. 生物技术通报, 2025, 41(7): 117-127. |
| [15] | 张学琼, 潘素君, 李魏, 戴良英. 植物磷酸盐转运蛋白在胁迫响应中的研究进展[J]. 生物技术通报, 2025, 41(7): 28-36. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||