








生物技术通报 ›› 2022, Vol. 38 ›› Issue (10): 54-65.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1384
收稿日期:2021-11-03
出版日期:2022-10-26
发布日期:2022-11-11
作者简介:陈臣,男,博士,副教授,研究方向:食品生物技术、食品风味;E-mail:基金资助:
CHEN Chen( ), HUANG Zhi-yang, YU Hai-yan, YUAN Hai-bin, TIAN Huai-xiang(
), HUANG Zhi-yang, YU Hai-yan, YUAN Hai-bin, TIAN Huai-xiang( )
)
Received:2021-11-03
Published:2022-10-26
Online:2022-11-11
摘要:
原核生物基因表达调控主要发生在转录水平,研究原核生物的转录调控有利于了解其基因表达调节机制。近年来,随着分子生物学及相关技术的突破,转录调控研究技术也不断发展,因此主要综述了原核生物转录调控的技术方法及其新进展,包括凝胶电泳迁移率实验、DNase I足迹技术、染色质免疫共沉淀技术、微量热泳动技术、等温滴定量热法及细菌单杂交系统,以期系统地了解这些方法的优缺点和适用性,帮助研究者更好的利用原核生物转录调控为人类造福。
陈臣, 黄芝阳, 于海燕, 袁海彬, 田怀香. 原核生物转录调控研究技术及进展[J]. 生物技术通报, 2022, 38(10): 54-65.
CHEN Chen, HUANG Zhi-yang, YU Hai-yan, YUAN Hai-bin, TIAN Huai-xiang. Research Technology and Progress in Transcriptional Regulation in Prokaryotes[J]. Biotechnology Bulletin, 2022, 38(10): 54-65.
| 研究技术 Method | 英文名称/缩写 Name/Abbreviation | 优缺点 Advantages/Disadvantages | 适用性 Applicability | 参考文献 Reference | |
|---|---|---|---|---|---|
| 体外方法 | 凝胶电泳迁移率实验 | EMSA | 方法简单、灵敏度高,放射性标记探针安全性低成本高,电泳运行条件下蛋白质-DNA复合体不稳定 | 适用于验证转录因子与假定DNA结合位点直接相互作用及结合位点突变对结合作用的影响 | [ |
| 等温滴定量热法 | ITC | 蛋白质无需固定化或修饰,样品消耗量少,可区分结合常数相近的配体相互作用及比较结构与结合作用的关系,对温度适应范围广但难以解释复杂系统中的相互作用 | 适用于成分简单的超高/超低亲和力相互作用系统及复杂的相互作用,可获得丰富的热力学信息 | [ | |
| DNase I footprinting技术 | DNase I footprinting | 分辨率高、可区分同一DNA片段多个不连续结合位点,但需要较多蛋白质才能产生清晰足迹,易产生超敏位点受到切割 | 适用于未纯化蛋白样品的检测,判断同一片段是否存在多个结合位点获得结合序列及比较各自亲和力 | [ | |
| 微量热泳动技术 | MST | 对相互作用的分子大小或质量无选择性,具有较好的适用性,对结合亲和力准确测定,可检测低至pM级别的结合亲和力,可在溶液环境中进行,无需固定分子避免结合假阳性 | 适用于结合亲和力弱,样品量小,样品所处环境复杂的情况 | [ | |
| 体内方法 | 染色质-免疫共沉淀技术 | ChIP | 接近细胞内真实情况,可研究转录因子对启动子结合的动态过程,但实验重复性不佳,获得良好实验结果对经验依赖性较高,对实验环境的要求严格 | 适用于确定转录因子修饰位置及低丰度转录因子结合分析 | [ |
| 细菌单杂交 | B1H | 细菌转化效率高构建文库质粒容量更大,无需复杂的仪器,转录因子需要能在大肠杆菌中表达,可能存在假阳性和假阴性的情况,结果需要进一步验证 | 适用于未纯化蛋白样品的检测,缺少相应仪器,用分子生物学手段进行筛选,转录因子要求能在大肠杆菌中表达,可发现新的转录因子 | [ | |
表1 原核生物转录调控研究技术总结
Table 1 Summary of research techniques on transcriptional regulation in prokaryotes
| 研究技术 Method | 英文名称/缩写 Name/Abbreviation | 优缺点 Advantages/Disadvantages | 适用性 Applicability | 参考文献 Reference | |
|---|---|---|---|---|---|
| 体外方法 | 凝胶电泳迁移率实验 | EMSA | 方法简单、灵敏度高,放射性标记探针安全性低成本高,电泳运行条件下蛋白质-DNA复合体不稳定 | 适用于验证转录因子与假定DNA结合位点直接相互作用及结合位点突变对结合作用的影响 | [ |
| 等温滴定量热法 | ITC | 蛋白质无需固定化或修饰,样品消耗量少,可区分结合常数相近的配体相互作用及比较结构与结合作用的关系,对温度适应范围广但难以解释复杂系统中的相互作用 | 适用于成分简单的超高/超低亲和力相互作用系统及复杂的相互作用,可获得丰富的热力学信息 | [ | |
| DNase I footprinting技术 | DNase I footprinting | 分辨率高、可区分同一DNA片段多个不连续结合位点,但需要较多蛋白质才能产生清晰足迹,易产生超敏位点受到切割 | 适用于未纯化蛋白样品的检测,判断同一片段是否存在多个结合位点获得结合序列及比较各自亲和力 | [ | |
| 微量热泳动技术 | MST | 对相互作用的分子大小或质量无选择性,具有较好的适用性,对结合亲和力准确测定,可检测低至pM级别的结合亲和力,可在溶液环境中进行,无需固定分子避免结合假阳性 | 适用于结合亲和力弱,样品量小,样品所处环境复杂的情况 | [ | |
| 体内方法 | 染色质-免疫共沉淀技术 | ChIP | 接近细胞内真实情况,可研究转录因子对启动子结合的动态过程,但实验重复性不佳,获得良好实验结果对经验依赖性较高,对实验环境的要求严格 | 适用于确定转录因子修饰位置及低丰度转录因子结合分析 | [ |
| 细菌单杂交 | B1H | 细菌转化效率高构建文库质粒容量更大,无需复杂的仪器,转录因子需要能在大肠杆菌中表达,可能存在假阳性和假阴性的情况,结果需要进一步验证 | 适用于未纯化蛋白样品的检测,缺少相应仪器,用分子生物学手段进行筛选,转录因子要求能在大肠杆菌中表达,可发现新的转录因子 | [ | |
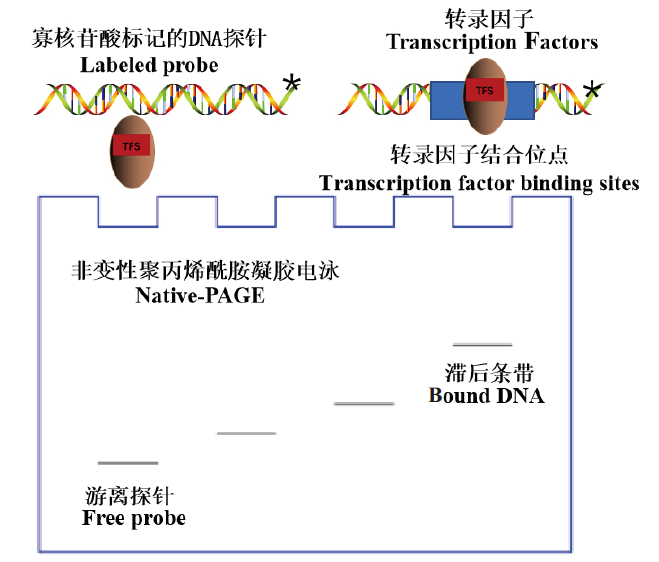
图1 EMSA实验的基本原理 星号表示标记的探针,TFs表示转录因子,TFBS表示转录因子结合位点
Fig. 1 Basic principle of EMSA experiment Asterisks indicate labeled probes,TFs indicate transcription factors,and TFBS indicate transcription factor binding sites
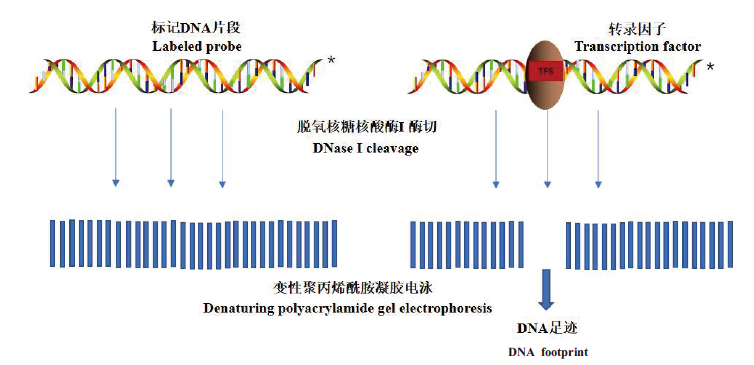
图2 DNase I footprinting技术基本原理 对DNA片段中的一条链进行标记,加入适当浓度脱氧核糖核酸酶I(DNaseI),与目的蛋白结合的DNA基序不被DNase I水解,经过对比未结合目的蛋白DNA片段的放射自显影图谱可得到中断的DNA梯度条带
Fig. 2 Basic principle of DNase I footprint technology Adding appropriate concentration of deoxyribonuclease I(DNase I)to label strand in the DNA fragment,the DNA motif bound to the target protein will not be hydrolyzed by DNase I. After comparing the autoradiograph of the DNA fragment not bound to the target protein,interrupted DNA gradient bands can be obtained
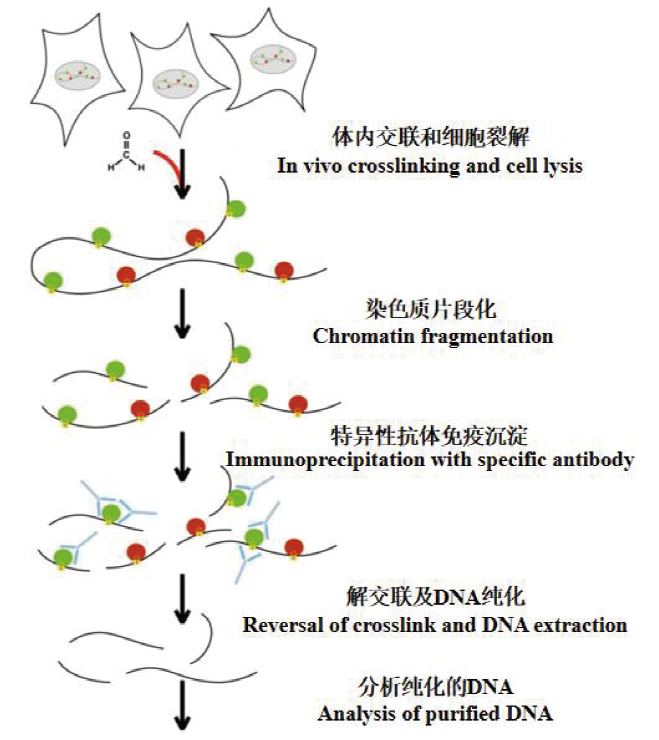
图3 ChIP实验的基本原理 在体内通过交联剂将目的蛋白与DNA交联,再将交联得到的染色质复合物片段化处理后通过目的蛋白特异性抗体沉淀富集目的蛋白结合的DNA片段,对复合物解交联、纯化并分析DNA产物
Fig. 3 Basic principle of ChIP experiment Cross-link the target protein with DNA in vivo through a cross-linking agent,and then fragment the DNA and protein complex. After that,precipitate with the specific antibody of the target protein to enrich the DNA fragments bound to the target protein. Reversal of crosslink and DNA extraction,finally analysis of purified DNA
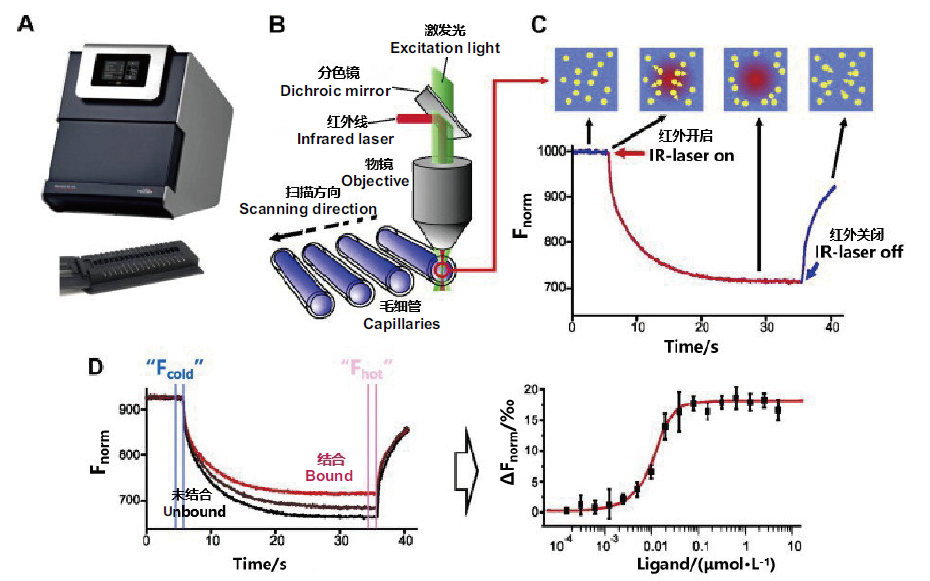
图4 MST实验的基本原理 A:荧光探测器;B:MST光学元件示意图;C:MST实验信号曲线;D:MST结合实验
Fig. 4 Basic principle of MST experiment A:Fluorescence detector. B:MST optical element schematic diagram. C:MST experiment signal curve. D:MST combination experiment
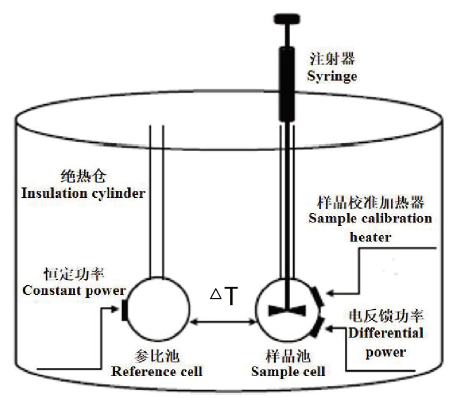
图5 ITC实验的基本原理 已知浓度的反应物滴定到样品中引起样品池中组分之间反应吸热或放热,温度补偿系统使样品池与参比池之间维持恒定的温差,通过记录数据模拟整合加热的等温线之后,可以得到反应焓变、结合亲和力和结合化学计量等信息
Fig. 5 Basic principle of ITC experiment The titration of reactants of known concentration into the sample causes the reaction between the components in the sample cell to be endothermic or exothermic. The temperature compensation system maintains a constant temperature difference between the sample cell and the reference cell,and the data are recorded to simulate the integrated heating isotherm. After that,information about the reaction enthalpy change,binding affinity and binding stoichiometry can be obtained.
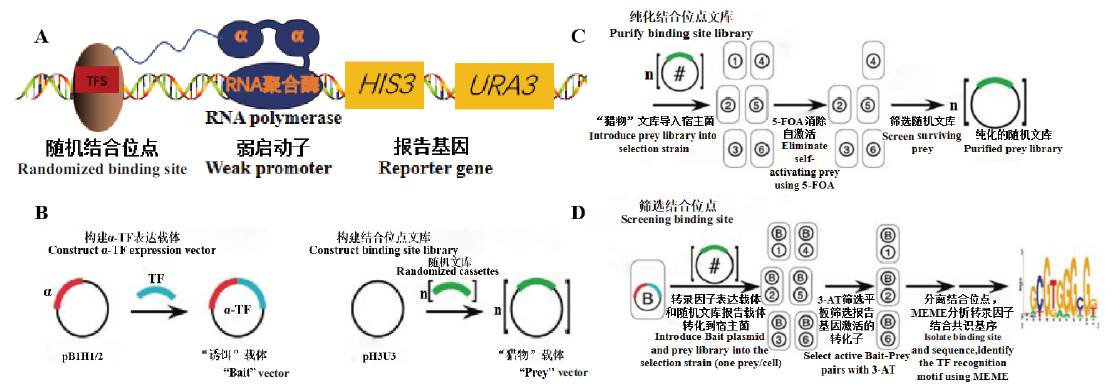
图6 细菌单杂交系统基本原理 A:转录因子识别结合位点启动报告基因表达;B:分别构建α-TF表达载体和结合位点文库;C:消除自激活现象纯化结合位点文库;D:筛选并分析结合位点
Fig. 6 Basic principle of bacterial one hybrid system A:Transcription factor recognition binding sites activate the expressions of the reporter genes. B:Construct α-TF expression vector and binding site library respectively. C:Eliminate self-activation to purify binding site library. D:Selection and analysis of binding sites
| [1] |
Fleischmann RD, Adams MD, White O, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd[J]. Science, 1995, 269(5223):496-512.
pmid: 7542800 |
| [2] |
Faria JP, Overbeek R, Xia FF, et al. Genome-scale bacterial transcriptional regulatory networks:reconstruction and integrated analysis with metabolic models[J]. Brief Bioinform, 2014, 15(4):592-611.
pmid: 23422247 |
| [3] |
Kono N, Arakawa K. Nanopore sequencing:review of potential applications in functional genomics[J]. Dev Growth Differ, 2019, 61(5):316-326.
doi: 10.1111/dgd.12608 URL |
| [4] | 陈竺, 黄薇, 傅刚, 等. 人类基因组计划现状与展望[J]. 自然杂志, 2000, 22(3):125-133, 188. |
| Chen Z, Huang W, Fu G, et al. Human genome project progress and prospect[J]. Nat Mag, 2000, 22(3):125-133, 188. | |
| [5] |
Majewska M, Wysokińska H, Kuźma Ł, et al. Eukaryotic and prokaryotic promoter databases as valuable tools in exploring the regulation of gene transcription:a comprehensive overview[J]. Gene, 2018, 644:38-48.
doi: 10.1016/j.gene.2017.10.079 URL |
| [6] |
Monico C, Capitanio M, Belcastro G, et al. Optical methods to study protein-DNA interactions in vitro and in living cells at the single-molecule level[J]. Int J Mol Sci, 2013, 14(2):3961-3992.
doi: 10.3390/ijms14023961 URL |
| [7] | 周子康, 许平. 全局转录调控在细胞工厂构建中的应用与进展[J]. 化工进展, 2021, 40(3):1248-1251. |
| Zhou ZK, Xu P. Application and progress of global transcription regulation in microbial cell factory construction[J]. Chem Ind Eng Prog, 2021, 40(3):1248-1251. | |
| [8] |
Hellman LM, Fried MG. Electrophoretic mobility shift assay(EMSA)for detecting protein-nucleic acid interactions[J]. Nat Protoc, 2007, 2(8):1849-1861.
pmid: 17703195 |
| [9] |
Unterholzner SJ, Rozhon W, Poppenberger B. Analysis of in vitro DNA interactions of brassinosteroid-controlled transcription factors using electrophoretic mobility shift assay[J]. Methods Mol Biol, 2017, 1564:133-144.
doi: 10.1007/978-1-4939-6813-8_11 pmid: 28124251 |
| [10] |
齐心洁, 王玥, 等. 等温滴定量热法在蛋白质-配体相互作用中的应用[J]. 生物技术通报, 2017, 33(5):40-49.
doi: 10.13560/j.cnki.biotech.bull.1985.2017.05.006 |
| Qi XJ, Wang Y, et al. Applications of isothermal titration calorimetry in protein-ligand interactions[J]. Biotechnol Bull, 2017, 33(5):40-49. | |
| [11] |
Velazquez-Campoy A, Freire E. Isothermal titration calorimetry to determine association constants for high-affinity ligands[J]. Nat Protoc, 2006, 1(1):186-191.
pmid: 17406231 |
| [12] |
Velazquez-Campoy A, Freire E. ITC in the post-genomic era... ? priceless[J]. Biophys Chem, 2005, 115(2-3):115-124.
doi: 10.1016/j.bpc.2004.12.015 URL |
| [13] | Leblanc B, Moss T. DNase I footprinting[M]// Tom Moss. DNA-Protein Interactions. New Jersey: Humana Press, 2001:31-38. |
| [14] | 李丽琴, 石童, 周国超, 等. MST技术在生命科学中的应用进展[J]. 现代生物医学进展, 2017, 17(32):6393-6397. |
| Li LQ, Shi T, Zhou GC, et al. Progress on the applications of microscale themophoresis technology in life science[J]. Prog Mod Biomed, 2017, 17(32):6393-6397. | |
| [15] |
Gade P, Kalvakolanu DV. Chromatin immunoprecipitation assay as a tool for analyzing transcription factor activity[J]. Methods Mol Biol, 2012, 809:85-104.
doi: 10.1007/978-1-61779-376-9_6 pmid: 22113270 |
| [16] |
Christensen RG, Gupta A, Zuo Z, et al. A modified bacterial one-hybrid system yields improved quantitative models of transcription factor specificity[J]. Nucleic Acids Res, 2011, 39(12):e83.
doi: 10.1093/nar/gkr239 URL |
| [17] |
Bak G, Han K, et al. Electrophoretic mobility shift assay of RNA-RNA complexes[J]. Methods Mol Biol, 2015, 1240:153-163.
doi: 10.1007/978-1-4939-1896-6_12 pmid: 25352144 |
| [18] |
Künne T, Westra ER, Brouns SJJ. Electrophoretic mobility shift assay of DNA and CRISPR-cas ribonucleoprotein complexes[J]. Methods Mol Biol, 2015, 1311:171-184.
doi: 10.1007/978-1-4939-2687-9_11 pmid: 25981473 |
| [19] |
Pan YC, Karns K, Herr AE. Microfluidic electrophoretic mobility shift assays for quantitative biochemical analysis[J]. Electrophoresis, 2014, 35(15):2078-2090.
doi: 10.1002/elps.201300500 pmid: 24591076 |
| [20] |
Vavrova A, Vrzal R, Dvorak Z. A nonradioactive electrophoretic mobility shift assay for measurement of pregnane X receptor binding activity to CYP3A4 response element[J]. ELECTROPHORESIS, 2013, 34(13):1863-1668.
pmid: 23977680 |
| [21] |
Cornelussen RNM, Gupta S, Knowlton AA. Regulation of prostaglandin A1-induced heat shock protein expression in isolated cardiomyocytes[J]. J Mol Cell Cardiol, 2001(8):1447-1454.
pmid: 11448133 |
| [22] | 葛兴枫, 李慧, 李少华, 等. 用改良的非同位素凝胶电泳迁移实验鉴定核酸适配体与靶蛋白的结合[J]. 生物技术通讯, 2015, 26(2):249-251. |
| Ge XF, Li H, Li SH, et al. A modified non-isotope EMSA technique for identification of aptamer binding to its target protein[J]. Lett Biotechnol, 2015, 26(2):249-251. | |
| [23] |
Tokunaga S, Stegeman JJ. Elimination of nonspecific bands in non-radioactive electrophoretic mobility shift assays using the digoxigenin system[J]. Anal Biochem, 2014, 465:70-72.
doi: 10.1016/j.ab.2014.06.020 pmid: 25004462 |
| [24] |
Fried MG, Bromberg JL. Factors that affect the stability of protein-DNA complexes during gel electrophoresis[J]. Electrophoresis, 1997, 18(1):6-11.
pmid: 9059813 |
| [25] |
Brown L, Villegas JM, Elean M, et al. YebC, a putative transcriptional factor involved in the regulation of the proteolytic system of Lactobacillus[J]. Sci Rep, 2017, 7(1):8579.
doi: 10.1038/s41598-017-09124-1 URL |
| [26] | 王丽滨. 肺炎链球菌糖代谢蛋白CcpA对荚膜多糖的调控研究[D]. 重庆: 重庆医科大学, 2015. |
| Wang LB. The regulation effect of CcpA protein on the biosynthesis of capsular polysaccharide in Streptococcus pneumoniae[D]. Chongqing: Chongqing Medical University, 2015. | |
| [27] |
Chen C, Lu YQ, Wang LL, et al. CcpA-dependent carbon catabolite repression regulates fructooligosaccharides metabolism in Lactobacillus plantarum[J]. Front Microbiol, 2018, 9:1114.
doi: 10.3389/fmicb.2018.01114 pmid: 29896178 |
| [28] |
Keyhani J, Keyhani E. Detection of DNA autoantibodies by electrophoretic mobility shift assay[J]. Methods Mol Biol, 2019, 1901:133-152.
doi: 10.1007/978-1-4939-8949-2_11 pmid: 30539574 |
| [29] |
Hampshire AJ, Rusling DA, Broughton-Head VJ, et al. Footprinting:a method for determining the sequence selectivity, affinity and kinetics of DNA-binding ligands[J]. Methods, 2007, 42(2):128-140.
pmid: 17472895 |
| [30] | 徐冬冬, 刘德培, 吕湘, 等. 固相DNaseⅠ足迹法研究DNA-蛋白质相互作用[J]. 生物化学与生物物理进展, 2001, 28(4):587-590. |
| Xu DD, Liu DP, Lv X, et al. A method for the study of DNA-protein interaction:solid-phase DNaseⅠ footprinting[J]. Prog Biochem Biophys, 2001, 28(4):587-590. | |
| [31] | Gong LC, Ren C, Xu Y. GlnR negatively regulates glutamate-dependent acid resistance in Lactobacillus brevis[J]. Appl Environ Microbiol, 2020, 86(7):e02615-e02619. |
| [32] |
Ihara K, Sato K, Hori H, et al. Expression of the alaE gene is positively regulated by the global regulator Lrp in response to intracellular accumulation of l-alanine in Escherichia coli[J]. J Biosci Bioeng, 2017, 123(4):444-450.
doi: 10.1016/j.jbiosc.2016.11.015 URL |
| [33] | Yang XP, Teng KL, Li LL, et al. Transcriptional regulator AcrR increases ethanol tolerance through regulation of fatty acid synthesis in Lactobacillus plantarum[J]. Appl Environ Microbiol, 2019, 85(22):e01690-e01619. |
| [34] | 王智. DNase1足纹法[J]. 基础医学与临床, 1990, 10(3):59-63. |
| Wang Z. DNase I-footprinting assay[J]. Basic Med Sci Clin, 1990, 10(3):59-63. | |
| [35] |
Wagner M, Jung J, Koslowski M, et al. Chromatin immunoprecipitation assay to identify genomic binding sites of regulatory factors[J]. Methods Mol Biol, 2016, 1366:53-65.
doi: 10.1007/978-1-4939-3127-9_6 pmid: 26585127 |
| [36] |
Orlando V, Paro R. Mapping Polycomb-repressed domains in the bithorax complex using in vivo formaldehyde cross-linked chromatin[J]. Cell, 1993, 75(6):1187-1198.
pmid: 7903220 |
| [37] | Wiehle L, Breiling A. Chromatin immunoprecipitation[M]// Lanzuolo C, Bodega B. Polycomb Group Proteins. New York: Humana Press, 2016:7-21. |
| [38] |
Dahl JA, Collas P. Q2ChIP, a quick and quantitative chromatin immunoprecipitation assay, unravels epigenetic dynamics of developmentally regulated genes in human carcinoma cells[J]. Stem Cells, 2007, 25(4):1037-1046.
pmid: 17272500 |
| [39] |
Walton CB, Matter ML. Chromatin immunoprecipitation assay:examining the interaction of NFkB with the VEGF promoter[J]. Methods Mol Biol, 2015, 1332:75-87.
doi: 10.1007/978-1-4939-2917-7_6 pmid: 26285747 |
| [40] |
Chen H, Lin RJ, Xie W, et al. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase[J]. Cell, 1999, 98(5):675-686.
pmid: 10490106 |
| [41] |
Ishihama A. Prokaryotic genome regulation:multifactor promoters, multitarget regulators and hierarchic networks[J]. FEMS Microbiol Rev, 2010, 34(5):628-645.
doi: 10.1111/j.1574-6976.2010.00227.x pmid: 20491932 |
| [42] |
Ratib NR, Sabio EY, Mendoza C, et al. Genome-wide identification of genes directly regulated by ChvI and a consensus sequence for ChvI binding in Sinorhizobium meliloti[J]. Mol Microbiol, 2018, 110(4):596-615.
doi: 10.1111/mmi.14119 URL |
| [43] |
Yun CS, Takahashi Y, Shintani M, et al. MvaT family proteins encoded on IncP-7 plasmid pCAR1 and the host chromosome regulate the host transcriptome cooperatively but differently[J]. Appl Environ Microbiol, 2015, 82(3):832-842.
doi: 10.1128/AEM.03071-15 URL |
| [44] | Shao XL, Zhang XN, Zhang YC, et al. RpoN-dependent direct regulation of quorum sensing and the type VI secretion system in Pseudomonas aeruginosa PAO1[J]. J Bacteriol, 2018, 200(16):e00205-e00218. |
| [45] |
Bard-Chapeau EA, Jeyakani J, Kok CH, et al. Ecotopic viral integration site 1(EVI1)regulates multiple cellular processes important for cancer and is a synergistic partner for FOS protein in invasive tumors[J]. PNAS, 2012, 109(6):2168-2173.
doi: 10.1073/pnas.1119229109 pmid: 22308434 |
| [46] |
王泓力, 焦雨铃. 染色质免疫共沉淀实验方法[J]. 植物学报, 2020, 55(4):475-480.
doi: 10.11983/CBB20076 |
| Wang HL, Jiao YL. Protocols for chromatin immunoprecipitation[J]. Chin Bull Bot, 2020, 55(4):475-480. | |
| [47] |
Breitsprecher D, Schlinck N, Witte D, et al. Aptamer binding studies using MicroScale thermophoresis[J]. Methods Mol Biol, 2016, 1380:99-111.
doi: 10.1007/978-1-4939-3197-2_8 pmid: 26552819 |
| [48] |
Jerabek-Willemsen M, André T, Wanner R, et al. MicroScale thermophoresis:interaction analysis and beyond[J]. J Mol Struct, 2014, 1077:101-113.
doi: 10.1016/j.molstruc.2014.03.009 URL |
| [49] |
Jerabek-Willemsen M, Wienken CJ, Braun D, et al. Molecular interaction studies using microscale thermophoresis[J]. Assay Drug Dev Technol, 2011, 9(4):342-353.
doi: 10.1089/adt.2011.0380 URL |
| [50] | Gudim I, Lofstad M, Hammerstad M, et al. Measurement of FNR-NrdI interaction by microscale thermophoresis(MST)[J]. Bio-protocol, 2017, 7(8): e2223. |
| [51] |
Entzian C, Schubert T. Studying small molecule-aptamer interactions using microScale thermophoresis(MST)[J]. Methods, 2016, 97:27-34.
doi: 10.1016/j.ymeth.2015.08.023 URL |
| [52] |
Wienken CJ, Baaske P, Duhr S, et al. Thermophoretic melting curves quantify the conformation and stability of RNA and DNA[J]. Nucleic Acids Res, 2011, 39(8):e52.
doi: 10.1093/nar/gkr035 URL |
| [53] |
Zillner K, Filarsky M, Rachow K, et al. Large-scale organization of ribosomal DNA chromatin is regulated by Tip5[J]. Nucleic Acids Res, 2013, 41(10):5251-5262.
doi: 10.1093/nar/gkt218 pmid: 23580549 |
| [54] |
Shang X, Marchioni F, Evelyn CR, et al. Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors[J]. PNAS, 2013, 110(8):3155-3160.
doi: 10.1073/pnas.1212324110 pmid: 23382194 |
| [55] |
van den Bogaart G, Meyenberg K, et al. Phosphatidylinositol 4, 5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold[J]. J Biol Chem, 2012, 287(20):16447-16453.
doi: 10.1074/jbc.M112.343418 pmid: 22447935 |
| [56] |
Papageorgiou AC, Adam PS, et al. HU histone-like DNA-binding protein from Thermus thermophilus:structural and evolutionary analyses[J]. Extremophiles, 2016, 20(5):695-709.
doi: 10.1007/s00792-016-0859-1 pmid: 27342116 |
| [57] |
Seidel SAI, Dijkman PM, Lea WA, et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions[J]. Methods, 2013, 59(3):301-315.
doi: 10.1016/j.ymeth.2012.12.005 pmid: 23270813 |
| [58] | 吴萌, 李竑, 陈铭. 两种实验技术在蛋白质-蛋白质相互作用检测中的应用[J]. 生命的化学, 2021(2):353-360. |
| Wu M, Li H, Chen M. An overview of BLI and MST applications in protein-protein interaction[J]. Chem Life, 2021(2):353-360. | |
| [59] |
Wang Q, Wang J, Song SX, et al. Microscale thermophoresis in the investigation of biomolecular interactions[J]. J Chin Pharm Sci, 2020, 29(9):656-665.
doi: 10.5246/jcps.2020.09.061 |
| [60] |
Keller S, Vargas C, Zhao HY, et al. High-precision isothermal titration calorimetry with automated peak-shape analysis[J]. Anal Chem, 2012, 84(11):5066-5073.
doi: 10.1021/ac3007522 pmid: 22530732 |
| [61] |
Boudker O, Oh S. Isothermal titration calorimetry of ion-coupled membrane transporters[J]. Methods, 2015, 76:171-182.
doi: S1046-2023(15)00024-9 pmid: 25676707 |
| [62] |
Zhuo L, Zhang Z, Pan Z, et al. CIRCE element evolved for the coordinated transcriptional regulation of bacterial duplicate groELs[J]. Biochim Biophys Acta Gene Regul Mech, 2018, 1861(10):928-937.
doi: 10.1016/j.bbagrm.2018.08.003 URL |
| [63] |
Wang W, Ji J, Li X, et al. Angucyclines as signals modulate the behaviors of Streptomyces coelicolor[J]. PNAS, 2014, 111(15):5688-5693.
doi: 10.1073/pnas.1324253111 URL |
| [64] |
Yamasaki K, Akutsu Y, Yamasaki T, et al. Enhanced affinity of racemic phosphorothioate DNA with transcription factor SATB1 arising from diastereomer-specific hydrogen bonds and hydrophobic contacts[J]. Nucleic Acids Res, 2020, 48(8):4551-4561.
doi: 10.1093/nar/gkaa170 pmid: 32187371 |
| [65] |
Weber A, Dettling M, Brunner H, et al. Isothermal titration calorimetry of molecularly imprinted polymer nanospheres[J]. Macromol Rapid Commun, 2002, 23(14):824-828.
doi: 10.1002/1521-3927(20021001)23:14<824::AID-MARC824>3.0.CO;2-P URL |
| [66] |
Brautigam CA, Zhao HY, et al. Integration and global analysis of isothermal titration calorimetry data for studying macromolecular interactions[J]. Nat Protoc, 2016(5):882-894.
doi: 10.1038/nprot.2016.044 pmid: 27055097 |
| [67] |
Meng XD, Wolfe SA. Identifying DNA sequences recognized by a transcription factor using a bacterial one-hybrid system[J]. Nat Protoc, 2006, 1(1):30-45.
pmid: 17406209 |
| [68] | 翟征远. 德氏乳杆菌保加利亚亚种CAUH1酸耐受机制的蛋白组学研究及抗酸胁迫基因Ldb0677和pyk的功能分析[D]. 北京: 中国农业大学, 2014. |
| Zhai ZY. Proteomic characterization of the acid tolerance response in Lactobacillus delbrueckii subsp. bulgaricus CAUH1and functional identification of acid stress-related genes Ldb0677 and pyk[D]. Beijing: China Agricultural University, 2014. | |
| [69] | Hebdon SD, Menon SK, Richter-Addo GB, et al. Regulatory targets of the response regulator RR_1586 from Clostridioides difficile identified using a bacterial one-hybrid screen[J]. J Bacteriol, 2018, 200(23):e00351-e00318. |
| [70] |
Zhai ZY, Douillard FP, An HR, et al. Proteomic characterization of the acid tolerance response in Lactobacillus delbrueckii subsp. bulgaricus CAUH1 and functional identification of a novel acid stress-related transcriptional regulator Ldb0677[J]. Environ Microbiol, 2014, 16(6):1524-1537.
doi: 10.1111/1462-2920.12280 URL |
| [71] | Zhu LJ, Christensen RG, Kazemian M, et al. FlyFactorSurvey:a database of Drosophila transcription factor binding specificities determined using the bacterial one-hybrid system[J]. Nucleic Acids Res, 2011, 39(Database issue):D111-D117. |
| [1] | 黄小龙, 孙贵连, 马丹丹, 闫慧清. 水稻幼苗酵母单杂文库构建及LAZY1上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 126-135. |
| [2] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [3] | 韩浩章, 张丽华, 李素华, 赵荣, 王芳, 王晓立. 盐碱胁迫诱导的猴樟酵母cDNA文库构建及CbP5CS上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 236-245. |
| [4] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [5] | 徐靖, 朱红林, 林延慧, 唐力琼, 唐清杰, 王效宁. 甘薯IbHQT1启动子的克隆及上游调控因子的鉴定[J]. 生物技术通报, 2023, 39(8): 213-219. |
| [6] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [7] | 陈晓, 于茗兰, 吴隆坤, 郑晓明, 逄洪波. 植物lncRNA及其对低温胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(7): 1-12. |
| [8] | 李英, 岳祥华. DNA甲基化在解析毛竹自然变异中的应用[J]. 生物技术通报, 2023, 39(7): 48-55. |
| [9] | 郭怡婷, 赵文菊, 任延靖, 赵孟良. 菊芋NAC转录因子家族基因的鉴定及分析[J]. 生物技术通报, 2023, 39(6): 217-232. |
| [10] | 冯珊珊, 王璐, 周益, 王幼平, 方玉洁. WOX家族基因调控植物生长发育和非生物胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(5): 1-13. |
| [11] | 王兵, 赵会纳, 余婧, 余世洲, 雷波. 植物侧枝发育的调控研究进展[J]. 生物技术通报, 2023, 39(5): 14-22. |
| [12] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [13] | 张新博, 崔浩亮, 史佩华, 高锦春, 赵顺然, 陶晨雨. 低起始量的免疫共沉淀技术研究进展[J]. 生物技术通报, 2023, 39(4): 227-235. |
| [14] | 杨岚, 张晨曦, 樊学伟, 王阳光, 王春秀, 李文婷. 鸡 BMP15 基因克隆、表达模式及启动子活性分析[J]. 生物技术通报, 2023, 39(4): 304-312. |
| [15] | 葛颜锐, 赵冉, 徐静, 李若凡, 胡云涛, 李瑞丽. 植物维管形成层发育及其调控的研究进展[J]. 生物技术通报, 2023, 39(3): 13-25. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||