








生物技术通报 ›› 2022, Vol. 38 ›› Issue (12): 149-155.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0400
收稿日期:2022-05-23
出版日期:2022-12-26
发布日期:2022-12-29
作者简介:陈铎,男,博士,研究方向:呼吸与危重症医学;E-mail: 基金资助:Received:2022-05-23
Published:2022-12-26
Online:2022-12-29
摘要:
本研究通过原核表达方法,探索新型冠状病毒核蛋白N端结构域的表达纯化,同时优化其蛋白晶体的生长条件,为新型冠状病毒核蛋白的结构生物研究提供基础。本研究合成N蛋白N端结构域基因序列,构建原核系统表达载体,利用大肠杆菌表达重组目的蛋白。Ni2+亲和层析和凝胶过滤层析的方法对N蛋白N端结构域重组蛋白进行纯化,SDS-PAGE电泳鉴定重组蛋白的纯度。本研究成功构建原核重组载体pET28b-N-N,大肠杆菌在37℃下进行扩增生长,16℃下进行诱导表达,80%目的蛋白以可溶蛋白的形式表达。Ni2+亲和纯化和凝胶过滤层析后,Quantity One软件进行蛋白胶积分分析,获得纯化后的积分占比,获得目的蛋白的纯度高达90%以上,利用Hampton的蛋白晶体试剂盒进行蛋白质晶体筛选,获得质量较高的N蛋白N端结构域蛋白质晶体。凝胶迁移试验(EMSA)验证了N蛋白N端结构域具有结合特定RNA片段的作用,明确了N蛋白N端结构域稳定新型冠状病毒基因组的功能。为进一步解析N蛋白N端结构域的三维结构以及后续抗体开发提供研究基础。
陈铎, 刘永哲. 新型冠状病毒核蛋白N端结构域的表达纯化以及晶体优化[J]. 生物技术通报, 2022, 38(12): 149-155.
CHEN Duo, LIU Yong-zhe. Prokaryotic Expression,Purification and Crystallization of N-terminal Domain of Nucleocapsid Protein in SARS-CoV-2[J]. Biotechnology Bulletin, 2022, 38(12): 149-155.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| N-N-F | GGAATTCCATATGAATAATACTGCGTCTTGGTT |
| N-N-R | CCGCTCGAGTTACCCTTCTGCGTAGAAGCCTT |
表1 引物序列
Table 1 Primer sequences
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| N-N-F | GGAATTCCATATGAATAATACTGCGTCTTGGTT |
| N-N-R | CCGCTCGAGTTACCCTTCTGCGTAGAAGCCTT |
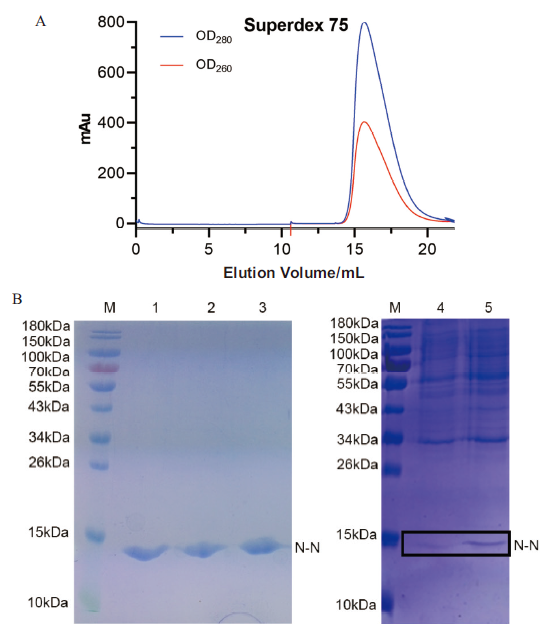
图3 N-N蛋白的表达纯化 A:N-N蛋白通过Superdex 75凝胶色谱分子筛纯化图;B:纯化后的N-N蛋白SDS-PAGE结果图。M为蛋白质marker;1-3:N-N蛋白;4:诱导前样品;5:诱导后样品
Fig. 3 Expression and purification of N-N protein A:The N-N protein samples were purified by Superdex 75 column. B:The purified N-N protein samples were analyzed on SDS-PAGE. M:Protein marker. 1-3:The N-N protein;4:Before-induced.5:After-induced
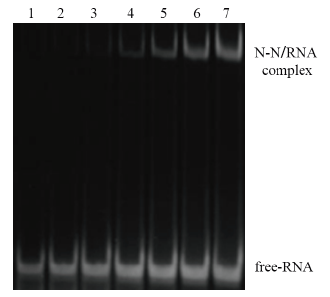
图4 N-N蛋白与RNA结合的EMSA结果 1:空白RNA对照;2-7:N-N蛋白与RNA孵育(N-N蛋白浓度分别对应1、2、3、4、5、6 mg/mL)
Fig. 4 EMSA result of binding of N-N protein and RNA 1:Normal RNA control. 2-7:Incubation of RNA and N-N protein(The N-N protein concentrations correspond to 1,2,3,4,5 and 6 mg/mL respectively)
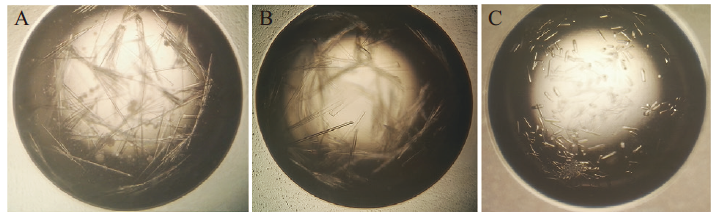
图5 N蛋白的晶体优化 A:PEG/Ion Screen 23号初筛晶体;B:添加Crystal Screen 45号复筛晶体;C:添加Additive Screen 25号最终获得晶体
Fig. 5 Optimization of N protein crystal A:Making Initial crystal by PEG/Ion Screen 23. B:Making further crystal by adding Crystal Screen 45. C:Making final crystal by adding Additive Screen 25
| [1] |
Dong ES, Du HR, Gardner L. An interactive web-based dashboard to track COVID-19 in real time[J]. Lancet Infect Dis, 2020, 20(5):533-534.
doi: S1473-3099(20)30120-1 pmid: 32087114 |
| [2] |
Huang CL, Wang YM, Li XW, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China[J]. Lancet, 2020, 395(10223):497-506.
doi: S0140-6736(20)30183-5 pmid: 31986264 |
| [3] |
Anand KB, Karade S, Sen S, et al. SARS-CoV-2:camazotz’s curse[J]. Med J Armed Forces India, 2020, 76(2):136-141.
doi: 10.1016/j.mjafi.2020.04.008 URL |
| [4] |
Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing[J]. Nature, 2020, 583(7816):459-468.
doi: 10.1038/s41586-020-2286-9 URL |
| [5] |
Romano M, Ruggiero A, Squeglia F, et al. A structural view of SARS-CoV-2 RNA replication machinery:RNA synthesis, proofreading and final capping[J]. Cells, 2020, 9(5):1267.
doi: 10.3390/cells9051267 URL |
| [6] |
Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19[J]. Nat Rev Microbiol, 2021, 19(3):141-154.
doi: 10.1038/s41579-020-00459-7 pmid: 33024307 |
| [7] |
Yang D, Leibowitz JL. The structure and functions of coronavirus genomic 3' and 5' ends[J]. Virus Res, 2015, 206:120-133.
doi: 10.1016/j.virusres.2015.02.025 pmid: 25736566 |
| [8] |
Uddin M, Mustafa F, Rizvi TA, et al. SARS-CoV-2/COVID-19:viral genomics, epidemiology, vaccines, and therapeutic interventions[J]. Viruses, 2020, 12(5):526.
doi: 10.3390/v12050526 URL |
| [9] |
Yao HP, Song YT, Chen Y, et al. Molecular architecture of the SARS-CoV-2 virus[J]. Cell, 2020, 183(3):730-738. e13.
doi: 10.1016/j.cell.2020.09.018 pmid: 32979942 |
| [10] |
Ye QZ, West AMV, Silletti S, et al. Architecture and self-assembly of the SARS-CoV-2 nucleocapsid protein[J]. Protein Sci, 2020, 29(9):1890-1901.
doi: 10.1002/pro.3909 URL |
| [11] | Peng Y, Du N, Lei YQ, et al. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design[J]. EMBO J, 2020, 39(20):e105938. |
| [12] | Jia Z, Liu C, Chen Y, et al. Crystal structures of the SARS-CoV-2 nucleocapsid protein C-terminal domain and development of nucleocapsid-targeting nanobodies[J]. FEBS J, 2021. Doi:10.1111/febs.16239. |
| [13] |
张西西, 张怡青, 李玉林, 等. 新型冠状病毒(SARS-CoV-2)N蛋白C端重组蛋白的原核表达、纯化及应用[J]. 生物技术通报, 2021, 37(5):92-97.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0902 URL |
| Zhang XX, Zhang YQ, Li YL, et al. Prokaryotic expression, purification and application of N protein C-terminal recombinant protein in novel coronavirus(SARS-CoV-2)[J]. Biotechnol Bull, 2021, 37(5):92-97. | |
| [14] |
Wu C, Qavi AJ, Moyle AB, et al. Domain-specific biochemical and serological characterization of SARS-CoV-2 nucleocapsid protein[J]. STAR Protoc, 2021, 2(4):100906.
doi: 10.1016/j.xpro.2021.100906 URL |
| [15] |
Zeng WH, Liu GF, Ma H, et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein[J]. Biochem Biophys Res Commun, 2020, 527(3):618-623.
doi: 10.1016/j.bbrc.2020.04.136 URL |
| [16] |
Gupta Y, Kumar S, Zak SE, et al. Antiviral evaluation of hydroxyethylamine analogs:Inhibitors of SARS-CoV-2 main protease(3CLpro), a virtual screening and simulation approach[J]. Bioorg Med Chem, 2021, 47:116393.
doi: 10.1016/j.bmc.2021.116393 URL |
| [17] |
Samper IC, McMahon CJ, Schenkel MS, et al. Electrochemical immunoassay for the detection of SARS-CoV-2 nucleocapsid protein in nasopharyngeal samples[J]. Anal Chem, 2022, 94(11):4712-4719.
doi: 10.1021/acs.analchem.1c04966 pmid: 35263100 |
| [18] |
Low JS, Vaqueirinho D, Mele F, et al. Clonal analysis of immunodominance and cross-reactivity of the CD4 T cell response to SARS-CoV-2[J]. Science, 2021, 372(6548):1336-1341.
doi: 10.1126/science.abg8985 pmid: 34006597 |
| [19] |
López-Muñoz AD, Kosik I, Holly J, et al. Cell surface SARS-CoV-2 nucleocapsid protein modulates innate and adaptive immunity[J]. bioRxiv, 2021. DOI:10.1101/2021.12.10.472169.
doi: 10.1101/2021.12.10.472169 URL |
| [20] |
Xie CZ, Ding HJ, Ding JZ, et al. Preparation of highly specific monoclonal antibodies against SARS-CoV-2 nucleocapsid protein and the preliminary development of antigen detection test strips[J]. J Med Virol, 2022, 94(4):1633-1640.
doi: 10.1002/jmv.27520 URL |
| [21] | Li DD, Li JM. Immunologic testing for SARS-CoV-2 infection from the antigen perspective[J]. J Clin Microbiol, 2021, 59(5):e02160-e02120. |
| [22] |
Wang YT, Long XY, Ding X, et al. Novel nucleocapsid protein-targeting phenanthridine inhibitors of SARS-CoV-2[J]. Eur J Med Chem, 2022, 227:113966.
doi: 10.1016/j.ejmech.2021.113966 URL |
| [23] |
Lucas C, Vogels CBF, Yildirim I, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity[J]. Nature, 2021, 600(7889):523-529.
doi: 10.1038/s41586-021-04085-y URL |
| [24] |
Pitcovski J, Gruzdev N, Abzach A, et al. Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine[J]. Vaccine, 2022, 40(8):1098-1107.
doi: 10.1016/j.vaccine.2022.01.025 URL |
| [25] |
Chatzileontiadou DSM, Szeto C, Jayasinghe D, et al. Protein purification and crystallization of HLA-A 02:01 in complex with SARS-CoV-2 peptides[J]. STAR Protoc, 2021, 2(3):100635.
doi: 10.1016/j.xpro.2021.100635 URL |
| [26] |
Cubuk J, Alston JJ, Incicco JJ, et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA[J]. Nat Commun, 2021, 12(1):1936.
doi: 10.1038/s41467-021-21953-3 pmid: 33782395 |
| [27] |
Savastano A, Ibáñez de Opakua A, Rankovic M, et al. Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates[J]. Nat Commun, 2020, 11(1):6041.
doi: 10.1038/s41467-020-19843-1 pmid: 33247108 |
| [28] |
Jack A, Ferro LS, Trnka MJ, et al. SARS-CoV-2 nucleocapsid protein forms condensates with viral genomic RNA[J]. PLoS Biol, 2021, 19(10):e3001425.
doi: 10.1371/journal.pbio.3001425 URL |
| [29] |
Parashar NC, Poddar J, Chakrabarti S, et al. Repurposing of SARS-CoV nucleocapsid protein specific nuclease resistant RNA aptamer for therapeutics against SARS-CoV-2[J]. Infect Genet Evol, 2020, 85:104497.
doi: 10.1016/j.meegid.2020.104497 URL |
| [30] | Tatar G, Ozyurt E, Turhan K. Computational drug repurposing study of the RNA binding domain of SARS-CoV-2 nucleocapsid protein with antiviral agents[J]. Biotechnol Prog, 2021, 37(2):e3110. |
| [1] | 李文硕, 王林松, 杜桂彩, 郭群群, 张廷婷, 杨宏, 李荣贵. 一种松材线虫醛脱氢酶的基因克隆及其生化性质[J]. 生物技术通报, 2022, 38(9): 207-214. |
| [2] | 覃雪晶, 王雨涵, 曹一博, 张凌云. 青杄PwHAP5基因原核表达及多克隆抗体制备[J]. 生物技术通报, 2022, 38(8): 142-149. |
| [3] | 易芳, 来鹏程, 郑希鳌, 胡帅, 高燕丽. Kod DNA聚合酶的制备及纯化研究[J]. 生物技术通报, 2022, 38(5): 183-190. |
| [4] | 汪巧菊, 胡雨萌, 温亚亚, 宋丽, 孟闯, 潘志明, 焦新安. 新型冠状病毒S1蛋白的表达及活性鉴定[J]. 生物技术通报, 2022, 38(3): 157-163. |
| [5] | 王小琴, 黄银萍, 王蔚倩, 吴萍, 全舒. 含非天然氨基酸定点突变的MLL3SET蛋白表达与纯化[J]. 生物技术通报, 2022, 38(3): 194-202. |
| [6] | 张丰文, 周丽亚, 董超, 史延茂. 纳豆中抗氧化肽的分离纯化与活性研究[J]. 生物技术通报, 2022, 38(2): 158-165. |
| [7] | 赵玉雪, 王芸, 余璐瑶, 刘京晶, 斯金平, 张新凤, 张磊. 植物中C-糖基转移酶的结构与应用[J]. 生物技术通报, 2022, 38(10): 18-28. |
| [8] | 曹汝菲, 李泽轩, 许欢, 张莎, 张敏敏, 戴枫, 段晓雷. 脆弱拟杆菌Pif1解旋酶的表达纯化与晶体生长[J]. 生物技术通报, 2021, 37(9): 180-190. |
| [9] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [10] | 张西西, 张怡青, 李玉林, 韩笑, 王国强, 王晓军, 王旭东, 王云龙. 新型冠状病毒(SARS-CoV-2)N蛋白C端重组蛋白的原核表达、纯化及应用[J]. 生物技术通报, 2021, 37(5): 92-97. |
| [11] | 王志新, 鲁雷震, 周景波, 封成玲, 贾紫伟, 宁亚维, 贾英民. 抗真菌肽研究进展[J]. 生物技术通报, 2021, 37(3): 206-218. |
| [12] | 高竞溪, 高珂星, 鲁非, 纪锋, 郭志刚. 与SARS-CoV有交叉免疫反应的SARS-CoV-2 S蛋白B细胞抗原表位预测[J]. 生物技术通报, 2021, 37(10): 169-178. |
| [13] | 唐禄, 董丽平, 尹茉莉, 刘磊, 董媛, 王会岩. 成纤维细胞生长因子20单克隆抗体的制备及鉴定[J]. 生物技术通报, 2021, 37(10): 179-185. |
| [14] | 陈汭, 付嘉钰, 刘浩宇, 李成, 赵玉佳, 胡靖飞, 瞿欢, 曹三杰, 文心田, 文翼平, 赵勤, 伍锐, 黄小波. 猪δ冠状病毒(PDCoV)N蛋白的原核表达及多克隆抗体制备[J]. 生物技术通报, 2020, 36(8): 104-110. |
| [15] | 孟利, 杜彩萍. 大鼠His-Akt1重组蛋白的真核表达、蛋白纯化及活性鉴定[J]. 生物技术通报, 2020, 36(12): 98-103. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
