








生物技术通报 ›› 2022, Vol. 38 ›› Issue (9): 207-214.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1450
李文硕( ), 王林松, 杜桂彩, 郭群群, 张廷婷, 杨宏, 李荣贵(
), 王林松, 杜桂彩, 郭群群, 张廷婷, 杨宏, 李荣贵( )
)
收稿日期:2021-11-21
出版日期:2022-09-26
发布日期:2022-10-11
作者简介:李文硕,女,硕士研究生,研究方向:松萎蔫病的致病机理;E-mail: 基金资助:
LI Wen-shuo( ), WANG Lin-song, DU Gui-cai, GUO Qun-qun, ZHANG Ting-ting, YANG Hong LI Rong-gui
), WANG Lin-song, DU Gui-cai, GUO Qun-qun, ZHANG Ting-ting, YANG Hong LI Rong-gui
Received:2021-11-21
Published:2022-09-26
Online:2022-10-11
摘要:
松材线虫(Bursaphelenchus xylophilus)是松萎蔫病的病原,可引起数十种松属树种的毁灭性破坏。通过RT-PCR 成功克隆了松材线虫体内一种醛脱氢酶基因aldh,aldh的长度为1 353 bp,编码451 个氨基酸残基,前期研究表明,杀线剂甲吡唑处理能使aldh表达水平显著上调。序列比对表明,与大多数其他醛脱氢酶中依靠半胱氨酸(Cys)残基发挥催化功能不同,aldh 编码的蛋白质保守的位置是亮氨酸(Leu)残基,但其C-端紧邻Leu残基的是组氨酸残基(His),而不是常见的Leu残基。构建了表达载体pET-15b-aldh并转化大肠杆菌BL21(DE3),IPTG诱导aldh在工程菌中实现了高表达,重组醛脱氢酶通过Ni-NTA亲和层析纯化后进行表征。以甲醛为底物的醛脱氢酶Km为27.87 mmol/L,最适pH值为7.5,最适温度为25℃,Fe3+和Ni2+可提高酶活性,Ca2+、Mn2+、Na+和K+可降低酶活性。重组醛脱氢酶对5种醛类化合物显示出不同的催化活性,其中香草醛是其最佳底物。该研究为深入了解醛脱氢酶在松材线虫致病过程中的作用打下了基础,也为新型工业用醛脱氢酶的研究提供了参考。
李文硕, 王林松, 杜桂彩, 郭群群, 张廷婷, 杨宏, 李荣贵. 一种松材线虫醛脱氢酶的基因克隆及其生化性质[J]. 生物技术通报, 2022, 38(9): 207-214.
LI Wen-shuo, WANG Lin-song, DU Gui-cai, GUO Qun-qun, ZHANG Ting-ting, YANG Hong LI Rong-gui. Gene Cloning of an Aldehyde Dehydrogenase from Bursaphelenchus xylophilus and Biochemical Characterization[J]. Biotechnology Bulletin, 2022, 38(9): 207-214.
| 引物名称 Name of primer | 序列Sequence(5'-3') |
|---|---|
| F1 | aaaCATATGggacagctccagagtttggt |
| R1 | aaaGGATCCtcactcgatggagatccgctttc |
表1 本试验所用引物
Table 1 PCR primers used in this study
| 引物名称 Name of primer | 序列Sequence(5'-3') |
|---|---|
| F1 | aaaCATATGggacagctccagagtttggt |
| R1 | aaaGGATCCtcactcgatggagatccgctttc |

图3 重组醛脱氢酶的表达与纯化 M:Marker;1:大肠杆菌E.coli BL21(DE3)空菌全蛋白;2:工程菌全蛋白;3:纯化的醛脱氢酶
Fig. 3 Expression and purification of the recombinant alde-hyde dehydrogenase M:Protein marker;1:total proteins of E. coli BL21(DE3);2:total proteins of E. coli BL21(DE3)harboring pET-15b-aldh;3:the purified recombinant aldehyde dehydrogenase
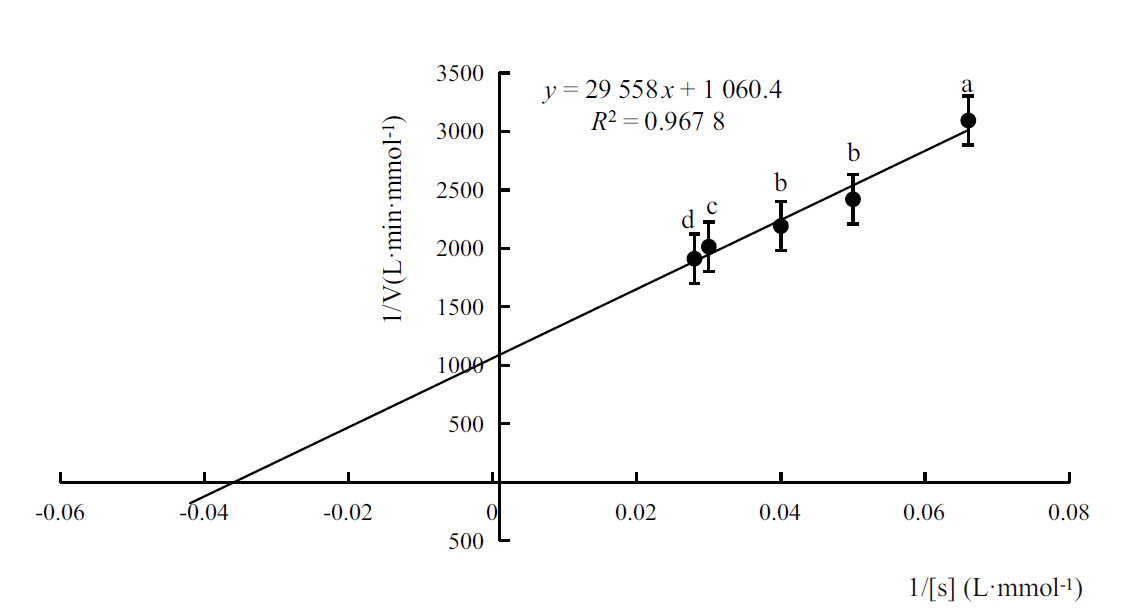
图4 通过Lineweaver-Burk计算醛脱氢酶的Km值 图中误差线表示标准偏差。小写字母表示不同测试结果间差异达到(P<0.05)显著水平,下同
Fig. 4 Km of the recombinant aldehyde dehydrogenase calculated by Lineweaver-Burk plot The error line in the figure refers to the standard deviation. The lowercase letters indicate that the difference among different test reached a significant level(P <0.05). The same meaning is suitable for the rest of this paper
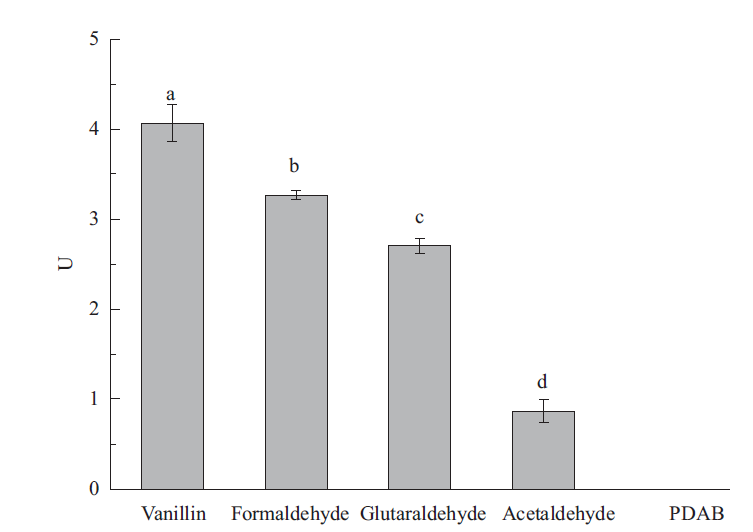
图8 醛脱氢酶对不同醛类的催化活性 Vanillin:香草醛;Formaldehyde:甲醛;Acetaldehyde:乙醛;Glutaraldehyde:戊二醛;4-(dimethylamino)-benzaldehyde(PDAB):4-(二甲氨基)-苯甲醛
Fig. 8 Catalyzed activities of aldehyde dehydrogenase on different aldehydes

图9 多序列对比不同来源的醛脱氢酶的一级结构
Fig.9 Multiple sequences alignments of primary structures of aldehyde dehydrogenases from different sources Bursaphelenchus xylophilus:GenBank NO.CAD5152421.1;Bursaphelenchus okinawaensis:GenBank NO.CAD5218566.1;Trypanosoma grayi:GenBank NO.XP_009312368.1;Caenorhabditis elegans:GenBank NO. NP_741553.1;Mus musculus:GenBank NO.NP_001318043.1;Strongyloides ratti:GenBank NO. XP_024502805.1;Pseudomonas sp. HR199:GenBank NO. CAA06926.1;Schistocerca gregaria:GenBank NO.QVD39485.1;Meloidogyne graminicola:GenBank NO. KAF7633361.1;Ancylostoma caninum:GenBank NO.RCN51637.1;Micrococcus luteus NCTC 2665:GenBank NO. ACS29670.1
| [1] | Mota MM, Futai K, Vieira P. Pine wilt disease and the pinewood nematode, Bursaphelenchus xylophilus[M]// Ciancio A, Mukerji KG. Integrated Management of Fruit Crops Nematodes. Dordrecht: Springer Netherlands, 2009:253-274. |
| [2] |
Meng FL, Wang J, Wang X, et al. Expression analysis of thaumatin-like proteins from Bursaphelenchus xylophilus and Pinus massoniana[J]. Physiol Mol Plant Pathol, 2017, 100:178-184.
doi: 10.1016/j.pmpp.2017.10.002 URL |
| [3] |
Futai K. Pine wood nematode, Bursaphelenchus xylophilus[J]. Annu Rev Phytopathol, 2013, 51:61-83.
doi: 10.1146/annurev-phyto-081211-172910 URL |
| [4] |
Macho AP, Zipfel C. Plant PRRs and the activation of innate immune signaling[J]. Mol Cell, 2014, 54(2):263-272.
doi: 10.1016/j.molcel.2014.03.028 pmid: 24766890 |
| [5] |
Li Y, Hu LJ, Wu XQ, et al. A Bursaphelenchus xylophilus effector, Bx-FAR-1, suppresses plant defense and affects nematode infection of pine trees[J]. Eur J Plant Pathol, 2020, 157(3):637-650.
doi: 10.1007/s10658-020-02031-8 URL |
| [6] |
Holbein J, Grundler FMW, Siddique S. Plant basal resistance to Nematodes:an update[J]. J Exp Bot, 2016, 67(7):2049-2061.
doi: 10.1093/jxb/erw005 pmid: 26842982 |
| [7] |
Han ZM, Hong YD, Zhao BG. A study on pathogenicity of bacteria carried by pine wood Nematodes[J]. J Phytopathol, 2003, 151(11/12):683-689.
doi: 10.1046/j.1439-0434.2003.00790.x URL |
| [8] |
Zhang W, Yu HY, Lv YX, et al. Gene family expansion of pinewood nematode to detoxify its host defence chemicals[J]. Mol Ecol, 2020, 29(5):940-955.
doi: 10.1111/mec.15378 pmid: 32031723 |
| [9] |
Li Z, Zhang QW, Zhou XG. A 2-Cys peroxiredoxin in response to oxidative stress in the pine wood nematode, Bursaphelenchus xylophilus[J]. Sci Rep, 2016, 6:27438.
doi: 10.1038/srep27438 URL |
| [10] |
Fu HY, Ren JH, Huang L, et al. Screening and functional analysis of the peroxiredoxin specifically expressed in Bursaphelenchus xylophilus-the causative agent of pine wilt disease[J]. Int J Mol Sci, 2014, 15(6):10215-10232.
doi: 10.3390/ijms150610215 URL |
| [11] |
Xu CJ, Li CYT, Kong ANT. Induction of phase I, II and III drug metabolism/transport by xenobiotics[J]. Arch Pharm Res, 2005, 28(3):249-268.
pmid: 15832810 |
| [12] |
Yan X, Cheng XY, et al. Comparative transcriptomics of two pathogenic pinewood nematodes yields insights into parasitic adaptation to life on pine hosts[J]. Gene, 2012, 505(1):81-90.
doi: 10.1016/j.gene.2012.05.041 pmid: 22705985 |
| [13] |
Guengerich FP. Cytochrome p450 and chemical toxicology[J]. Chem Res Toxicol, 2008, 21(1):70-83.
pmid: 18052394 |
| [14] |
Xu XL, Wu XQ, Ye JR, et al. Molecular characterization and functional analysis of three pathogenesis-related cytochrome P450 genes from Bursaphelenchus xylophilus(Tylenchida:Aphelenchoidoidea)[J]. Int J Mol Sci, 2015, 16(3):5216-5234.
doi: 10.3390/ijms16035216 URL |
| [15] |
Wang LS, Zhang TT, Pan ZS, et al. The alcohol dehydrogenase with a broad range of substrate specificity regulates vitality and reproduction of the plant-parasitic nematode Bursaphelenchus xylophilus[J]. Parasitology, 2019, 146(4):497-505.
doi: 10.1017/S0031182018001695 URL |
| [16] |
Koppaka V, Thompson DC, Chen Y, et al. Aldehyde dehydrogenase inhibitors:a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application[J]. Pharmacol Rev, 2012, 64(3):520-539.
doi: 10.1124/pr.111.005538 URL |
| [17] |
Guo QQ, Du GC, Qi HT, et al. A nematicidal tannin from Punica granatum L. rind and its physiological effect on pine wood nematode(Bursaphelenchus xylophilus)[J]. Pestic Biochem Physiol, 2017, 135:64-68.
doi: 10.1016/j.pestbp.2016.06.003 URL |
| [18] |
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Anal Biochem, 1976, 72:248-254.
pmid: 942051 |
| [19] |
Okibe N, Amada K, Hirano S, et al. Gene cloning and characterization of aldehyde dehydrogenase from a petroleum-degrading bacterium, strain HD-1[J]. J Biosci Bioeng, 1999, 88(1):7-11.
pmid: 16232565 |
| [20] |
Leal NA, Havemann GD, Bobik TA. PduP is a coenzyme-a-acylating propionaldehyde dehydrogenase associated with the polyhedral bodies involved in B12-dependent 1, 2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2[J]. Arch Microbiol, 2003, 180(5):353-361.
doi: 10.1007/s00203-003-0601-0 URL |
| [21] | 赵振东, 李冬梅, 胡樨萼, 等. 抗松材线虫病马尾松种源化学成分与抗性机理研究(第Ⅱ报)- -马尾松种源抗性与中性萜类化合物组成差异关系研究[J]. 林产化学与工业, 2001, 21(1):56-60. |
| Zhao ZD, Li DM, Hu XE, et al. Study on chemical components and resistance mechanism to pine wood nematode of Masson pine provenance(ⅱ)-study on the components of neutral terpenoids and their differences among different resistant provenances of Pinus massoniana[J]. Chem Ind For Prod, 2001, 21(1):56-60. | |
| [22] | 喻方圆, 刘建兵, 胡晓健. 马尾松苗木中松醇的分布及其对干旱胁迫的响应[J]. 南京林业大学学报:自然科学版, 2009, 33(2):13-16. |
| Yu FY, Liu JB, Hu XJ. The distribution of pinitol in the Masson pine seedlings and its relation with drought stress[J]. J Nanjing For Univ Nat Sci Ed, 2009, 33(2):13-16. | |
| [23] | IKEDA T, ODA K. The occurrence of attractiveness for Monochamus alternatus hope(Coleoptera:Cerambycidae)in nematode-infected pine trees[J]. Nihon Ringakkai Shi/journal Jpn For Soc, 1980, 62:432-434. |
| [24] |
Zhao YH, Xu SY, Lu HB, et al. Effects of the plant volatile trans-2-hexenal on the dispersal ability, nutrient metabolism and enzymatic activities of Bursaphelenchus xylophilus[J]. Pestic Biochem Physiol, 2017, 143:147-153.
doi: 10.1016/j.pestbp.2017.08.004 URL |
| [25] |
Stiti N, Podgórska K, Bartels D. Aldehyde dehydrogenase enzyme ALDH3H1 from Arabidopsis thaliana:identification of amino acid residues critical for cofactor specificity[J]. Biochim Biophys Acta, 2014, 1844(3):681-693.
doi: 10.1016/j.bbapap.2014.01.008 pmid: 24463048 |
| [26] |
Corbier C, Della Seta F, Branlant G. A new chemical mechanism catalyzed by a mutated aldehyde dehydrogenase[J]. Biochemistry, 1992, 31(49):12532-12535.
pmid: 1463740 |
| [27] |
Schreiner L, Bauer P, Buettner A. Resolving the smell of wood - identification of odour-active compounds in Scots pine(Pinus sylvestris L.)[J]. Sci Rep, 2018, 8(1):8294.
doi: 10.1038/s41598-018-26626-8 pmid: 29844440 |
| [28] |
Kim JW, Im S, Jeong HR, et al. Neuroprotective effects of Korean red pine(Pinus densiflora)bark extract and its phenolics[J]. J Microbiol Biotechnol, 2018, 28(5):679-687.
doi: 10.4014/jmb.1801.01053 URL |
| [29] |
Li XM, Li YX, Wei DM, et al. Characterization of a broad-range aldehyde dehydrogenase involved in alkane degradation in Geobacillus thermodenitrificans NG80-2[J]. Microbiol Res, 2010, 165(8):706-712.
doi: 10.1016/j.micres.2010.01.006 URL |
| [1] | 苗永美, 苗翠苹, 于庆才. 枯草芽孢杆菌BBs-27发酵液性质及脂肽对黄色镰刀菌的抑菌作用[J]. 生物技术通报, 2023, 39(9): 255-267. |
| [2] | 张曼, 张叶卓, 何其邹洪, 鄂一岚, 李晔. 植物细胞壁结构及成像技术研究进展[J]. 生物技术通报, 2023, 39(7): 113-122. |
| [3] | 谢田朋, 张佳宁, 董永骏, 张建, 景明. 早期抽薹对当归根际土壤微环境的影响[J]. 生物技术通报, 2023, 39(7): 206-218. |
| [4] | 赵赛赛, 张小丹, 贾晓妍, 陶大炜, 刘可玉, 宁喜斌. 高产硝酸盐还原酶Staphylococcus simulans ZSJ6的复合诱变选育及其酶学性质研究[J]. 生物技术通报, 2023, 39(4): 103-113. |
| [5] | 杨俊钊, 张新蕊, 赵国柱, 郑菲. 新型GH5家族多结构域纤维素酶的结构与功能研究[J]. 生物技术通报, 2023, 39(4): 71-80. |
| [6] | 覃雪晶, 王雨涵, 曹一博, 张凌云. 青杄PwHAP5基因原核表达及多克隆抗体制备[J]. 生物技术通报, 2022, 38(8): 142-149. |
| [7] | 贺丽娜, 冯源, 石慧敏, 叶建仁. 具有杀线活性马尾松内生细菌的筛选与鉴定[J]. 生物技术通报, 2022, 38(8): 159-166. |
| [8] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [9] | 赵林艳, 官会林, 王克书, 卢燕磊, 向萍, 魏富刚, 杨绍周, 徐武美. 土壤含水量对三七连作土壤微生物群落的影响[J]. 生物技术通报, 2022, 38(7): 215-223. |
| [10] | 赵忠娟, 杨凯, 扈进冬, 魏艳丽, 李玲, 徐维生, 李纪顺. 盐胁迫条件下哈茨木霉ST02对椒样薄荷生长及根区土壤理化性质的影响[J]. 生物技术通报, 2022, 38(7): 224-235. |
| [11] | 易芳, 来鹏程, 郑希鳌, 胡帅, 高燕丽. Kod DNA聚合酶的制备及纯化研究[J]. 生物技术通报, 2022, 38(5): 183-190. |
| [12] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [13] | 王小琴, 黄银萍, 王蔚倩, 吴萍, 全舒. 含非天然氨基酸定点突变的MLL3SET蛋白表达与纯化[J]. 生物技术通报, 2022, 38(3): 194-202. |
| [14] | 常晴, 束月蓉, 王文韬, 蒋昊, 延泉德, 钱政, 高雪纯, 吴金鸿, 张勇. 来自Yeosuana marina sp. JLT21内切型海藻酸裂解酶的异源表达及酶学表征[J]. 生物技术通报, 2022, 38(2): 123-131. |
| [15] | 张丰文, 周丽亚, 董超, 史延茂. 纳豆中抗氧化肽的分离纯化与活性研究[J]. 生物技术通报, 2022, 38(2): 158-165. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||