








生物技术通报 ›› 2022, Vol. 38 ›› Issue (5): 183-190.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1210
收稿日期:2021-09-19
出版日期:2022-05-26
发布日期:2022-06-10
作者简介:易芳,女,硕士研究生,研究方向:植物分子生物学;E-mail: 基金资助:
YI Fang( ), LAI Peng-cheng, ZHENG Xi-ao, HU Shuai, GAO Yan-li(
), LAI Peng-cheng, ZHENG Xi-ao, HU Shuai, GAO Yan-li( )
)
Received:2021-09-19
Published:2022-05-26
Online:2022-06-10
摘要:
Kod DNA聚合酶作为常见的高保真DNA聚合酶,具有较强的DNA延伸能力以及3'-5'外切核酸酶校正活性。本研究通过对重组Kod DNA聚合酶分离纯化条件的优化,以期提高其表达和纯化效率以及片段扩增能力。利用异丙基硫代-β-D-半乳糖苷(isopropyl β-D-thiogalactoside,IPTG)对pET-30a-Kod进行诱导表达后,利用酶溶法和超声波破碎法对细胞破碎并提取粗酶液,然后利用Ni-NTA柱洗脱纯化获得较纯Kod DNA酶,再经透析得到高纯度Kod DNA酶,利用PCR反应和Sanger测序手段来检测自提纯化的Kod DNA聚合酶活性及保真性。同时,本研究对IPTG浓度和洗脱液中咪唑浓度等重要参数进行优化。结果表明,在28℃条件下0.1 mmol/L IPTG诱导16-18 h后,Kod DNA聚合酶高效表达;当咪唑浓度为200 mmol/L时蛋白洗脱效果最佳。PCR反应体系中Mg2+浓度为1.5 mmol/L,Kod DNA聚合酶浓度为0.061 25 μg/µL时,Kod DNA聚合酶效率较高,能有效地扩增3 194 bp的目的条带;通过序列比对,PCR产物序列中未引入任何突变。经优化制备后的Kod DNA聚合酶在酶活性和扩增保真性上均能达到商用高保真DNA聚合酶水平。本研究为节省实验室PCR成本,进一步开发和利用Kod DNA聚合酶奠定一定的基础。
易芳, 来鹏程, 郑希鳌, 胡帅, 高燕丽. Kod DNA聚合酶的制备及纯化研究[J]. 生物技术通报, 2022, 38(5): 183-190.
YI Fang, LAI Peng-cheng, ZHENG Xi-ao, HU Shuai, GAO Yan-li. Research on the Preparation and Purification of Kod DNA Polymerase[J]. Biotechnology Bulletin, 2022, 38(5): 183-190.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物大小 Product size/bp |
|---|---|---|
| AtVSR6 gDNA-F | ATGAGTCTCCAACCAATGAGAC | 3 194 |
| AtVSR6 gDNA-R | ACATTTCCCCAACCAATTCATTC- TCTGT |
表1 PCR扩增引物信息
Table 1 Information of the primers used for PCR
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 产物大小 Product size/bp |
|---|---|---|
| AtVSR6 gDNA-F | ATGAGTCTCCAACCAATGAGAC | 3 194 |
| AtVSR6 gDNA-R | ACATTTCCCCAACCAATTCATTC- TCTGT |
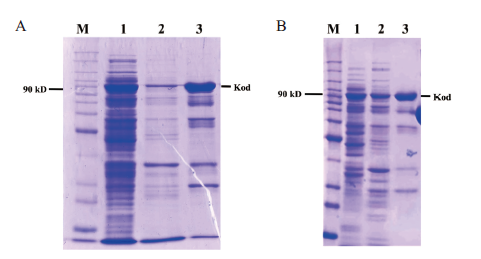
图1 不同浓度诱导剂IPTG对Kod DNA聚合酶表达的影响 不同浓度IPTG对Kod DNA聚合酶表达的影响。M:蛋白分子质量标准;A:0.1 mmol/L IPTG诱导Kod DNA聚合酶表达。1:Kod DNA聚合酶粗提液(CSKod);2:超声破碎后离心的残渣重悬液(CMKod);3:透析后经储存缓冲液1∶1稀释后的Kod DNA聚合酶原液。B:0.2 mmol/L IPTG诱导Kod DNA聚合酶表达。1:Kod DNA聚合酶粗提液(CSKod);2:超声破碎后离心的残渣重悬液(CMKod);3:透析后经储存缓冲液1∶1稀释后的Kod DNA聚合酶原液
Fig.1 Effects of different concentrations of IPTG on the expressions of Kod DNA polymerases The effects of different concentrations of IPTG on the expressions of Kod DNA polymerase. M:Protein molecular weight standard. A:0.1 mmol/L IPTG induces the expression of Kod DNA polymerase. 1:The crude extract of Kod DNA polymerase(CSKod);2:suspension of residue after sonication and centrifugation(CMKod);3:Kod DNA polymerase stock solution diluted with storage buffer at 1∶1 after dialysis. B. 0.2 mmol/L IPTG induces the expression of Kod DNA polymerase. 1:The crude extract of Kod DNA polymerase(CSKod);2:suspension of residue after sonication and centrifugation(CMKod);3:Kod DNA polymerase stock solution diluted with storage buffer at 1∶1 after dialysis
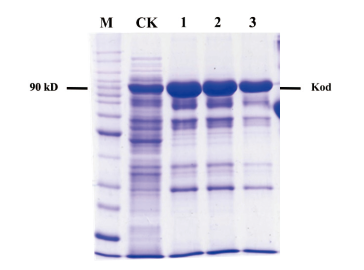
图2 不同浓度咪唑对Kod DNA聚合酶纯化的影响 含有不同浓度咪唑洗脱液对Kod DNA聚合酶纯化回收产量的影响。M:蛋白分子质量标准;1-3:500 mmol/L、200 mmol/L、100 mmol/L咪唑洗脱液;CK:Kod DNA聚合酶粗提液(CSKod)
Fig.2 Effects of different concentrations of imidazole on the purifications of Kod DNA polymerases The effect of different imidazole concentrations contained in elution buffer on the purification yield of Kod DNA polymerase. M:Protein molecule quality standard;1-3:500 mmol/L,200 mmol/L 100 mmol/L imidazole eluate;CK:the crude extracti of Kod DNA polymerase(CSKod)
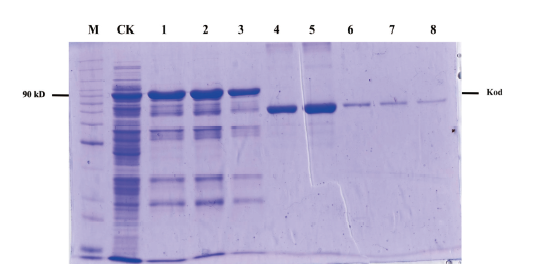
图3 优化条件后Kod DNA聚合酶的制备效果 优化诱导和提取条件后,Kod DNA聚合酶的制备效果。M:蛋白分子质量标准;CK:Kod DNA聚合酶粗提液(CSKod);1-3:200 mmol/L咪唑洗脱液洗脱第1次,第2次和第3次收集的Kod DNA聚合酶;4-8:浓度为0.5 mg/mL、1 mg/mL、0.25 mg/mL、0.125 mg/mL、0.0625 mg/mL的牛血清蛋白(BSA)
Fig.3 Preparation effect of Kod DNA polymerase after comprehensive optimization conditions Kod preparation after comprehensive optimization induction and purification conditions. M:Protein molecule quality standard. CK:The crude extraction of Kod DNA polymerase(CSKod);1-3:elution times using 200 mmol/L imidazole;4-8:Different concentrations of BSA(0.5 mg/mL,1 mg/mL,0.25 mg/mL,0.125 mg/mL,0.0625 mg/mL)
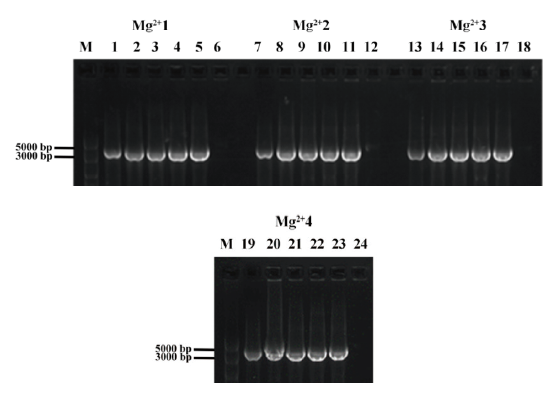
图4 Kod DNA聚合酶PCR反应体系中酶浓度及Mg2+浓度对目的基因扩增的影响 M:5 000 bp DNA分子质量标准;1-24:扩增长度约为3 194 bp的AtVSR6片段;1、7、13、19:使用市售商品化GO Tag高保真DNA聚合酶扩增结果;2-6、8-12、20-24:分别使用浓度为0.061 25 μg/µL、0.125 μg/µL、0.25 μg/µL、0.5 μg/µL、1 μg/µL的Kod DNA聚合酶扩增结果;Mg2+1、Mg2+2、Mg2+3、Mg2+4:分别使用浓度为1.5 mmol/L、1.75 mmol/L、2.0 mmol/L和2.25 mmol/L
Fig. 4 Effects of enzyme content and Mg2+ concentration on target gene amplification in Kod DNA polymerase reaction system M:DL5000 DNA marker;1-24:amplification of AtVSR6 fragments with a length of about 3 194 bp;1,7,13,19:amplification product with commercial GO Tag high-fidelity DNA polymerase;2-6,8-12,20-24:amplified results using different concentrations of Kod DNA polymerase(0.061 25 μg/µL,0.125 μg/µL,0.25 μg/µL,0.5μg/µL,and 1μg/µL);Mg2+1. Mg2+2,Mg2+3,Mg2+4:different concentrations of Mg2+(1.5 mmol/L,1.75 mmol/L,2.0 mmol/L and 2.25 mmol/L)
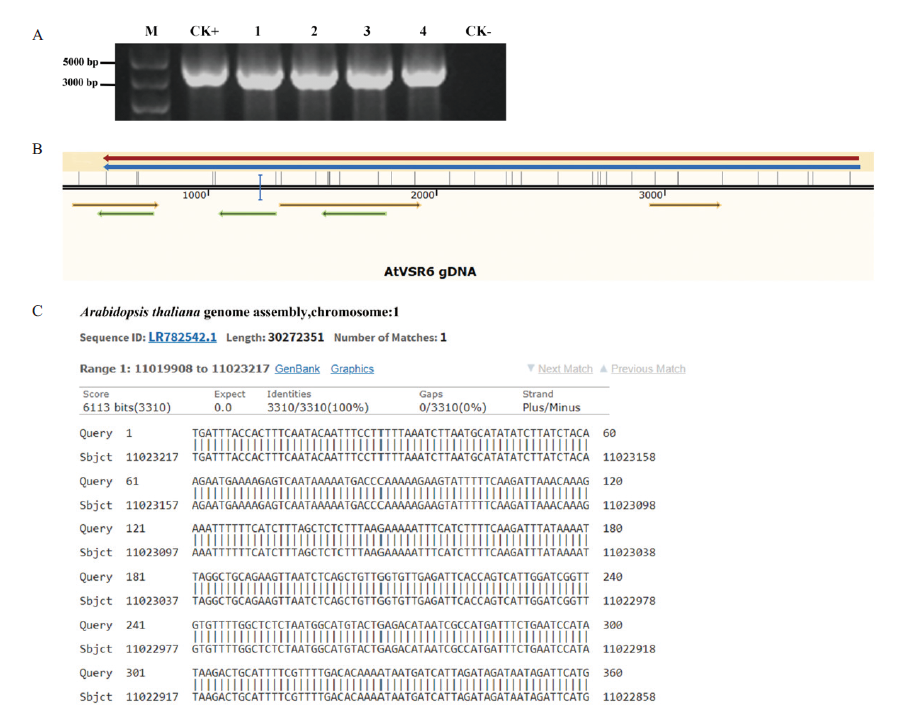
图5 Kod DNA聚合酶的保真性验证 A:M:5000 bp DNA分子质量标准;1-4:扩增片段长度为3 194 bp的AtVSR6片段;CK+:阳性对照;CK-:阴性对照。B,C:拟南芥AtVSR6基因测序结果与NCBI数据库中AtVSR6基因序列的比对结果
Fig.5 High-fidelity verification of Kod DNA polymerases A:M:DL5000 DNA marker;1-4:amplification of AtVSR6 fragments with a length of about 3 194 bp;CK+:positive control;CK-:negative control. B,C:The BLAST result between sequencing result of AtVSR6 gene in A. thaliana and AtVSR6 gene sequence in NCBI database
| [1] |
Bębenek A, Ziuzia-Graczyk I. Fidelity of DNA replication-a matter of proofreading[J]. Curr Genet, 2018, 64(5):985-996.
doi: 10.1007/s00294-018-0820-1 pmid: 29500597 |
| [2] |
Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase[J]. Science, 1988, 239(4839):487-491.
doi: 10.1126/science.2448875 pmid: 2448875 |
| [3] | 张立华, 刘莉平. 浅析PCR技术中使用的DNA聚合酶[J]. 生物学教学, 2015, 40(9):67-69. |
| Zhang LH, Liu LP. Analysis of DNA polymerase used in PCR technology[J]. Biol Teach, 2015, 40(9):67-69. | |
| [4] | Green MR, Sambrook J. E. coli DNA polymerase I and the klenow fragment[J]. Cold Spring Harb Protoc, 2020, 2020(5):100743. |
| [5] |
Chien A, Edgar DB, Trela JM. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus[J]. J Bacteriol, 1976, 127(3):1550-1557.
doi: 10.1128/jb.127.3.1550-1557.1976 pmid: 8432 |
| [6] |
Din RU, Khan MI, Jan A, et al. A novel approach for high-level expression and purification of GST-fused highly thermostable Taq DNA polymerase in Escherichia coli[J]. Arch Microbiol, 2020, 202(6):1449-1458.
doi: 10.1007/s00203-020-01860-9 URL |
| [7] |
Erlich HA, Arnheim N. Genetic analysis using the polymerase chain reaction[J]. Annu Rev Genet, 1992, 26(1):479-506.
doi: 10.1146/annurev.ge.26.120192.002403 URL |
| [8] | 汪峻岭, 李照丹, 徐兴伟, 等. PCR技术、抗酸染色法在肺结核病理学诊断中应用比较[J]. 中外医疗, 2016, 35(15):77-78. |
| Wang JL, Li ZD, Xu XW, et al. Application comparison of PCR technique and acid-fast staining in diagnosis of pulmonary tuberculosis pathology[J]. China Foreign Med Treat, 2016, 35(15):77-78. | |
| [9] | 李娟, 慕力, 韩兴乔, 等. PCR-SSP/PCR-SSO两种KIR基因分型方法的比较[J]. 生物医学工程与临床, 2021, 25(2):225-231. |
| Li J, Mu L, Han XQ, et al. Comparison of kir genotyping between pcr-ssp and pcr-sso[J]. Biomed Eng Clin Med, 2021, 25(2):225-231. | |
| [10] | 曾元凤, 肖移生, 梁莉, 等. 长片段基因FMNL2 PCR实验的体会[J]. 南昌大学学报:医学版, 2013, 53(4):19-21. |
| Zeng YF, Xiao YS, Liang L, et al. PCR amplification of long fragment gene FMNL2[J]. J Nanchang Univ:Med Sci, 2013, 53(4):19-21. | |
| [11] |
Kay T. Overview of thermostable DNA polymerases for classical PCR applications:from molecular and biochemical fundamentals to commercial systems[J]. Appl Microbiol Biotechnol, 2013, 97(24):10243-10254.
doi: 10.1007/s00253-013-5290-2 URL |
| [12] | 曹淑中, 裘丽珍. DNA结合蛋白DbpA影响DNA聚合酶性能的研究[J]. 复旦学报:自然科学版, 2015, 54(4):469-477, 541. |
| Cao SZ, Qiu LZ. Research of impact of DNA binding protein DbpA on the performance of pfu DNA polymerase[J]. J Fudan Univ:Nat Sci, 2015, 54(4):469-477, 541. | |
| [13] |
Takagi M, Nishioka M, Kakihara H, et al. Characterization of DNA polymerase from Pyrococcus sp. strain KOD1 and its application to PCR[J]. Appl Environ Microbiol, 1997, 63(11):4504-4510.
doi: 10.1128/aem.63.11.4504-4510.1997 URL |
| [14] |
Benson LM, Null AP, Muddiman DC. Advantages of Thermococcus kodakaraenis(KOD)DNA Polymerase for PCR-mass spectrometry based analyses[J]. J Am Soc Mass Spectrom, 2003, 14(6):601-604.
doi: 10.1016/S1044-0305(03)00148-X URL |
| [15] | 肖朝文, 杜娟, 李晚忱. Taq DNA聚合酶制备技术的优化[J]. 四川农业大学学报, 2004, 22(4):318-321. |
| Xiao CW, Du J, Li WC. Optimization of preparation techniques of taq DNA polymerase[J]. J Sichuan Agric Univ, 2004, 22(4):318-321. | |
| [16] | 王天云, 秦川, 杨瑞, 等. 基因重组Taq DNA聚合酶的制备[J]. 新乡医学院学报, 2007, 24(6):551-553. |
| Wang TY, Qin C, Yang R, et al. Preparation of recombinant Taq DNA polymerase[J]. J Xinxiang Med Coll, 2007, 24(6):551-553. | |
| [17] | 何钢, 王义强, 李樊, 等. 大肠杆菌表达重组Taq DNA聚合酶的分离和纯化[J]. 中南林学院学报, 2006, 26(5):50-54. |
| He G, Wang YQ, Li F, et al. Separation and purification of taq DNA polymerase in E. coli[J]. J Central South For Univ, 2006, 26(5):50-54. | |
| [18] | 丁燕华, 刘树涛, 齐庆远. Taq DNA聚合酶的热纯化制备[J]. 安徽农业科学, 2011, 39(17):10153-10155. |
| Ding YH, Liu ST, Qi QY. Preparation of taq DNA polymerase by thermal purification[J]. J Anhui Agric Sci, 2011, 39(17):10153-10155. | |
| [19] | 刘钦松, 刘孟刚, 张丛丛, 等. 重组Taq DNA聚合酶的表达和纯化鉴定[J]. 江西农业学报, 2012, 24(5):124-126. |
| Liu QS, Liu MG, Zhang CC, et al. Expression, purification and identification of recombinant taq DNA polymerase[J]. Acta Agric Jiangxi, 2012, 24(5):124-126. | |
| [20] | 祁浩, 刘新利. 大肠杆菌表达系统和酵母表达系统的研究进展[J]. 安徽农业科学, 2016, 44(17):4-6, 52. |
| Qi H, Liu XL. Research progress of expression systems of Escherichia coli and yeast[J]. J Anhui Agric Sci, 2016, 44(17):4-6, 52. | |
| [21] | 张云鹏, 温彤, 姜伟. 大肠杆菌和酵母表达系统的研究进展[J]. 生物技术进展, 2014, 4(6):389-393. |
|
Zhang YP, Wen T, Jiang W. The research progress of Escherichia coli expression systems and yeast expression systems[J]. Curr Biotechnol, 2014, 4(6):389-393.
doi: 10.2174/2211550105666151107001338 URL |
|
| [22] |
Yeon YJ, Park HJ, Park HY, et al. Effect of His-tag location on the catalytic activity of 3-hydroxybutyrate dehydrogenase[J]. Biotechnol Bioprocess Eng, 2014, 19(5):798-802.
doi: 10.1007/s12257-014-0089-2 URL |
| [23] |
Chen Z, Li Y, Yuan Q. Study the effect of His-tag on chondroitinase ABC I based on characterization of enzyme[J]. Int J Biol Macromol, 2015, 78:96-101.
doi: 10.1016/j.ijbiomac.2015.03.068 URL |
| [24] |
Chen Y, Li Y, Liu P, et al. Optimized expression in Pichia pastoris eliminates common protein contaminants from subsequent His-tag purification[J]. Biotechnol Lett, 2014, 36(4):711-718.
doi: 10.1007/s10529-013-1411-3 URL |
| [25] | 周彦霞, 孔慧芳, 赵商岐, 等. pET30a-EgG1Y162质粒的构建及目的蛋白的表达和纯化[J]. 中国病原生物学杂志, 2021, 16(3):287-291. |
| Zhou YX, Kong HF, Zhao SQ, et al. Construction of a pET30a-EgG1Y162 plasmid and purification, identification, and expression of the recombinant protein[J]. J Pathog Biol, 2021, 16(3):287-291. | |
| [26] | 胡兰兰, 田亚楠, 陈福生, 等. 重组大肠杆菌E. coli BL21/pET28a(+)-mle产苹果酸乳酸酶发酵条件的优化[J]. 中国酿造, 2018, 37(6):161-164. |
| Hu LL, Tian YN, Chen FS, et al. Optimization of fermentation conditions for malolactic enzyme production by recombinant E. coli BL21/p ET28a(+)-mle[J]. China Brew, 2018, 37(6):161-164. | |
| [27] |
Stefani M, Dobson CM. Protein aggregation and aggregate toxicity:new insights into protein folding, misfolding diseases and biological evolution[J]. J Mol Med, 2003, 81(11):678-699.
doi: 10.1007/s00109-003-0464-5 URL |
| [28] |
Ventura S. Sequence determinants of protein aggregation:tools to increase protein solubility[J]. Microb Cell Fact, 2005, 4(1):11.
doi: 10.1186/1475-2859-4-11 URL |
| [29] | 牟筱, 宗惠, 宫皓, 等. 重组大肠杆菌表达外源蛋白包涵体复性的研究进展[J]. 甘肃畜牧兽医, 2018, 48(4):35-37. |
| Mou X, Zong H, Gong H, et al. Progress in renaturation of foreign protein inclusion bodies expressed in recombinant Escherichia coli[J]. Gansu Animal Husb Vet, 2018, 48(4):35-37. | |
| [30] |
Dyson MR, Shadbolt SP, Vincent KJ, et al. Production of soluble mammalian proteins in Escherichia coli:identification of protein features that correlate with successful expression[J]. BMC Biotechnol, 2004, 4:32.
doi: 10.1186/1472-6750-4-32 URL |
| [31] | 陈颜亮, 郑治, 王剑龙, 等. 带Myc、His标签的SPAG4L真核表达载体的构建与表达[J]. 生物工程学报, 2013, 29(11):1654-1662. |
| Chen YL, Zheng Z, Wang JL, et al. Construction of eukaryotic expression vector of SPAG4L tagged with Myc and His[J]. Chin J Biotechnol, 2013, 29(11):1654-1662. | |
| [32] | 贾平. 高纯度Taq DNA聚合酶制备技术研究[D]. 南京: 南京理工大学, 2013. |
| Jia P. Study on preparation of high-purity taq DNA polymerase[D]. Nanjing: Nanjing University of Science and Technology, 2013. |
| [1] | 陈晓雨, 张建, 张新亚, 唐雨婷, 邵钰晨, 罗志丹, 卢辰. 一种快速精确测定Tth DNA聚合酶活性的方法[J]. 生物技术通报, 2021, 37(5): 281-286. |
| [2] | 罗中钦, 程琳, 张茜, 陈国华. 丝状真菌PCR模板DNA的快速制备方法[J]. 生物技术通报, 2015, 31(9): 79-83. |
| [3] | 梁雪莲;关彩蝶;柳勇;廖伯强;. 耐热DNA聚合酶基因双元载体的初步构建[J]. , 2009, 0(10): 189-193. |
| [4] | 张国广;陈亮;吴亦亮;曾雅明;. 利用高保真酶扩增特性调整基因读码框的方法[J]. , 2006, 0(03): 54-57. |
| [5] | B.G.Cassidy. 花生条纹病毒外壳蛋白基因在烟草中的表达与抗性[J]. , 1996, 0(06): 16-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||