








生物技术通报 ›› 2022, Vol. 38 ›› Issue (8): 24-31.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0102
沈月1,2( ), 陶宝杰1,2, 华夏1,2, 吕冰2, 刘立军1, 陈云1,2(
), 陶宝杰1,2, 华夏1,2, 吕冰2, 刘立军1, 陈云1,2( )
)
收稿日期:2022-01-22
出版日期:2022-08-26
发布日期:2022-09-14
作者简介:沈月,女,硕士,研究方向:植物细胞信号转导;E-mail: 基金资助:
SHEN Yue1,2( ), TAO Bao-jie1,2, HUA Xia1,2, LV Bing2, LIU Li-jun1, CHEN Yun1,2(
), TAO Bao-jie1,2, HUA Xia1,2, LV Bing2, LIU Li-jun1, CHEN Yun1,2( )
)
Received:2022-01-22
Published:2022-08-26
Online:2022-09-14
摘要:
独脚金内酯是调控植物根系生长的新型植物激素,在刺激寄生植物种子萌发、促进丛枝菌根真菌菌丝分枝以及调节植物分枝等过程中发挥着重要的作用。根系是植物感知土壤信号以及吸收养分、水分和矿质元素等的重要器官。外部环境和内部激素均能影响植物根系的生长发育。独脚金内酯可以抑制生长素转运而增加分生区及过渡区大小调控主根伸长、抑制侧根原基的发生和侧根发育,该过程同时依赖于细胞分裂素受体AHK3,也可以促进乙烯的合成和生长素转运及其受体TIR1的表达而调控根毛伸长。综述了独脚金内酯的结构、功能及其与生长素和细胞分裂素等植物激素互作调控主根伸长、侧根发生、根毛的发生和伸长等过程,以期为阐明独脚金内酯调控植物根系生长的机制提供理论与实践依据。
沈月, 陶宝杰, 华夏, 吕冰, 刘立军, 陈云. 独脚金内酯与激素互作调控根系生长的研究进展[J]. 生物技术通报, 2022, 38(8): 24-31.
SHEN Yue, TAO Bao-jie, HUA Xia, LV Bing, LIU Li-jun, CHEN Yun. Research Progress in the Interactions of Strigolactone with Hormones on Regulating Root Growth[J]. Biotechnology Bulletin, 2022, 38(8): 24-31.
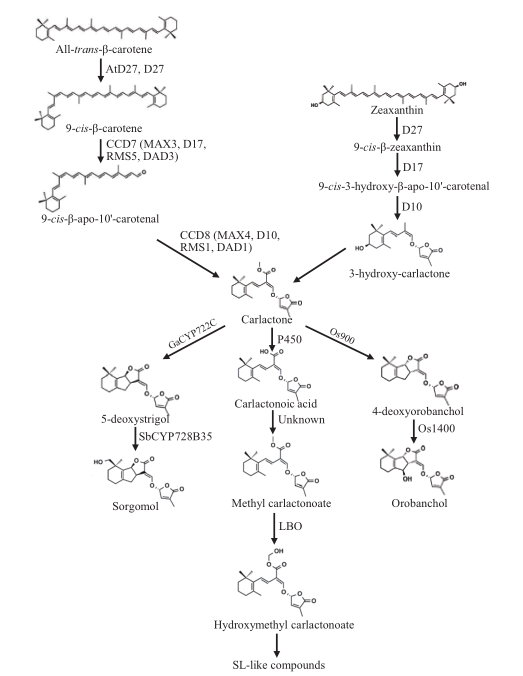
图2 独脚金内酯的生物合成通路 独脚金内酯合成基因在不同物种中的命名。拟南芥:MAX;水稻:D;豌豆:RMS;矮牵牛:DAD。生物合成通路引自文献[9,18]
Fig.2 Biosynthesis pathway of strigolactone The genes of strigolactone biosynthesis are identified and named MAX(MORE AXILLARY GROWTH)in Arabidopsis,D(DWARF)in rice,RMS(RAMOSUS)in pea and DAD(DECREASED APICAL DOMINANCE)in petunia. Biosynthesis pathway cited from references[9,18]
| 功能 Function | 生理/分子反应 Physiological/molecular response | 参考文献 Reference |
|---|---|---|
| 丛枝菌根真菌菌丝分枝 | BRC1表达上调,促进养分胁迫下营养物质的吸收 | [ |
| 植物分枝 | BRC1表达上调 | [ |
| 种子萌发 | 激活CYP707A1表达,促进DELLA降解 | [ |
| 主根发育及伸长 | 依赖MAX2,与生长素互作 | [ |
| 侧根的形成 | PIN1表达降低,与生长素、细胞分裂素互作 | [ |
| 不定根的形成 | 生长素含量及敏感性降低、细胞分裂素含量增加 | [ |
| 根毛的发生与伸长 | 依赖MAX2,与生长素、乙烯互作 | [ |
| 叶片衰老 | SAG表达上调 | [ |
| 调节植物次生生长 | 细胞分裂增强、形成层活性提高 | [ |
| 光形态建成 | 与隐花色素、光敏色素STH7/BBX20互作 | [ |
| 养分胁迫 | PDR1表达上调 | [ |
| 干旱胁迫 | 活性氧清除剂、诱导气孔关闭减少蒸腾作用 | [ |
| 低温胁迫 | D10表达上调 | [ |
| 盐胁迫 | MDA、H2O2、ROS等含量降低 | [ |
| 生物胁迫 | 增加对病原菌的敏感性 | [ |
表1 独脚金内酯的功能及其生理或分子反应
Table 1 Functions and physiological/molecular responses of strigolactone
| 功能 Function | 生理/分子反应 Physiological/molecular response | 参考文献 Reference |
|---|---|---|
| 丛枝菌根真菌菌丝分枝 | BRC1表达上调,促进养分胁迫下营养物质的吸收 | [ |
| 植物分枝 | BRC1表达上调 | [ |
| 种子萌发 | 激活CYP707A1表达,促进DELLA降解 | [ |
| 主根发育及伸长 | 依赖MAX2,与生长素互作 | [ |
| 侧根的形成 | PIN1表达降低,与生长素、细胞分裂素互作 | [ |
| 不定根的形成 | 生长素含量及敏感性降低、细胞分裂素含量增加 | [ |
| 根毛的发生与伸长 | 依赖MAX2,与生长素、乙烯互作 | [ |
| 叶片衰老 | SAG表达上调 | [ |
| 调节植物次生生长 | 细胞分裂增强、形成层活性提高 | [ |
| 光形态建成 | 与隐花色素、光敏色素STH7/BBX20互作 | [ |
| 养分胁迫 | PDR1表达上调 | [ |
| 干旱胁迫 | 活性氧清除剂、诱导气孔关闭减少蒸腾作用 | [ |
| 低温胁迫 | D10表达上调 | [ |
| 盐胁迫 | MDA、H2O2、ROS等含量降低 | [ |
| 生物胁迫 | 增加对病原菌的敏感性 | [ |
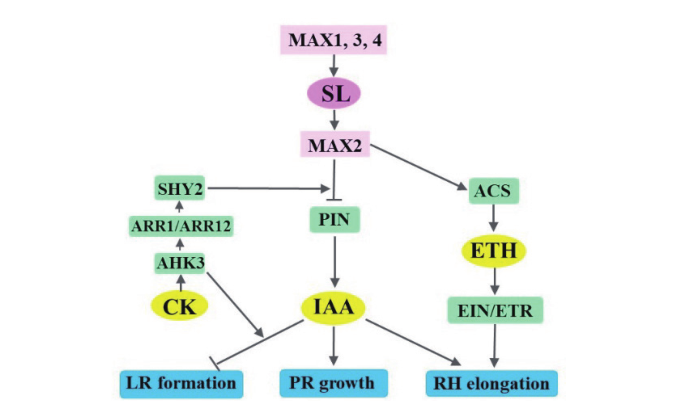
图3 独脚金内酯、生长素、细胞分裂素和乙烯对根系生长的协同调节 PR:主根;LR:侧根;RH:根毛;IAA:生长素;CK:细胞分裂素;ETH:乙烯;SL:独脚金内酯
Fig.3 Synergetic regulation of the root growth by strigola-ctone,auxin,cytokinin and ethylene PR:Primary root;LR:lateral root;RH:root hair;IAA:auxin;CK:cytokinin;ETH:ethylene;SL:strigolactone
| [1] |
Cook CE, Whichard LP, Turner B, et al. Germination of witchweed(Striga lutea Lour. ):isolation and properties of a potent stimulant[J]. Science, 1966, 154(3753):1189-1190.
pmid: 17780042 |
| [2] |
Yokota T, Sakai H, Okuno K, et al. Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover[J]. Phytochemistry, 1998, 49(7):1967-1973.
doi: 10.1016/S0031-9422(98)00419-1 URL |
| [3] |
Akiyama K, Matsuzaki KI, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi[J]. Nature, 2005, 435(7043):824-827.
doi: 10.1038/nature03608 URL |
| [4] |
Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching[J]. Nature, 2008, 455(7210):189-194.
doi: 10.1038/nature07271 URL |
| [5] |
Umehara M, Hanada A, Yoshida S, et al. Inhibition of shoot branching by new terpenoid plant hormones[J]. Nature, 2008, 455(7210):195-200.
doi: 10.1038/nature07272 URL |
| [6] |
Ruyter-Spira C, Al-Babili S, van der Krol S, et al. The biology of strigolactones[J]. Trends Plant Sci, 2013, 18(2):72-83.
doi: 10.1016/j.tplants.2012.10.003 pmid: 23182342 |
| [7] |
Zwanenburg B, Pospíšil T, Zeljković SĆ. Strigolactones:new plant hormones in action[J]. Planta, 2016, 243(6):1311-1326.
doi: 10.1007/s00425-015-2455-5 pmid: 26838034 |
| [8] |
Yoneyama K. Recent progress in the chemistry and biochemistry of strigolactones[J]. J Pestic Sci, 2020, 45(2):45-53.
doi: 10.1584/jpestics.D19-084 pmid: 32508512 |
| [9] |
Yoneyama K, Brewer PB. Strigolactones, how are they synthesized to regulate plant growth and development?[J]. Curr Opin Plant Biol, 2021, 63:102072.
doi: 10.1016/j.pbi.2021.102072 URL |
| [10] |
姚瑞枫, 谢道昕. 独脚金内酯信号途径的新发现——抑制子也是转录因子[J]. 植物学报, 2020, 55(4):397-402.
doi: 10.11983/CBB20099 |
| Yao RF, Xie DX. New insight into strigolactone signaling[J]. Chin Bull Bot, 2020, 55(4):397-402. | |
| [11] |
Mashiguchi K, Seto Y, Yamaguchi S. Strigolactone biosynthesis, transport and perception[J]. Plant J, 2021, 105(2):335-350.
doi: 10.1111/tpj.15059 URL |
| [12] |
Matusova R, Rani K, Verstappen FWA, et al. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway[J]. Plant Physiol, 2005, 139(2):920-934.
doi: 10.1104/pp.105.061382 URL |
| [13] |
Baz L, Mori N, Mi JN, et al. 3-hydroxycarlactone, a novel product of the strigolactone biosynthesis core pathway[J]. Mol Plant, 2018, 11(10):1312-1314.
doi: 10.1016/j.molp.2018.06.008 URL |
| [14] |
Abe S, Sado A, Tanaka K, et al. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro[J]. Proc Natl Acad Sci USA, 2014, 111(50):18084-18089.
doi: 10.1073/pnas.1410801111 URL |
| [15] |
Zhang YX, Cheng X, Wang YT, et al. The tomato MAX1 homolog, SlMAX1, is involved in the biosynthesis of tomato strigolactones from carlactone[J]. New Phytol, 2018, 219(1):297-309.
doi: 10.1111/nph.15131 URL |
| [16] |
Wakabayashi T, Shida K, Kitano Y, et al. CYP722C from Gossypium arboreum catalyzes the conversion of carlactonoic acid to 5-deoxystrigol[J]. Planta, 2020, 251(5):97.
doi: 10.1007/s00425-020-03390-6 pmid: 32306106 |
| [17] |
Wakabayashi T, Ishiwa S, Shida K, et al. Identification and characterization of sorgomol synthase in sorghum strigolactone biosynthesis[J]. Plant Physiol, 2021, 185(3):902-913.
doi: 10.1093/plphys/kiaa113 pmid: 33793911 |
| [18] |
Al-Babili S, Bouwmeester HJ. Strigolactones, a novel carotenoid-derived plant hormone[J]. Annu Rev Plant Biol, 2015, 66:161-186.
doi: 10.1146/annurev-arplant-043014-114759 pmid: 25621512 |
| [19] | 马敏艳. 独脚金内酯(±)-GR24及其类似物的合成研究[D]. 西安: 陕西师范大学, 2016. |
| Ma MY. Synthesis of strigolactone(±)-GR24 and its analogus[D]. Xi'an: Shaanxi Normal University, 2016. | |
| [20] |
Bromhead LJ, Visser J, McErlean CSP. Enantioselective synthesis of the strigolactone mimic(+)-GR24[J]. J Org Chem, 2014, 79(3):1516-1520.
doi: 10.1021/jo402722p pmid: 24422520 |
| [21] |
Dun EA, de Saint Germain A, Rameau C, et al. Dynamics of strigolactone function and shoot branching responses in Pisum sativum[J]. Mol Plant, 2013, 6(1):128-140.
doi: 10.1093/mp/sss131 URL |
| [22] |
Gutjahr C, Parniske M. Cell and developmental biology of arbuscular mycorrhiza symbiosis[J]. Annu Rev Cell Dev Biol, 2013, 29:593-617.
doi: 10.1146/annurev-cellbio-101512-122413 URL |
| [23] |
Schmitz AM, Harrison MJ. Signaling events during initiation of arbuscular mycorrhizal symbiosis[J]. J Integr Plant Biol, 2014, 56(3):250-261.
doi: 10.1111/jipb.12155 |
| [24] |
Schwartz SH, Qin XQ, Loewen MC. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching[J]. J Biol Chem, 2004, 279(45):46940-46945.
doi: 10.1074/jbc.M409004200 pmid: 15342640 |
| [25] |
Marzec M. Strigolactones and gibberellins:a new couple in the phytohormone world?[J]. Trends Plant Sci, 2017, 22(10):813-815.
doi: S1360-1385(17)30174-7 pmid: 28844847 |
| [26] |
Jain A, Poling MD, Karthikeyan AS, et al. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis[J]. Plant Physiol, 2007, 144(1):232-247.
doi: 10.1104/pp.106.092130 URL |
| [27] |
Ruyter-Spira C, Kohlen W, Charnikhova T, et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis:another belowground role for strigolactones?[J]. Plant Physiol, 2011, 155(2):721-734.
doi: 10.1104/pp.110.166645 pmid: 21119044 |
| [28] |
Bennett T, Sieberer T, Willett B, et al. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport[J]. Curr Biol, 2006, 16(6):553-563.
doi: 10.1016/j.cub.2006.01.058 pmid: 16546078 |
| [29] |
Brewer PB, Dun EA, Ferguson BJ, et al. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis[J]. Plant Physiol, 2009, 150(1):482-493.
doi: 10.1104/pp.108.134783 URL |
| [30] |
Crawford S, Shinohara N, Sieberer T, et al. Strigolactones enhance competition between shoot branches by dampening auxin transport[J]. Development, 2010, 137(17):2905-2913.
doi: 10.1242/dev.051987 pmid: 20667910 |
| [31] |
Kapulnik Y, Delaux PM, Resnick N, et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis[J]. Planta, 2011, 233(1):209-216.
doi: 10.1007/s00425-010-1310-y pmid: 21080198 |
| [32] |
Kumar M, Pandya-Kumar N, Kapulnik Y, et al. Strigolactone signaling in root development and phosphate starvation[J]. Plant Signal Behav, 2015, 10(7):e1045174.
doi: 10.1080/15592324.2015.1045174 URL |
| [33] |
Pandya-Kumar N, Shema R, Kumar M, et al. Strigolactone analog GR24 triggers changes in PIN2 polarity, vesicle trafficking and actin filament architecture[J]. New Phytol, 2014, 202(4):1184-1196.
doi: 10.1111/nph.12744 pmid: 24571327 |
| [34] |
Jiang LX, Matthys C, Marquez-Garcia B, et al. Strigolactones spatially influence lateral root development through the cytokinin signaling network[J]. J Exp Bot, 2016, 67(1):379-389.
doi: 10.1093/jxb/erv478 URL |
| [35] |
Rasmussen A, Mason MG, de Cuyper C, et al. Strigolactones suppress adventitious rooting in Arabidopsis and pea[J]. Plant Physiol, 2012, 158(4):1976-1987.
doi: 10.1104/pp.111.187104 pmid: 22323776 |
| [36] |
Foo E, Bullier E, Goussot M, et al. The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea[J]. Plant Cell, 2005, 17(2):464-474.
doi: 10.1105/tpc.104.026716 URL |
| [37] |
Hayward A, Stirnberg P, Beveridge C, et al. Interactions between auxin and strigolactone in shoot branching control[J]. Plant Physiol, 2009, 151(1):400-412.
doi: 10.1104/pp.109.137646 pmid: 19641034 |
| [38] |
Agusti J, Herold S, Schwarz M, et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants[J]. Proc Natl Acad Sci USA, 2011, 108(50):20242-20247.
doi: 10.1073/pnas.1111902108 URL |
| [39] |
Kapulnik Y, Resnick N, Mayzlish-Gati E, et al. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis[J]. J Exp Bot, 2011, 62(8):2915-2924.
doi: 10.1093/jxb/erq464 pmid: 21307387 |
| [40] |
Mayzlish-Gati E, De-Cuyper C, Goormachtig S, et al. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis[J]. Plant Physiol, 2012, 160(3):1329-1341.
doi: 10.1104/pp.112.202358 pmid: 22968830 |
| [41] |
Koltai H, Dor E, Hershenhorn J, et al. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers[J]. J Plant Growth Regul, 2010, 29(2):129-136.
doi: 10.1007/s00344-009-9122-7 URL |
| [42] |
Yamada Y, Furusawa S, Nagasaka S, et al. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency[J]. Planta, 2014, 240(2):399-408.
doi: 10.1007/s00425-014-2096-0 URL |
| [43] |
de Saint Germain A, Ligerot Y, Dun EA, et al. Strigolactones stimulate internode elongation independently of gibberellins[J]. Plant Physiol, 2013, 163(2):1012-1025.
doi: 10.1104/pp.113.220541 URL |
| [44] |
Thussagunpanit J, Nagai Y, Nagae M, et al. Involvement of STH7 in light-adapted development in Arabidopsis thaliana promoted by both strigolactone and karrikin[J]. Biosci Biotechnol Biochem, 2017, 81(2):292-301.
doi: 10.1080/09168451.2016.1254536 URL |
| [45] |
Jia KP, Luo Q, He SB, et al. Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis[J]. Mol Plant, 2014, 7(3):528-540.
doi: 10.1093/mp/sst093 URL |
| [46] |
Kretzschmar T, Kohlen W, Sasse J, et al. A Petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching[J]. Nature, 2012, 483(7389):341-344.
doi: 10.1038/nature10873 URL |
| [47] |
Liu JW, He HZ, Vitali M, et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots:exploring the interaction between strigolactones and ABA under abiotic stress[J]. Planta, 2015, 241(6):1435-1451.
doi: 10.1007/s00425-015-2266-8 URL |
| [48] |
Min Z, Li RY, Chen L, et al. Alleviation of drought stress in grapevine by foliar-applied strigolactones[J]. Plant Physiol Biochem, 2019, 135:99-110.
doi: 10.1016/j.plaphy.2018.11.037 URL |
| [49] |
Sedaghat M, Tahmasebi-Sarvestani Z, Emam Y, et al. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought[J]. Plant Physiol Biochem, 2017, 119:59-69.
doi: 10.1016/j.plaphy.2017.08.015 URL |
| [50] |
Ha CV, Leyva-González MA, Osakabe Y, et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress[J]. Proc Natl Acad Sci USA, 2014, 111(2):851-856.
doi: 10.1073/pnas.1322135111 URL |
| [51] |
Ling FL, Su QW, Jiang H, et al. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings[J]. Sci Rep, 2020, 10(1):6183.
doi: 10.1038/s41598-020-63352-6 URL |
| [52] |
Cheng X, Floková K, Bouwmeester H, et al. The role of endogenous strigolactones and their interaction with ABA during the infection process of the parasitic weed Phelipanche ramosa in tomato plants[J]. Front Plant Sci, 2017, 8:392.
doi: 10.3389/fpls.2017.00392 pmid: 28392795 |
| [53] |
Torres-Vera R, García JM, Pozo MJ, et al. Do strigolactones contribute to plant defence?[J]. Mol Plant Pathol, 2014, 15(2):211-216.
doi: 10.1111/mpp.12074 pmid: 24112811 |
| [54] |
Koren D, Resnick N, Gati EM, et al. Strigolactone signaling in the endodermis is sufficient to restore root responses and involves SHORT HYPOCOTYL 2(SHY2)activity[J]. New Phytol, 2013, 198(3):866-874.
doi: 10.1111/nph.12189 URL |
| [55] |
Kumar M, Pandya-Kumar N, Dam A, et al. Arabidopsis response to low-phosphate conditions includes active changes in actin filaments and PIN2 polarization and is dependent on strigolactone signalling[J]. J Exp Bot, 2015, 66(5):1499-1510.
doi: 10.1093/jxb/eru513 URL |
| [56] |
Dello Ioio R, Linhares FS, Scacchi E, et al. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation[J]. Curr Biol, 2007, 17(8):678-682.
doi: 10.1016/j.cub.2007.02.047 URL |
| [57] |
Rehman NU, Li X, Zeng PC, et al. Harmony but not uniformity:role of strigolactone in plants[J]. Biomolecules, 2021, 11(11):1616.
doi: 10.3390/biom11111616 URL |
| [58] |
Li WQ, Nguyen KH, Ha CV, et al. Crosstalk between the cytokinin and MAX2 signaling pathways in growth and callus formation of Arabidopsis thaliana[J]. Biochem Biophys Res Commun, 2019, 511(2):300-306.
doi: 10.1016/j.bbrc.2019.02.038 URL |
| [59] |
Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root hair development in Arabidopsis thaliana[J]. Plant J, 1995, 8(6):943-948.
pmid: 8580964 |
| [60] |
Rahman A, Hosokawa S, Oono Y, et al. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators[J]. Plant Physiol, 2002, 130(4):1908-1917.
doi: 10.1104/pp.010546 URL |
| [61] | 纠松涛, 徐岩, 张才喜, 等. 独脚金内酯及其调控植物根系生长发育的研究进展[J]. 分子植物育种, 2021, 19(15):5164-5171. |
| Jiu ST, Xu Y, Zhang CX, et al. Reasearch advancements on strigolactones and its regulatory effect on root growth and development in plants[J]. Mol Plant Breed, 2021, 19(15):5164-5171. | |
| [62] |
Ito S, Yamagami D, Umehara M, et al. Regulation of strigolactone biosynthesis by gibberellin signaling[J]. Plant Physiol, 2017, 174(2):1250-1259.
doi: 10.1104/pp.17.00301 URL |
| [63] |
Lantzouni O, Klermund C, Schwechheimer C. Largely additive effects of gibberellin and strigolactone on gene expression in Arabidopsis thaliana seedlings[J]. Plant J, 2017, 92(5):924-938.
doi: 10.1111/tpj.13729 URL |
| [1] | 高聪, 萧楚健, 鲁帅, 王苏蓉, 袁卉华, 曹云英. 氧化石墨烯对拟南芥生长的促进作用[J]. 生物技术通报, 2022, 38(6): 120-128. |
| [2] | 周希萌, 付春, 马长乐, 王兴军, 赵传志. 作物分枝的分子调控研究进展[J]. 生物技术通报, 2021, 37(3): 107-114. |
| [3] | 郭宾会, 戴毅, 宋丽. 干旱下植物激素影响作物根系发育的研究进展[J]. 生物技术通报, 2018, 34(7): 48-56. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||