








生物技术通报 ›› 2023, Vol. 39 ›› Issue (10): 136-147.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0339
陈浩婷1( ), 张玉静1, 刘洁1, 代泽敏1, 刘伟1, 石玉1, 张毅1(
), 张玉静1, 刘洁1, 代泽敏1, 刘伟1, 石玉1, 张毅1( ), 李天来1,2(
), 李天来1,2( )
)
收稿日期:2023-04-11
出版日期:2023-10-26
发布日期:2023-11-28
通讯作者:
张毅,男,博士,教授,研究方向:设施蔬菜栽培与生理;E-mail: harmony1228@163.com;作者简介:陈浩婷,女,博士研究生,研究方向:设施蔬菜栽培与生理;E-mail: 746852776@qq.com
基金资助:
CHEN Hao-ting1( ), ZHANG Yu-jing1, LIU Jie1, DAI Ze-min1, LIU Wei1, SHI Yu1, ZHANG Yi1(
), ZHANG Yu-jing1, LIU Jie1, DAI Ze-min1, LIU Wei1, SHI Yu1, ZHANG Yi1( ), LI Tian-lai1,2(
), LI Tian-lai1,2( )
)
Received:2023-04-11
Published:2023-10-26
Online:2023-11-28
摘要:
本研究旨在分析番茄转录因子WRKY6在低磷胁迫应答中的作用,为研究番茄耐低磷分子机制及挖掘提高番茄低磷耐受性和磷素利用率的基因资源奠定理论基础。试验以野生型番茄Ailsa Craig为材料,以其cDNA为模板克隆基因SlWRKY6,利用根癌农杆菌介导转化法构建RNAi-SlWRKY6和OE-SlWRKY6转基因番茄株系,并对野生型、RNAi-SlWRKY6和OE-SlWRKY63种不同基因型的番茄植株进行低磷胁迫处理,在处理第18天对叶片与根系进行表型观察和生理生化指标测定。表型观察结果显示,与RNAi-SlWRKY6转基因番茄相比,OE-SlWRKY6转基因番茄植株较矮壮,叶片数量较多,受低磷胁迫影响较小,表现出较强的耐低磷性。生理生化指标分析结果表明,与野生型相比,OE-SlWRKY6转基因番茄根系与叶片的有机磷和总磷含量显著升高,根系酸性磷酸酶活性和部分有机酸含量显著增加,磷转运体表达量显著降低。而RNAi-SlWRKY6转基因番茄受到低磷胁迫后,这些指标的变化趋势与过表达株系相反。低磷胁迫后,LePT1的相对表达量在OE-SlWRKY6植株根系中呈下降趋势,与RNAi-SlWRKY6植株中变化相反。在野生型、RNAi-SlWRKY6和OE-SlWRKY6植株根系中LePT2的相对表达量均呈整体上升趋势,而LePT3则呈整体下降趋势。由上述可知,SlWRKY6能够响应低磷胁迫,其表达量高低与番茄植株的低磷耐受性呈正相关,且可能影响磷转运体基因对低磷的响应。该研究结果可为进一步揭示番茄WRKY转录因子家族的功能及其对低磷胁迫的响应机制提供理论依据。
陈浩婷, 张玉静, 刘洁, 代泽敏, 刘伟, 石玉, 张毅, 李天来. 低磷胁迫下番茄转录因子WRKY6功能分析[J]. 生物技术通报, 2023, 39(10): 136-147.
CHEN Hao-ting, ZHANG Yu-jing, LIU Jie, DAI Ze-min, LIU Wei, SHI Yu, ZHANG Yi, LI Tian-lai. Functional Analysis of WRKY6 Gene in Tomato Under Low-phosphorus Stress[J]. Biotechnology Bulletin, 2023, 39(10): 136-147.
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| SlWRKY6-F | CAACCACCCATTACCACCAG |
| SlWRKY6-R | GTTTGGATTTTGGGCTGTG |
| LePT1;1-F | TTGGTGGTGATTATCCCCTTTC |
| LePT1;1-R | GGCAGTTTCAGGCATCTTCATA |
| LePT1;2-F | AAAATGGGACGAAAAAAGGTG |
| LePT1;2-R | GATGGTAGCGGACAAAGGGT |
| LePT1;3-F | TTCGATTTTGGCTTGGTTTTG |
| LePT1;3-R | GTGCTGTCTCAGGCATCTTCA |
| Actin-F | ACCACTGAGCACAATGTTACCG |
| Actin-R | GTCCTCTTCCAGCCATCCA |
| JC-WRKY6-F | ATGACGCACAATCCCACTATC |
| JC-WRKY6-R | CTACCGTTGACTGATCACTTCC |
| JC-RNAiWRKY6-F | GGAAGGTGGCTCCTACAAATG |
| JC-RNAiWRKY6-R | CTTCCGATGAAGAGCGAGAATG |
表1 引物列表
Table 1 Table of primers
| 引物名称 Primer name | 引物序列 Primer sequence(5'-3') |
|---|---|
| SlWRKY6-F | CAACCACCCATTACCACCAG |
| SlWRKY6-R | GTTTGGATTTTGGGCTGTG |
| LePT1;1-F | TTGGTGGTGATTATCCCCTTTC |
| LePT1;1-R | GGCAGTTTCAGGCATCTTCATA |
| LePT1;2-F | AAAATGGGACGAAAAAAGGTG |
| LePT1;2-R | GATGGTAGCGGACAAAGGGT |
| LePT1;3-F | TTCGATTTTGGCTTGGTTTTG |
| LePT1;3-R | GTGCTGTCTCAGGCATCTTCA |
| Actin-F | ACCACTGAGCACAATGTTACCG |
| Actin-R | GTCCTCTTCCAGCCATCCA |
| JC-WRKY6-F | ATGACGCACAATCCCACTATC |
| JC-WRKY6-R | CTACCGTTGACTGATCACTTCC |
| JC-RNAiWRKY6-F | GGAAGGTGGCTCCTACAAATG |
| JC-RNAiWRKY6-R | CTTCCGATGAAGAGCGAGAATG |

图1 番茄的遗传转化和T2代番茄的 PCR 验证 A、E:共培养;B、F:筛选及分化;C、G:生根;D、H:T2代番茄 PCR 鉴定;M:DNA marker DL2000;P:质粒对照;CK:阴性对照;WT:对照植株;D(1-7)和H(1-8):分别是过表达株系和干扰株系的阳性苗
Fig. 1 Genetic transformation in tomato and PCR validation in the tomato with the T2 generation A, E: Co-culture. B, F: Screening and differentiation. C, G: Rooting. D, H: PCR identification of T2 generation tomato. M: DNA marker DL 2000. P: Plasmid groups. CK: Negative control. WT: Controlled plant. D(1-7)and H(1-8): Positive strains of overexpressing and interfering strains, respectively
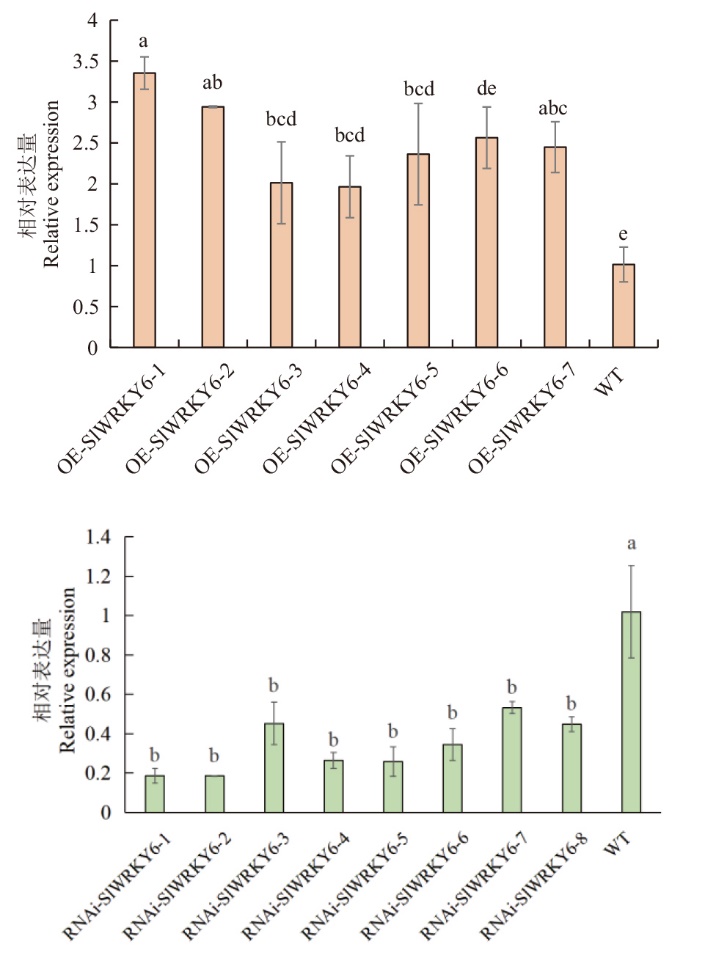
图2 RT-qPCR检测T2代转基因番茄植株RNAi-WRKY6和OE-WRKY6中WRKY6的表达量 不同小写字母表示不同处理间差异显著(P<0.05)。下同
Fig. 2 Expressions of WRKY6 in RNAi-WRKY6 and OE-WRKY6 in T2 generation transgenic tomato plants detected by RT-qPCR Different letters indicate significant differences among different treatments(P < 0.05). The same below
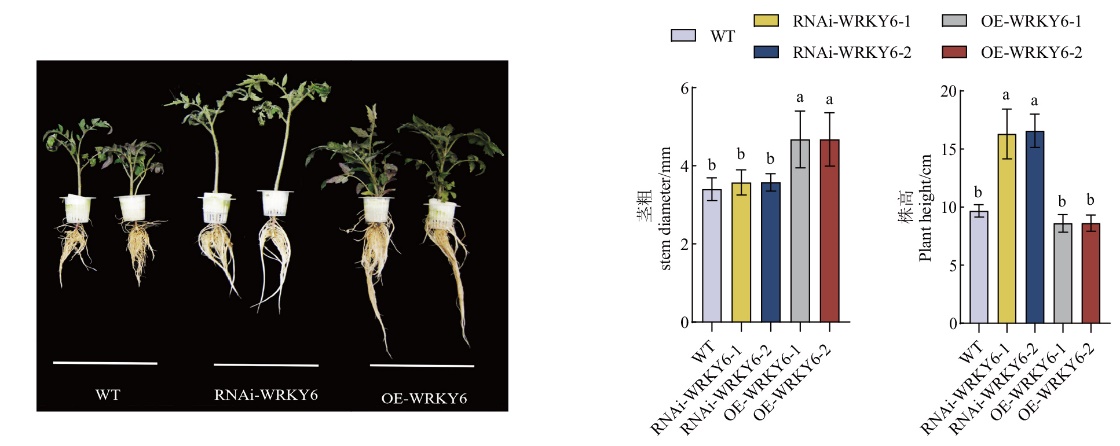
图3 低磷条件下转基因番茄植株RNAi-WRKY6和OE-WRKY6的表型观察RNAi-WRKY6从左往右依次为RNAi-WRKY6-1、RNAi-WRKY6-2;OE-WRKY6从左往右依次为OE-WRKY6-1、OE-WRKY6-2
Fig. 3 Phenotypes of transgenic tomato plant RNAi-WRKY6 and OE-WRKY6 under low phosphorus conditions RNAi-WRKY6 from left to right is RNAi-WRKY6-1, RNAi-WRKY6-2; OE-WRKY6 from left to right is OE-WRKY6-1, OE-WRKY6-2
| 处理 Treatment | 地上鲜重 Abovegro und fresh weight/g | 地下鲜重 Undergro- und fresh weight/g | 地上干重 Abovegr- ound dry weight/g | 地下干重 Underground dry weight/g | 总鲜重 Total fresh weight/g | 总干重 Total dry weight/g |
|---|---|---|---|---|---|---|
| WT | 1.88±0.25b | 0.95±0.08b | 0.22±0.05b | 0.08±0.01b | 2.83±0.18b | 0.30±0.05b |
| RNAi-SlWRKY6-1 | 1.71±0.34b | 1.35±0.16b | 0.20±0.04b | 0.08±0.01b | 3.06±0.49b | 0.28±0.05b |
| RNAi-SlWRKY6-2 | 1.68+0.33b | 1.34+0.14b | 0.20+0.04b | 0.09+0.01b | 3.02+0.45b | 0.28+0.05b |
| OE-SlWRKY6-1 | 3.38±0.08a | 1.94±0.14a | 0.46±0.04a | 0.14±0.01a | 5.33±0.19a | 0.60±0.05a |
| OE-SlWRKY6-2 | 3.40+014a | 1.93+0.13a | 0.45+0.05a | 0.14+0.01a | 5.33+0.22a | 0.59+0.06a |
表2 低磷条件下转基因番茄植株RNAi-WRKY6和OE-WRKY6生物量的测定
Table 2 Biomass of transgenic tomato plants RNAi-WRKY6 and OE-WRKY6 under low phosphorus conditions
| 处理 Treatment | 地上鲜重 Abovegro und fresh weight/g | 地下鲜重 Undergro- und fresh weight/g | 地上干重 Abovegr- ound dry weight/g | 地下干重 Underground dry weight/g | 总鲜重 Total fresh weight/g | 总干重 Total dry weight/g |
|---|---|---|---|---|---|---|
| WT | 1.88±0.25b | 0.95±0.08b | 0.22±0.05b | 0.08±0.01b | 2.83±0.18b | 0.30±0.05b |
| RNAi-SlWRKY6-1 | 1.71±0.34b | 1.35±0.16b | 0.20±0.04b | 0.08±0.01b | 3.06±0.49b | 0.28±0.05b |
| RNAi-SlWRKY6-2 | 1.68+0.33b | 1.34+0.14b | 0.20+0.04b | 0.09+0.01b | 3.02+0.45b | 0.28+0.05b |
| OE-SlWRKY6-1 | 3.38±0.08a | 1.94±0.14a | 0.46±0.04a | 0.14±0.01a | 5.33±0.19a | 0.60±0.05a |
| OE-SlWRKY6-2 | 3.40+014a | 1.93+0.13a | 0.45+0.05a | 0.14+0.01a | 5.33+0.22a | 0.59+0.06a |
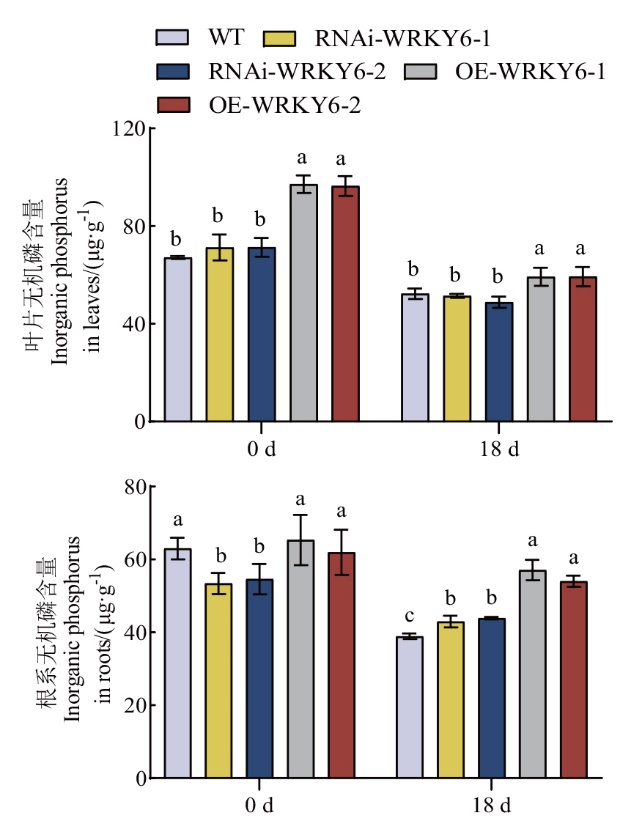
图4 低磷条件下转基因番茄植株RNAi-WRKY6和OE-WRKY6无机磷含量的测定
Fig. 4 Determination of inorganic phosphorus content in transgenic tomato plants RNAi-WRKY6 and OE-WRKY6 under low phosphorus condition
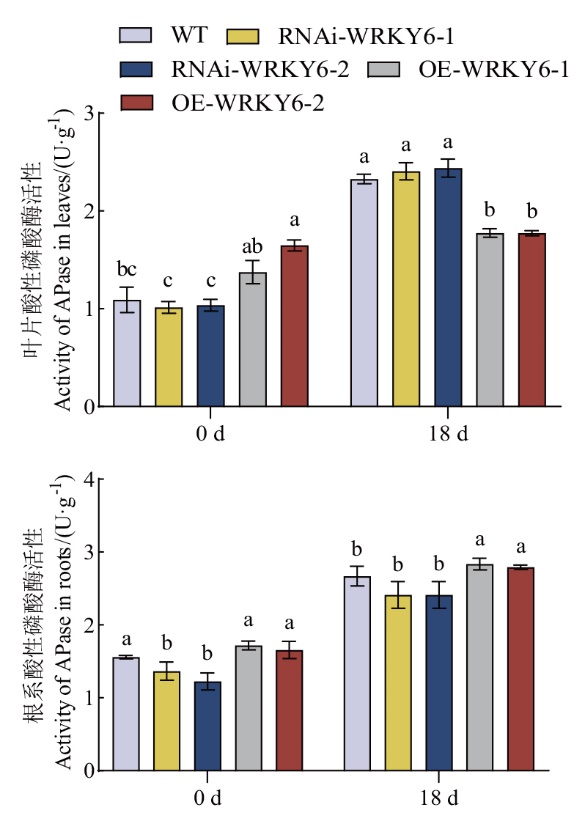
图5 低磷条件下转基因番茄植株RNAi-WRKY6和OE-WRKY6酸性磷酸酶活性的测定
Fig 5 Determination of acid phosphatase activity in transgenic tomato plant RNAi-WRKY6 and OE-WRKY6 under low phosphorus condition
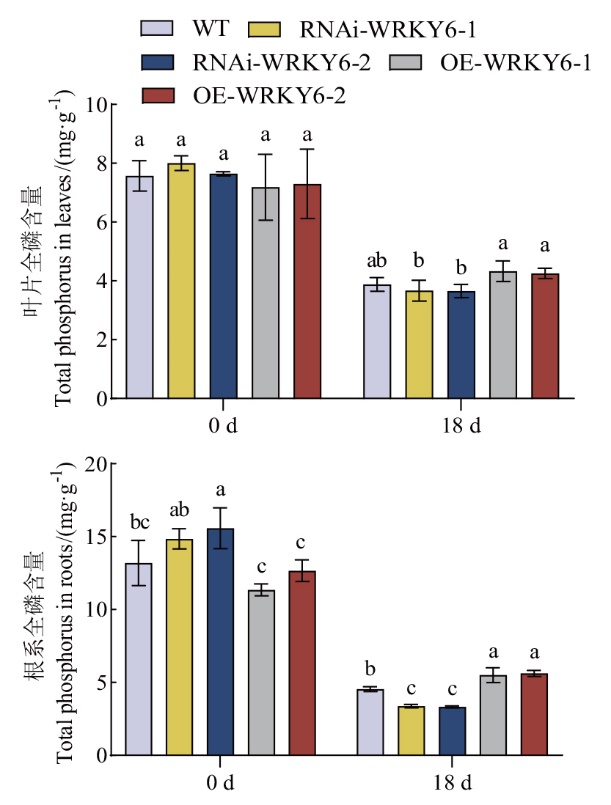
图6 低磷条件下转基因番茄植株RNAi-WRKY6和OE-WRKY6全磷含量的测定
Fig. 6 Determination of total phosphorus content of RNAi-WRKY6 and OE-WRKY6 transgenic tomato plants under low phosphorus condition
| 处理 Treatment | 草酸Oxalic aid/(mg·g-1) | 苹果酸Malic acid/(mg·g-1) | 柠檬酸Citric acid/(μg·g-1) | 琥珀酸Succinic acid/(μg·g-1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 d | 18 d | 0 d | 18 d | 0 d | 18 d | 0 d | 18 d | ||
| WT | 12.91±0.35a | 7.47±1.00b | 6.86±0.13ab | 1.69±0.78a | 117.11±15.46a | 11.11±0.93a | 248.86±22.46a | 161.85±77.35b | |
| RNAi-WRKY6-1 | 13.14±0.17a | 3.17±0.06c | 6.51±0.32b | 0.71±0.17b | 80.13±16.67b | 7.67±0.81b | 185.79±44.50bc | 78.35±10.86c | |
| RNAi-WRKY6-2 | 13.13±0.23a | 3.07±0.06c | 6.41±0.06b | 0.61±0.04b | 77.02±10.20b | 6.93±0.35b | 180.53±28.81c | 70.30±3.98c | |
| OE-WRKY6-1 | 13.14±0.29a | 9.22±0.90a | 5.90±0.48a | 2.56±0.25a | 110.70±29.50a | 11.22±2.33a | 229.93±32.63ab | 294.19±20.52a | |
| OE-WRKY6-2 | 12.74±0.21a | 9.25±0.32a | 5.67±0.32ab | 2.48±0.09a | 98.16±12.68ab | 10.92±1.92a | 233.98±17.91ab | 293.02±1.09a | |
表3 低磷条件下转基因番茄植株RNAi-WRKY6和OE-WRKY6根系有机酸含量的测定
Table 3 Determination of organic acid content in the roots of transgenic tomato plant RNAi-WRKY6 and OE-WRKY6 under low phosphorus condition
| 处理 Treatment | 草酸Oxalic aid/(mg·g-1) | 苹果酸Malic acid/(mg·g-1) | 柠檬酸Citric acid/(μg·g-1) | 琥珀酸Succinic acid/(μg·g-1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 d | 18 d | 0 d | 18 d | 0 d | 18 d | 0 d | 18 d | ||
| WT | 12.91±0.35a | 7.47±1.00b | 6.86±0.13ab | 1.69±0.78a | 117.11±15.46a | 11.11±0.93a | 248.86±22.46a | 161.85±77.35b | |
| RNAi-WRKY6-1 | 13.14±0.17a | 3.17±0.06c | 6.51±0.32b | 0.71±0.17b | 80.13±16.67b | 7.67±0.81b | 185.79±44.50bc | 78.35±10.86c | |
| RNAi-WRKY6-2 | 13.13±0.23a | 3.07±0.06c | 6.41±0.06b | 0.61±0.04b | 77.02±10.20b | 6.93±0.35b | 180.53±28.81c | 70.30±3.98c | |
| OE-WRKY6-1 | 13.14±0.29a | 9.22±0.90a | 5.90±0.48a | 2.56±0.25a | 110.70±29.50a | 11.22±2.33a | 229.93±32.63ab | 294.19±20.52a | |
| OE-WRKY6-2 | 12.74±0.21a | 9.25±0.32a | 5.67±0.32ab | 2.48±0.09a | 98.16±12.68ab | 10.92±1.92a | 233.98±17.91ab | 293.02±1.09a | |

图7 RNAi-WRKY6和OE-WRKY6转基因番茄根系中磷转运体表达量的测定
Fig. 7 Determination of phosphorus transporter expressions in the roots of transgenic tomato plant RNAi-WRKY6 and OE-WRKY6
| [1] | 柳美玉, 曹红霞, 杜贞其, 等. 营养液浓度对番茄营养生长期干物质累积及养分吸收的影响[J]. 西北农林科技大学学报: 自然科学版, 2017, 45(4): 119-126, 133. |
| Liu MY, Cao HX, Du ZQ, et al. Effects of nutrient concentration on dry matter accumulation and nutrients absorption of tomato[J]. J Northwest A F Univ Nat Sci Ed, 2017, 45(4): 119-126, 133. | |
| [2] |
王开, 李文学. 玉米耐受低磷胁迫的分子机制研究进展[J]. 生物技术通报, 2016, 32(10): 52-57.
doi: 10.13560/j.cnki.biotech.bull.1985.2016.10.002 |
|
Wang K, Li WX. Progresses on molecular mechanisms of low-phosphorus tolerance in maize[J]. Biotechnol Bull, 2016, 32(10): 52-57.
doi: 10.13560/j.cnki.biotech.bull.1985.2016.10.002 |
|
| [3] | 王保明, 陈永忠, 王湘南, 等. 植物低磷胁迫响应及其调控机制[J]. 福建农林大学学报: 自然科学版, 2015, 44(6): 567-575. |
| Wang BM, Chen YZ, Wang XN, et al. The response to low phosphorus stress and its regulation mechanism in plants[J]. J Fujian Agric For Univ Nat Sci Ed, 2015, 44(6): 567-575. | |
| [4] |
Kalaji HM, Oukarroum A, Alexandrov V, et al. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements[J]. Plant Physiol Biochem, 2014, 81: 16-25.
doi: 10.1016/j.plaphy.2014.03.029 URL |
| [5] |
李若楠, 武雪萍, 张彦才, 等. 减量施磷对温室菜地土壤磷素积累、迁移与利用的影响[J]. 中国农业科学, 2017, 50(20): 3944-3952.
doi: 10.3864/j.issn.0578-1752.2017.20.010 |
|
Li RN, Wu XP, Zhang YC, et al. Effects of reduced phosphorus fertilization on soil phosphorus accumulation, leaching and utilization in greenhouse vegetable production[J]. Sci Agric Sin, 2017, 50(20): 3944-3952.
doi: 10.3864/j.issn.0578-1752.2017.20.010 |
|
| [6] |
Sun X, Wang Y, Sui N. Transcriptional regulation of bHLH during plant response to stress[J]. Biochem Biophys Res Commun, 2018, 503(2): 397-401.
doi: 10.1016/j.bbrc.2018.07.123 URL |
| [7] |
Fang YJ, Zheng YQ, Lu W, et al. Roles of miR319-regulated TCPs in plant development and response to abiotic stress[J]. Crop J, 2021, 9(1): 17-28.
doi: 10.1016/j.cj.2020.07.007 |
| [8] |
Rushton PJ, Somssich IE, Ringler P, et al. WRKY transcription factors[J]. Trends Plant Sci, 2010, 15(5): 247-258.
doi: 10.1016/j.tplants.2010.02.006 pmid: 20304701 |
| [9] |
Jiang JJ, Ma SH, Ye NH, et al. WRKY transcription factors in plant responses to stresses[J]. J Integr Plant Biol, 2017, 59(2): 86-101.
doi: 10.1111/jipb.12513 |
| [10] |
Wang F, Deng MJ, Xu JM, et al. Molecular mechanisms of phosphate transport and signaling in higher plants[J]. Semin Cell Dev Biol, 2018, 74: 114-122.
doi: S1084-9521(16)30386-X pmid: 28648582 |
| [11] |
Devaiah BN, Karthikeyan AS, Raghothama KG. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis[J]. Plant Physiol, 2007, 143(4): 1789-1801.
doi: 10.1104/pp.106.093971 URL |
| [12] |
Su T, Xu Q, Zhang FC, et al. WRKY42 modulates phosphate homeostasis through regulating phosphate translocation and acquisition in Arabidopsis[J]. Plant Physiol, 2015, 167(4): 1579-1591.
doi: 10.1104/pp.114.253799 URL |
| [13] |
Zhang J, Gu M, Liang R, et al. OsWRKY21 and OsWRKY 108 function redundantly to promote phosphate accumulation through maintaining the constitutive expression of OsPHT1; 1 under phosphate-replete conditions[J]. New Phytol, 2021, 229(3): 1598-1614.
doi: 10.1111/nph.v229.3 URL |
| [14] |
Zhang Y, Chen HT, Liang Y, et al. Comparative transcriptomic and metabolomic analyses reveal the protective effects of silicon against low phosphorus stress in tomato plants[J]. Plant Physiol Biochem, 2021, 166: 78-87.
doi: 10.1016/j.plaphy.2021.05.043 URL |
| [15] | 周涛, 王娟, 胡佳蕙, 等. 番茄转录因子基因SlWRKY6的克隆与原核表达分析[J]. 西北植物学报, 2020, 40(11): 1824-1832. |
| Zhou T, Wang J, Hu JH, et al. Cloning and prokaryotic expression analysis of a WRKY transcription factor gene SlWRKY6 in Solanum lycopersicum[J]. Acta Bot Boreali Occidentalia Sin, 2020, 40(11): 1824-1832. | |
| [16] |
王茹, 陈超, 于丽杰, 等. 番茄SlWRKY6基因克隆及其在重金属胁迫下的表达分析[J]. 华北农学报, 2021, 36(1): 54-62.
doi: 10.7668/hbnxb.20191310 |
| Wang R, Chen C, Yu LJ, et al. Cloning of tomato SlWRKY6 gene and its expression analysis under heavy metal stress[J]. Acta Agric Boreali Sin, 2021, 36(1): 54-62. | |
| [17] | 王兵爽, 李淑君, 张舒桓, 等. 西瓜根系分泌酸性磷酸酶对有机肥营养的响应[J]. 土壤学报, 2019, 56(2): 454-465. |
| Wang BS, Li SJ, Zhang SH, et al. Responses of acid phosphatase secreted by watermelon roots to organic manure nutrition[J]. Acta Pedol Sin, 2019, 56(2): 454-465. | |
| [18] | 杨光凯, 薛诗怡, 李嘉祯, 等. 红宝石苹果果实有机酸组分及苹果酸代谢酶活性分析[J]. 果树学报, 2023, 40(5): 884-892. |
| Yang GK, Xue SY, Li JZ, et al. Analysis of organic acid components and malic acid metabolizing enzyme activity in Hongbaoshi apple fruits[J]. J Fruit Sci, 2023, 40(5): 884-892. | |
| [19] | 梁颖, 石玉, 赵鑫, 等. 低磷条件下硅对番茄幼苗生长及生理特性的影响[J]. 浙江大学学报: 农业与生命科学版, 2020, 46(2): 151-160. |
| Liang Y, Shi Y, Zhao X, et al. Effects of silicon on the growth and physiological properties of tomato seedlings under low phosphorus condition[J]. J Zhejiang Univ Nat Sci Ed, 2020, 46(2): 151-160. | |
| [20] | 崔博文, 乔光, 范付华, 等. 不同种源马尾松种质耐低磷的主成分与灰色关联度分析[J]. 西南大学学报: 自然科学版, 2017, 39(8): 49-56. |
| Cui BW, Qiao G, Fan FH, et al. Principal component analysis and grey correlation analysis for low phosphorus tolerance evaluation in Masson pine(Pinus massoniana)of different provenances[J]. J Southwest Univ Nat Sci Ed, 2017, 39(8): 49-56. | |
| [21] | 张丽梅, 郭再华, 张琳, 等. 缺磷对不同耐低磷玉米基因型酸性磷酸酶活性的影响[J]. 植物营养与肥料学报, 2015, 21(4): 898-910. |
| Zhang LM, Guo ZH, Zhang L, et al. Effects of phosphate deficiency on acid phosphatase activities of different maize genotypes tolerant to low- p stress[J]. J Plant Nutr Fertil, 2015, 21(4): 898-910. | |
| [22] |
Zhang C, Liu YX, Wang BT, et al. CRISPR/Cas9 targeted knockout FvPHO2 can increase phosphorus content and improve fruit quality of woodland strawberry[J]. Sci Hortic, 2023, 317: 112078.
doi: 10.1016/j.scienta.2023.112078 URL |
| [23] |
黄杰, 张郎织, 邢玉芬, 等. 低磷胁迫对崖州硬皮豆生长及酸性磷酸酶活性的影响[J]. 草地学报, 2021, 29(7): 1462-1468.
doi: 10.11733/j.issn.1007-0435.2021.07.011 |
| Huang J, Zhang LZ, Xing YF, et al. Effects of low phosphorus stress on the growth and acid phosphatase activity of Macrotyloma uni-florum(lam.) Verdc.Yazhou[J]. Acta Agrestia Sin, 2021, 29(7): 1462-1468. | |
| [24] | 徐壮, 王婉瑕, 徐磊, 等. 水稻磷素吸收与转运分子机制研究进展[J]. 植物营养与肥料学报, 2018, 24(5): 1378-1385. |
| Xu Z, Wang WX, Xu L, et al. Research progress in molecular mechanism of rice phosphorus uptake and translocation[J]. J Plant Nutr Fertil, 2018, 24(5): 1378-1385. | |
| [25] |
徐静, 张锡洲, 李廷轩, 等. 野生大麦对土壤磷吸收及其酸性磷酸酶活性的基因型差异[J]. 草业学报, 2015, 24(1): 88-98.
doi: 10.11686/cyxb20150112 |
| Xu J, Zhang XZ, Li TX, et al. Phosphorus absorption and acid phosphatase activity in wild barley genotypes with different phosphorus use efficiencies[J]. Acta Prataculturae Sin, 2015, 24(1): 88-98. | |
| [26] |
柯梅, 侯钰荣, 张一弓. 碱胁迫下冰草根系pH值与有机酸含量变化[J]. 新疆农业科学, 2021, 58(10): 1929-1937.
doi: 10.6048/j.issn.1001-4330.2021.10.021 |
| Ke M, Hou YR, Zhang YG. Study on the relationship between pH and organic acid in the root of Agropyron cristatum tawukumu under alkaline stress[J]. Xinjiang Agric Sci, 2021, 58(10): 1929-1937. | |
| [27] | 农玉琴, 陆金梅, 骆妍妃, 等. 不同磷水平下玉米-大豆间作对根系有机酸分泌特征及磷吸收的影响[J]. 中国土壤与肥料, 2022(7): 41-48. |
| Nong YQ, Lu JM, Luo YF, et al. Effects of maize and soybean intercropping on root exudation of organic acids and phosphorus uptake under different phosphorus rates[J]. Soil Fertil Sci China, 2022(7): 41-48. | |
| [28] | 苗淑杰, 乔云发, 刘晓冰. 磷影响大豆根系分泌有机酸总量和不同根区有机酸量[J]. 大豆科学, 2011, 30(1): 127-130. |
| Miao SJ, Qiao YF, Liu XB. Phosphorus affected organic acid exudation from soybean root[J]. Soybean Sci, 2011, 30(1): 127-130. | |
| [29] | 田江, 梁翠月, 陆星, 等. 根系分泌物调控植物适应低磷胁迫的机制[J]. 华南农业大学学报, 2019, 40(5): 175-185. |
| Tian J, Liang CY, Lu X, et al. Mechanism of root exudates regulating plant responses to phosphorus deficiency[J]. J South China Agric Univ, 2019, 40(5): 175-185. | |
| [30] |
Ligaba A, Shen H, Shibata K, et al. The role of phosphorus in aluminium-induced citrate and malate exudation from rape(Brassica napus)[J]. Physiol Plant, 2004, 120(4): 575-584.
doi: 10.1111/ppl.2004.120.issue-4 URL |
| [31] | 张晓艳, 杨忠仁, 张凤兰, 等. 干旱胁迫对地梢瓜琥珀酸合成代谢的影响[J]. 西北农林科技大学学报: 自然科学版, 2020, 48(4): 137-145. |
| Zhang XY, Yang ZR, Zhang FL, et al. Effect of drought stress on succinic acid biosynthesis in Cynanchum thesioides[J]. J Northwest A F Univ Nat Sci Ed, 2020, 48(4): 137-145. | |
| [32] | 沈文玥. 低磷胁迫下野大豆和大豆幼苗叶片养分再利用比较研究[D]. 长春: 东北师范大学, 2022. |
| Shen WY. Comparative study on leaf nutrient reuse of wild soybean and soybean seedlings under low phosphorus conditions[D]. Changchun: Northeast Normal University, 2022. | |
| [33] | 王萍, 陈爱群, 余玲, 等. 植物磷转运蛋白基因及其表达调控的研究进展[J]. 植物营养与肥料学报, 2006, 12(4): 584-591. |
| Wang P, Chen AQ, Yu L, et al. Advance of plant phosphate transporter genes and their regulated expression[J]. Plant Nutr Fertil Sci, 2006, 12(4): 584-591. | |
| [34] | 孙艳, 洪婉婷, 韩阳, 等. 植物内部磷循环利用提高磷效率的研究进展[J]. 植物营养与肥料学报, 2021, 27(12): 2216-2228. |
| Sun Y, Hong WT, Han Y, et al. Targeting internal phosphorus re-utilization to improve plant phosphorus use efficiency[J]. J Plant Nutr Fertil, 2021, 27(12): 2216-2228. | |
| [35] | 董旭, 王雪, 石磊, 等. 植物磷转运子PHT1家族研究进展[J]. 植物营养与肥料学报, 2017, 23(3): 799-810. |
| Dong X, Wang X, Shi L, et al. Advances in plant PHT1 phosphate transporter family research[J]. J Plant Nutr Fertil, 2017, 23(3): 799-810. |
| [1] | 黄小龙, 孙贵连, 马丹丹, 闫慧清. 水稻幼苗酵母单杂文库构建及LAZY1上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 126-135. |
| [2] | 韩浩章, 张丽华, 李素华, 赵荣, 王芳, 王晓立. 盐碱胁迫诱导的猴樟酵母cDNA文库构建及CbP5CS上游调控因子筛选[J]. 生物技术通报, 2023, 39(9): 236-245. |
| [3] | 吕秋谕, 孙培媛, 冉彬, 王佳蕊, 陈庆富, 李洪有. 苦荞转录因子基因FtbHLH3的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 194-203. |
| [4] | 徐靖, 朱红林, 林延慧, 唐力琼, 唐清杰, 王效宁. 甘薯IbHQT1启动子的克隆及上游调控因子的鉴定[J]. 生物技术通报, 2023, 39(8): 213-219. |
| [5] | 李博, 刘合霞, 陈宇玲, 周兴文, 朱宇林. 金花茶CnbHLH79转录因子的克隆、亚细胞定位及表达分析[J]. 生物技术通报, 2023, 39(8): 241-250. |
| [6] | 陈晓, 于茗兰, 吴隆坤, 郑晓明, 逄洪波. 植物lncRNA及其对低温胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(7): 1-12. |
| [7] | 刘珍银, 段郅臻, 彭婷, 王童欣, 王健. 基于三角梅的病毒诱导基因沉默体系的建立与优化[J]. 生物技术通报, 2023, 39(7): 123-130. |
| [8] | 郭怡婷, 赵文菊, 任延靖, 赵孟良. 菊芋NAC转录因子家族基因的鉴定及分析[J]. 生物技术通报, 2023, 39(6): 217-232. |
| [9] | 冯珊珊, 王璐, 周益, 王幼平, 方玉洁. WOX家族基因调控植物生长发育和非生物胁迫响应的研究进展[J]. 生物技术通报, 2023, 39(5): 1-13. |
| [10] | 王兵, 赵会纳, 余婧, 余世洲, 雷波. 植物侧枝发育的调控研究进展[J]. 生物技术通报, 2023, 39(5): 14-22. |
| [11] | 史建磊, 宰文珊, 苏世闻, 付存念, 熊自立. 番茄青枯病抗性相关miRNA的鉴定与表达分析[J]. 生物技术通报, 2023, 39(5): 233-242. |
| [12] | 车永梅, 郭艳苹, 刘广超, 叶青, 李雅华, 赵方贵, 刘新. 菌株C8和B4的分离鉴定及其耐盐促生效果和机制[J]. 生物技术通报, 2023, 39(5): 276-285. |
| [13] | 张新博, 崔浩亮, 史佩华, 高锦春, 赵顺然, 陶晨雨. 低起始量的免疫共沉淀技术研究进展[J]. 生物技术通报, 2023, 39(4): 227-235. |
| [14] | 胡明月, 杨宇, 郭仰东, 张喜春. 低温胁迫下番茄SlMYB96的功能分析[J]. 生物技术通报, 2023, 39(4): 236-245. |
| [15] | 葛颜锐, 赵冉, 徐静, 李若凡, 胡云涛, 李瑞丽. 植物维管形成层发育及其调控的研究进展[J]. 生物技术通报, 2023, 39(3): 13-25. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||