








生物技术通报 ›› 2024, Vol. 40 ›› Issue (3): 286-295.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0840
收稿日期:2023-08-29
出版日期:2024-03-26
发布日期:2024-04-08
通讯作者:
李鹏飞,男,博士,助理研究员,研究方向:医学微生物学;E-mail: lpf1234@suda.edu.cn;作者简介:雷棋怡,女,硕士研究生,研究方向:内科学;E-mail: leiqiyi0701@163.com
基金资助:
LEI Qi-yi( ), XU Yang(
), XU Yang( ), LI Peng-fei(
), LI Peng-fei( )
)
Received:2023-08-29
Published:2024-03-26
Online:2024-04-08
摘要:
【目的】 探讨脆弱拟杆菌(Bacteroides fragilis)中六型蛋白分泌系统(T6SS)对肠道屏障的影响及作用机制。【方法】 采用自杀载体构建B. fragilis T6SS缺陷株。建立葡聚糖硫酸钠(DSS)诱导的肠炎小鼠模型,并分别补充PBS,B. fragilis WT和B. fragilis ΔT6SS,随后比较三组小鼠疾病特征、肠道屏障完整性的差异。利用荧光定量PCR和免疫组化检测小鼠紧密连接蛋白的表达。采用非靶向代谢组学比较各组小鼠肠道差异代谢物。【结果】 T6SS缺失不影响B. fragilis的生物活性。与PBS对照组相比,B. fragilis WT明显改善小鼠的体重丢失、结肠长度等疾病指标,表现出对DSS诱导的肠炎的保护性,而B. fragilis ΔT6SS随着T6SS的敲除丧失了对DSS诱导的肠炎的保护性。小鼠血清中异硫氰酸荧光素葡聚糖含量和肠道病理切片均表明B. fragilis T6SS能改善小鼠肠道屏障的完整性。荧光定量PCR和免疫组化均表明B. fragilis T6SS影响肠道紧密连接蛋白的表达。非靶向代谢组学分析表明,与B. fragilis ΔT6SS组相比,B. fragilis WT组显著上调96个肠道差异代谢产物,其中多个代谢产物富集到胆碱能突触代谢和甘油磷脂代谢相关通路。【结论】 拟杆菌T6SS改变肠道代谢组并提高肠道细胞紧密连接蛋白的表达,改善肠道屏障的通透性,参与到拟杆菌对肠道屏障的保护性。
雷棋怡, 徐杨, 李鹏飞. 脆弱拟杆菌六型分泌系统对肠道屏障的影响及机制[J]. 生物技术通报, 2024, 40(3): 286-295.
LEI Qi-yi, XU Yang, LI Peng-fei. Influence and Mechanism of Bacteroides fragilis Type VI Secretory System on the Intestinal Barrier[J]. Biotechnology Bulletin, 2024, 40(3): 286-295.
| 引物名称Primer name | 引物序列Sequence | 引物用途Primer purpose |
|---|---|---|
| pLGB-13 vector | F: TCAAGTTGTGCGTGATGTG | pLGB-13载体引物 |
| R: GGATTCATACAAGCGGTC | pLGB-13 vector primer | |
| T6SS 5' flanking | F: CGCGGATCC CCGCATACGCCGGAAGT | T6SS基因上游同源臂扩增引物 |
| R: CAGTTTTAATCAGGGAACGAACA | 5' flanking amplification of T6SS genes | |
| T6SS 3' flanking | F: cgtgttcgttccctgattaaaactgTA CCTGAGCCGATACGACAAA | T6SS基因下游同源臂扩增引物 |
| R: tgcctgcagTGCTACGGGCATAAACCCT | 3' flanking amplification of T6SS genes | |
| T6SS mutation | F: TCCCAGCAGGGTAGCGTC | T6SS缺陷株筛选引物 |
| R: AGTGCATTCTGAATGTCCGTATT | Mutant selection | |
| Claudin1 | F: GGGGACAACATCGTGACCG | Claudin1荧光定量引物 |
| R: AGGAGTCGAAGACTTTGCACT | qPCR primer for Claudin1 amplification | |
| Claudin2 | F: CAACTGGTGGGCTACATCCTA | Claudin2荧光定量引物 |
| R: CCCTTGGAAAAGCCAACCG | qPCR primer for Claudin2 amplification | |
| Claudin3 | F: ACCAACTGCGTACAAGACGAG | Claudin3荧光定量引物 |
| R: CAGAGCCGCCAACAGGAAA | qPCR primer for Claudin3 amplification | |
| Claudin4 | F: TGGAGGACGAGACCGTCAA | Claudin4荧光定量引物 |
| R: CACGGGCACCATAATCAGCA | qPCR primer for Claudin4 amplification | |
| Occludin | F: TTGAAAGTCCACCTCCTTACAGA | Occludin荧光定量引物 |
| R: CCGGATAAAAAGAGTACGCTGG | qPCR primer for Occludin amplification | |
| ZO-1 | F: GCTTTAGCGAACAGAAGGAGC | ZO-1荧光定量引物 |
| R: TTCATTTTTCCGAGACTTCACCA | qPCR primer for ZO-1 amplification | |
| Jam | F: TCACGTTCAGTTCTGTGACCC | Jam荧光定量引物 |
| R: TGACCTCCCCGTAGTTCTGG | qPCR primer for Jam amplification | |
| Muc1 | F: GGCATTCGGGCTCCTTTCTT | Muc1荧光定量引物 |
| R: TGGAGTGGTAGTCGATGCTAAG | qPCR primer for Muc1 amplification | |
| Gapdh | F: GTCTCCTCTGACTTCAACAGCG | Gapdh荧光定量引物 |
| R: ACCACCCTGTTGCTGTAGCCAA | qPCR primer for Gapdh amplification |
表1 引物列表
Table 1 Primer sequence
| 引物名称Primer name | 引物序列Sequence | 引物用途Primer purpose |
|---|---|---|
| pLGB-13 vector | F: TCAAGTTGTGCGTGATGTG | pLGB-13载体引物 |
| R: GGATTCATACAAGCGGTC | pLGB-13 vector primer | |
| T6SS 5' flanking | F: CGCGGATCC CCGCATACGCCGGAAGT | T6SS基因上游同源臂扩增引物 |
| R: CAGTTTTAATCAGGGAACGAACA | 5' flanking amplification of T6SS genes | |
| T6SS 3' flanking | F: cgtgttcgttccctgattaaaactgTA CCTGAGCCGATACGACAAA | T6SS基因下游同源臂扩增引物 |
| R: tgcctgcagTGCTACGGGCATAAACCCT | 3' flanking amplification of T6SS genes | |
| T6SS mutation | F: TCCCAGCAGGGTAGCGTC | T6SS缺陷株筛选引物 |
| R: AGTGCATTCTGAATGTCCGTATT | Mutant selection | |
| Claudin1 | F: GGGGACAACATCGTGACCG | Claudin1荧光定量引物 |
| R: AGGAGTCGAAGACTTTGCACT | qPCR primer for Claudin1 amplification | |
| Claudin2 | F: CAACTGGTGGGCTACATCCTA | Claudin2荧光定量引物 |
| R: CCCTTGGAAAAGCCAACCG | qPCR primer for Claudin2 amplification | |
| Claudin3 | F: ACCAACTGCGTACAAGACGAG | Claudin3荧光定量引物 |
| R: CAGAGCCGCCAACAGGAAA | qPCR primer for Claudin3 amplification | |
| Claudin4 | F: TGGAGGACGAGACCGTCAA | Claudin4荧光定量引物 |
| R: CACGGGCACCATAATCAGCA | qPCR primer for Claudin4 amplification | |
| Occludin | F: TTGAAAGTCCACCTCCTTACAGA | Occludin荧光定量引物 |
| R: CCGGATAAAAAGAGTACGCTGG | qPCR primer for Occludin amplification | |
| ZO-1 | F: GCTTTAGCGAACAGAAGGAGC | ZO-1荧光定量引物 |
| R: TTCATTTTTCCGAGACTTCACCA | qPCR primer for ZO-1 amplification | |
| Jam | F: TCACGTTCAGTTCTGTGACCC | Jam荧光定量引物 |
| R: TGACCTCCCCGTAGTTCTGG | qPCR primer for Jam amplification | |
| Muc1 | F: GGCATTCGGGCTCCTTTCTT | Muc1荧光定量引物 |
| R: TGGAGTGGTAGTCGATGCTAAG | qPCR primer for Muc1 amplification | |
| Gapdh | F: GTCTCCTCTGACTTCAACAGCG | Gapdh荧光定量引物 |
| R: ACCACCCTGTTGCTGTAGCCAA | qPCR primer for Gapdh amplification |
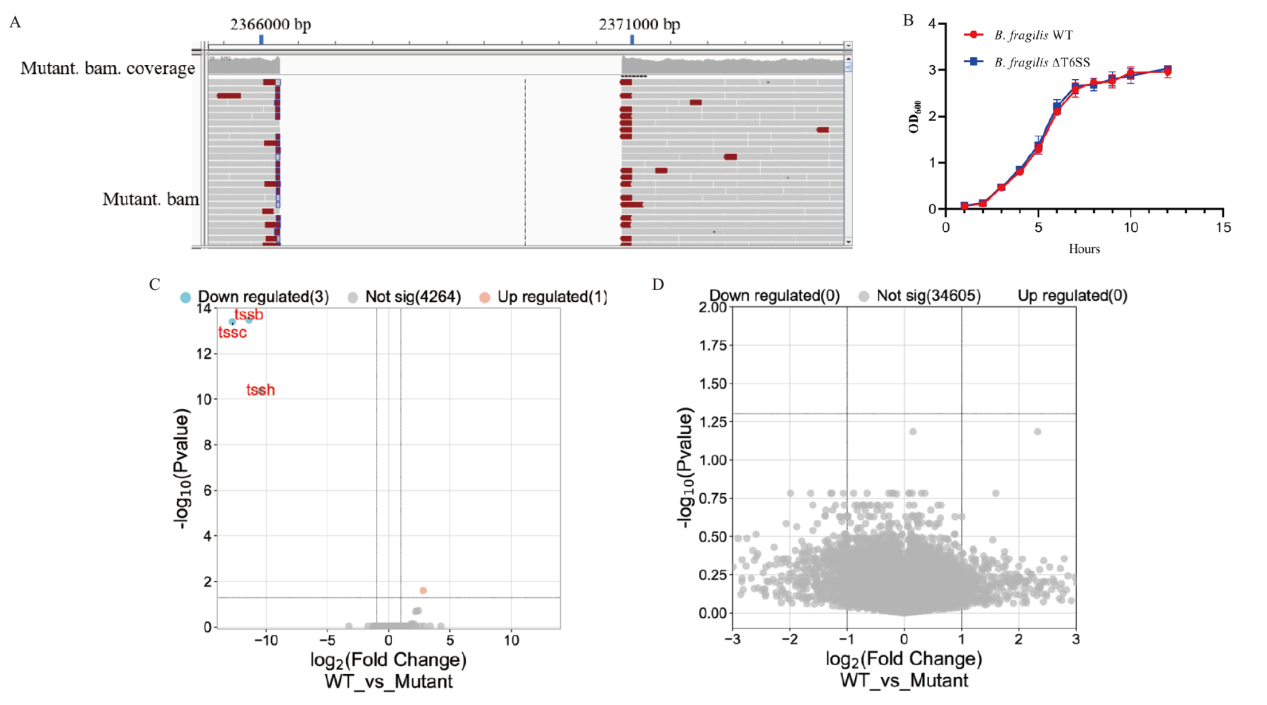
图2 B. fragilis T6SS缺陷株的构建 A:全基因组测序证实缺陷株T6SS tssB, tssC, tssH基因序列的缺失。B:B. fragilis野生株和缺陷株的生长曲线。C-D:B. fragilis野生株和缺陷株的细菌转录组和代谢组分析
Fig. 2 Construction of B. fragilis ΔT6SS strain A: Whole genome sequencing showing deletion of tssB, tssC and tssH. B: Growth curve of WT and mutated B. fragilis strains. C-D: Bacterial transcriptomic and metabolism analysis between B. fragilis WT and T6SS mutant

图3 B. fragilis T6SS参与到对DSS诱导肠道炎症的保护性 A:体重下降;B:结肠组织长度代表性图片;C:结肠长度
Fig. 3 B. fragilis T6SS involved in protection on DSS-induced colitis A: Body weight loss; B: representative pictures of colonic tissue length; C: colon length. * P < 0. 05
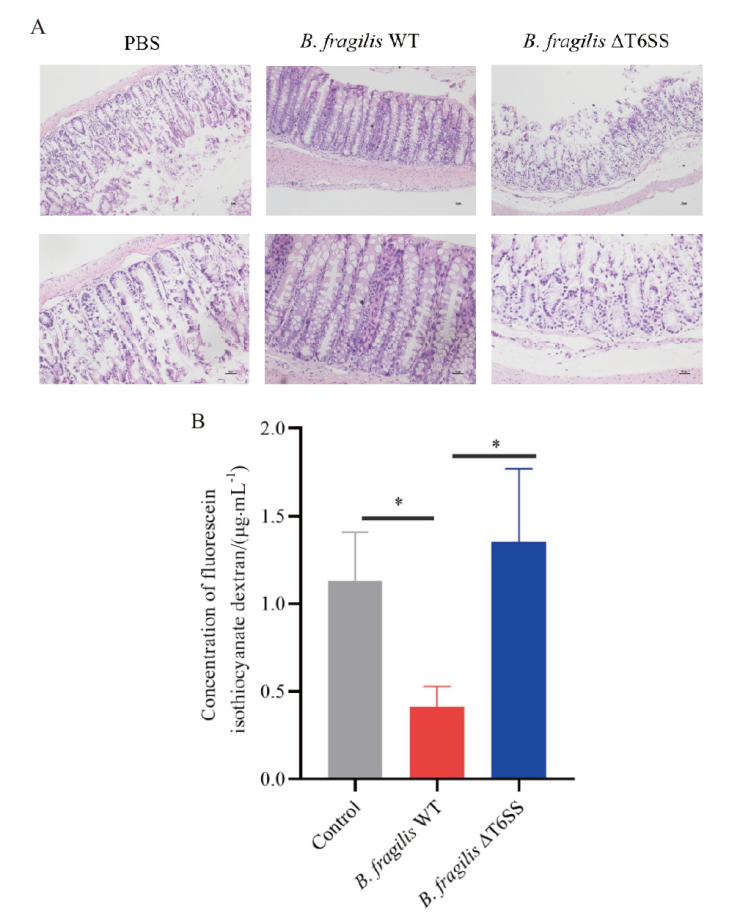
图4 B. fragilis T6SS有助于DSS诱导肠道炎症中肠道屏障的维持 A:小鼠结肠组织HE染色;B:小鼠血清中荧光素葡聚糖浓度
Fig. 4 B. fragilis T6SS contributes to the maintenance of the intestinal barrier in the DSS-induced colitis A: HE staining of colonic tissues in mice. B: Concentration of fluorescein isothiocyanate-dextran in mouse serum. * P < 0. 05

图5 B. fragilis T6SS有助于上调肠道细胞紧密连接蛋白的表达 A:定量PCR;B:免疫组化
Fig. 5 B. fragilis T6SS upregulates the expressions of intestinal tight junction proteins A: RT-qPCR. B: Immunohistochemistry. * P<0. 05, ** P < 0.01

图6 T6SS对肠道代谢组学的影响 A:PCA图;B:差异代谢物火山图;C:差异代谢物热图
Fig. 6 Effect of T6SS on intestinal metabolome A: PCA plot. B: Volcano blot. C: Heatmap of differentially expressed metabolites
| [1] |
Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases[J]. Intest Res, 2015, 13(1): 11-18.
doi: 10.5217/ir.2015.13.1.11 pmid: 25691839 |
| [2] |
Haussner F, Chakraborty S, Halbgebauer R, et al. Challenge to the intestinal mucosa during sepsis[J]. Front Immunol, 2019, 10: 891.
doi: 10.3389/fimmu.2019.00891 pmid: 31114571 |
| [3] |
Wang YF, Li JW, Wang DP, et al. Anti-hyperglycemic agents in the adjuvant treatment of sepsis: improving intestinal barrier function[J]. Drug Des Devel Ther, 2022, 16: 1697-1711.
doi: 10.2147/DDDT.S360348 URL |
| [4] |
Naymagon S, Naymagon L, Wong SY, et al. Acute graft-versus-host disease of the gut: considerations for the gastroenterologist[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(12): 711-726.
doi: 10.1038/nrgastro.2017.126 pmid: 28951581 |
| [5] |
Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease[J]. Nat Immunol, 2016, 17(5): 505-513.
doi: 10.1038/ni.3400 pmid: 26998764 |
| [6] |
Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota[J]. FEMS Microbiol Rev, 2014, 38(5): 996-1047.
doi: 10.1111/1574-6976.12075 pmid: 24861948 |
| [7] |
Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens[J]. Immunol Rev, 2017, 279(1): 90-105.
doi: 10.1111/imr.12563 pmid: 28856737 |
| [8] | Zafar H, Jr Saier MH. Gut Bacteroides species in health and disease[J]. Gut Microbes, 2021, 13(1): 1-20. |
| [9] |
Coyne MJ, Roelofs KG, Comstock LE. Type VI secretion systems of human gut Bacteroidales segregate into three genetic architectures, two of which are contained on mobile genetic elements[J]. BMC Genomics, 2016, 17: 58.
doi: 10.1186/s12864-016-2377-z pmid: 26768901 |
| [10] |
Wang J, Brodmann M, Basler M. Assembly and subcellular localization of bacterial type VI secretion systems[J]. Annu Rev Microbiol, 2019, 73: 621-638.
doi: 10.1146/annurev-micro-020518-115420 pmid: 31226022 |
| [11] |
García-Bayona L, Comstock LE. Bacterial antagonism in host-associated microbial communities[J]. Science, 2018, 361(6408): eaat2456.
doi: 10.1126/science.aat2456 URL |
| [12] |
Kostow N, Welch MD. Plasma membrane protrusions mediate host cell-cell fusion induced by Burkholderia thailandensis[J]. Mol Biol Cell, 2022, 33(8): ar70.
doi: 10.1091/mbc.E22-02-0056 URL |
| [13] |
Qin L, Wang XQ, Gao YL, et al. Roles of EvpP in Edwardsiella piscicida- macrophage interactions[J]. Front Cell Infect Microbiol, 2020, 10: 53.
doi: 10.3389/fcimb.2020.00053 URL |
| [14] | Logan SL, Thomas J, Yan JY, et al. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts[J]. Proc Natl Acad Sci USA, 2018, 115(16): E3779-E3787. |
| [15] | Coyne MJ, Zitomersky NL, McGuire AM, et al. Evidence of extensive DNA transfer between bacteroidales species within the human gut[J]. mBio, 2014, 5(3): e01305-e01314. |
| [16] | García-Bayona L, Comstock LE. Streamlined genetic manipulation of diverse Bacteroides and Parabacteroides isolates from the human gut microbiota[J]. mBio, 2019, 10(4): e01762-19. |
| [17] | Ito T, Gallegos R, Matano LM, et al. Genetic and biochemical analysis of anaerobic respiration in Bacteroides fragilis and its importance in vivo[J]. mBio, 2020, 11(1): e03238-19. |
| [18] |
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11[J]. Mol Biol Evol, 2021, 38(7): 3022-3027.
doi: 10.1093/molbev/msab120 pmid: 33892491 |
| [19] |
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees[J]. Mol Biol Evol, 1987, 4(4): 406-425.
doi: 10.1093/oxfordjournals.molbev.a040454 pmid: 3447015 |
| [20] |
Want EJ, Wilson ID, Gika H, et al. Global metabolic profiling procedures for urine using UPLC-MS[J]. Nat Protoc, 2010, 5(6): 1005-1018.
doi: 10.1038/nprot.2010.50 pmid: 20448546 |
| [21] |
Dunn WB, Broadhurst D, Begley P, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry[J]. Nat Protoc, 2011, 6(7): 1060-1083.
doi: 10.1038/nprot.2011.335 pmid: 21720319 |
| [22] |
Rosenfeldt V, Benfeldt E, Valerius NH, et al. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis[J]. J Pediatr, 2004, 145(5): 612-616.
doi: 10.1016/j.jpeds.2004.06.068 URL |
| [23] |
Haroun E, Kumar PA, Saba L, et al. Intestinal barrier functions in hematologic and oncologic diseases[J]. J Transl Med, 2023, 21(1): 233.
doi: 10.1186/s12967-023-04091-w pmid: 37004099 |
| [24] |
Chen Y, Yang B, Ross RP, et al. Orally administered CLA ameliorates DSS-induced colitis in mice via intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokine and gut microbiota modulation[J]. J Agric Food Chem, 2019, 67(48): 13282-13298.
doi: 10.1021/acs.jafc.9b05744 URL |
| [25] |
Martín R, Chamignon C, Mhedbi-Hajri N, et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response[J]. Sci Rep, 2019, 9(1): 5398.
doi: 10.1038/s41598-019-41738-5 |
| [26] |
Sofi MH, Wu YX, Ticer T, et al. A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD[J]. JCI Insight, 2021, 6(3): e136841.
doi: 10.1172/jci.insight.136841 URL |
| [27] |
Jenke A, Ruf EM, Hoppe T, et al. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy[J]. Arch Dis Child Fetal Neonatal Ed, 2012, 97(3): F217-F218.
doi: 10.1136/archdischild-2011-300838 URL |
| [28] | Mater DDG, Langella P, Corthier G, et al. A probiotic Lactobacillus strain can acquire vancomycin resistance during digestive transit in mice[J]. J Mol Microbiol Biotechnol, 2008, 14(1-3): 123-127. |
| [29] |
Wegh CAM, Geerlings SY, Knol J, et al. Postbiotics and their potential applications in early life nutrition and beyond[J]. Int J Mol Sci, 2019, 20(19): 4673.
doi: 10.3390/ijms20194673 URL |
| [30] |
Russell AB, Wexler AG, Harding BN, et al. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism[J]. Cell Host Microbe, 2014, 16(2): 227-236.
doi: S1931-3128(14)00261-3 pmid: 25070807 |
| [31] |
Le NH, Pinedo V, Lopez J, et al. Killing of Gram-negative and Gram-positive bacteria by a bifunctional cell wall-targeting T6SS effector[J]. Proc Natl Acad Sci USA, 2021, 118(40): e2106555118.
doi: 10.1073/pnas.2106555118 URL |
| [32] |
Wexler AG, Bao YQ, Whitney JC, et al. Human symbionts inject and neutralize antibacterial toxins to persist in the gut[J]. Proc Natl Acad Sci USA, 2016, 113(13): 3639-3644.
doi: 10.1073/pnas.1525637113 pmid: 26957597 |
| [33] |
Ting SY, Martínez-García E, Huang S, et al. Targeted depletion of bacteria from mixed populations by programmable adhesion with antagonistic competitor cells[J]. Cell Host Microbe, 2020, 28(2): 313-321.e6.
doi: 10.1016/j.chom.2020.05.006 URL |
| [1] | 许沛冬, 易剑锋, 陈迪, 潘磊, 谢丙炎, 赵文军. 贝莱斯芽孢杆菌生防次级代谢产物研究进展[J]. 生物技术通报, 2024, 40(3): 75-88. |
| [2] | 沙珊珊, 董世荣, 杨玉菊. 肠道菌群及代谢物调控宿主肠道免疫的研究进展[J]. 生物技术通报, 2023, 39(8): 126-136. |
| [3] | 熊淑琪. 胆汁酸生理功能及其与肠道微生物互作研究进展[J]. 生物技术通报, 2023, 39(4): 187-200. |
| [4] | 王伟宸, 赵进, 黄薇颐, 郭芯竹, 李婉颖, 张卓. 芽胞杆菌代谢产物防治三种常见植物病原真菌的研究进展[J]. 生物技术通报, 2023, 39(3): 59-68. |
| [5] | 和梦颖, 刘文彬, 林震鸣, 黎尔彤, 汪洁, 金小宝. 一株抗革兰阳性菌的戈登氏菌WA4-43全基因组测序与分析[J]. 生物技术通报, 2023, 39(2): 232-242. |
| [6] | 王松, 简晓平, 潘婉舒, 张永光, 王涛, 游玲. 玉米小曲酒糟发酵饲料对育肥猪肠道菌群的影响[J]. 生物技术通报, 2022, 38(9): 248-257. |
| [7] | 陈天赐, 武少兰, 杨国辉, 江丹霞, 江玉姬, 陈炳智. 无柄灵芝醇提物对小鼠睡眠及肠道菌群的影响[J]. 生物技术通报, 2022, 38(8): 225-232. |
| [8] | 何亚伦, 曾丽荣, 刘雄, 张铃, 王琼. 高剂量单宁酸对小鼠肠道屏障和肠道菌群的影响[J]. 生物技术通报, 2022, 38(4): 278-287. |
| [9] | 钟明月, 刘春妍, 颜妍, 张晓慧, 原海升, 徐国全, 张和平, 王玉珍. 乳双歧杆菌V9对高脂饮食诱导的NAFLD大鼠的改善作用[J]. 生物技术通报, 2022, 38(3): 181-187. |
| [10] | 王楠, 苏誉, 刘文杰, 封明, 毛瑜, 张新国. 植物内生菌中抗耐药微生物活性成分的研究进展[J]. 生物技术通报, 2021, 37(8): 263-274. |
| [11] | 梁振霆, 唐婷. 内生菌对植物次生代谢产物的生物合成影响和抗逆功能研究[J]. 生物技术通报, 2021, 37(8): 35-45. |
| [12] | 金秋霞, 王思宏, 金丽华. 果蝇肠道干细胞及肠道菌群的研究进展[J]. 生物技术通报, 2021, 37(4): 245-250. |
| [13] | 李海超, 谢飞, 张园琦, 关若冰. 不同抗、感水稻品种对褐飞虱肠道菌群的影响[J]. 生物技术通报, 2021, 37(3): 1-9. |
| [14] | 殷志斌, 黄文洁, 伍欣宙, 晏石娟. 空间分辨代谢组学进展和挑战[J]. 生物技术通报, 2021, 37(1): 32-51. |
| [15] | 胡紫媛, 夏嫱. 昆虫肠道菌群组学研究及功能和应用进展[J]. 生物技术通报, 2021, 37(1): 102-112. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||