








生物技术通报 ›› 2024, Vol. 40 ›› Issue (7): 235-246.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0158
周江鸿( ), 夏菲, 仲丽, 仇兰芬, 李广, 刘倩, 张国锋, 邵金丽, 李娜, 车少臣(
), 夏菲, 仲丽, 仇兰芬, 李广, 刘倩, 张国锋, 邵金丽, 李娜, 车少臣( )
)
收稿日期:2024-02-18
出版日期:2024-07-26
发布日期:2024-07-30
通讯作者:
车少臣,男,高级工程师,研究方向:园林植物病害防治;E-mail: cheshaochen@163.com作者简介:周江鸿,男,博士,正高级工程师,研究方向:园林植物病害防治;E-mail: zhoujh416@163.com
基金资助:
ZHOU Jiang-hong( ), XIA Fei, ZHONG Li, QIU Lan-fen, LI Guang, LIU Qian, ZHANG Guo-feng, SHAO Jin-li, LI Na, CHE Shao-chen(
), XIA Fei, ZHONG Li, QIU Lan-fen, LI Guang, LIU Qian, ZHANG Guo-feng, SHAO Jin-li, LI Na, CHE Shao-chen( )
)
Received:2024-02-18
Published:2024-07-26
Online:2024-07-30
摘要:
【目的】 从黄栌枝干内部分离到的细菌CCBC3-3-1菌株对黄栌枯萎病菌(Verticillium dahliae)表现出较强的拮抗作用,从基因组层面深入研究其拮抗机理,为进一步研发生防菌剂提供理论依据。【方法】 利用PacBio RS测序平台完成CCBC3-3-1的全基因组测序,根据16S rDNA序列信息构建系统发育树,并与同属近缘种菌株进行比较基因组学分析;对其发酵液进行LC-MS非靶标代谢组检测。【结果】 CCBC3-3-1基因组总长度为5.16 Mb,由1条环状双链染色体和3个环状质粒组成,GC含量48.08%,含有5 013个编码基因。经过antiSMASH预测,CCBC3-3-1基因组中有8个抗生素及次生代谢物合成相关的基因簇,其中2个与已知基因簇相似度较低,5个基因簇功能未知,其发酵液中检测出6种已知抗生素类物质。比较基因组学分析结果表明CCBC3-3-1基因组与泛菌属内4个近缘种均有显著差异,而且CCBC3-3-1在16S rDNA系统发育树上形成一个相对独立的分支。【结论】 CCBC3-3-1是泛菌属的一个新变异种Pantoea sp.,能够产生多种抗生素类物质,在黄栌枯萎病生物防治方面具有重要应用潜力。
周江鸿, 夏菲, 仲丽, 仇兰芬, 李广, 刘倩, 张国锋, 邵金丽, 李娜, 车少臣. 黄栌枯萎病拮抗细菌CCBC3-3-1的全基因组测序及比较基因组分析[J]. 生物技术通报, 2024, 40(7): 235-246.
ZHOU Jiang-hong, XIA Fei, ZHONG Li, QIU Lan-fen, LI Guang, LIU Qian, ZHANG Guo-feng, SHAO Jin-li, LI Na, CHE Shao-chen. Whole Genome Sequencing and Comparative Genomic Analysis of Antagonistic Bacterium CCBC3-3-1 against Verticillium dahlia[J]. Biotechnology Bulletin, 2024, 40(7): 235-246.
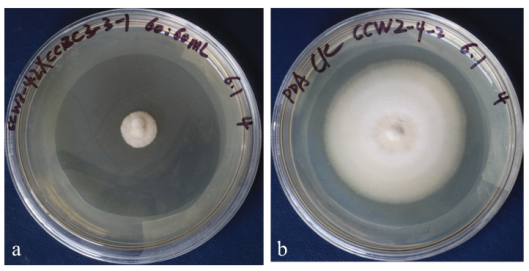
图1 黄栌内生细菌CCBC3-3-1无菌发酵液对黄栌枯萎病菌的拮抗作用 a:CCBC3-3-1无菌发酵液对黄栌枯萎病菌(V. dahlia)的抑制作用。b: 对照
Fig. 1 Antagonism of the sterile fermentation broth of endogenous bacterium CCBC3-3-1 in Cotinus cog-gygria on V. dahlia a: The sterile fermentation broth of CCBC3-3-1 effectively inhibited the growth of V. dahlia. b: Control
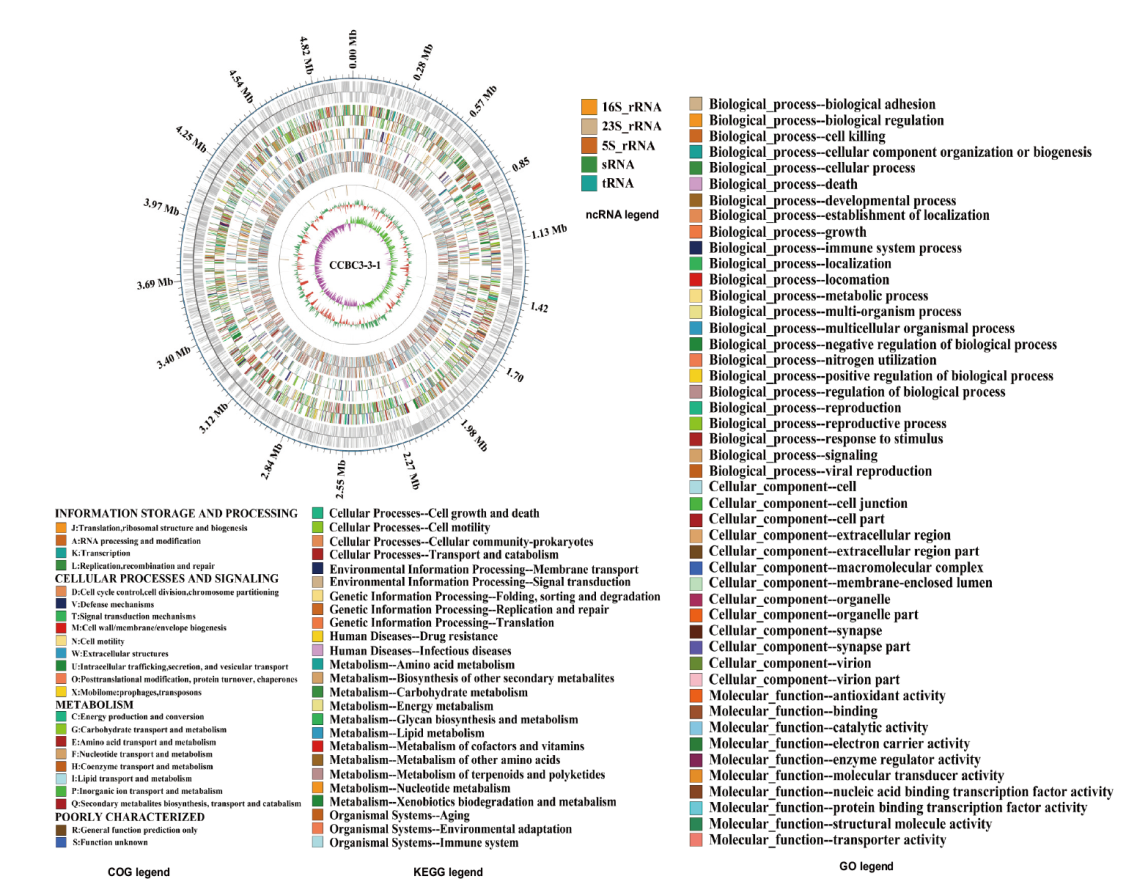
图2 菌株CCBC3-3-1的染色体基因组图谱 从外向内依次为: 1: 基因组的刻度, 大小为M(million bp); 2: 编码基因; 3: COG数据库注释结果; 4: KEGG数据库注释结果; 5: GO数据库的注释结果; 6:非编码RNA; 7: GC含量(向内的红色部分表示该区域GC含量低于全基因组平均GC含量,向外的绿色部分与之相反,且峰值越高表示与平均GC含量差值越大); 8: GC偏移(具体算法为G-C/G+C,用来衡量G和C的相对含量,如果G>C,则GC偏移值为正值,用向内的粉色部分表示;G<C,则为负值,用向外的浅绿色部分表示)
Fig. 2 Chromosome genome map of strain CCBC3-3-1 From outer to inner: 1: Genome size(million bp); 2: coding genes; 3: gene function annotation in the COG database; 4: gene function annotation in the KEGG database; 5: gene function annotation in the GO database; 6: nc RNA; 7: GC content(The inward red part indicates that the GC content in this region is lower than the average GC content of the entire genome, while the outward green part is the opposite. The higher the peak, the greater the difference between the GC content and the average GC content); 8: GC-skew(The algorithm is G-C/G+C, which is used to measure the relative content of G and C. If G>C, the GC-skew value is positive, represented by the inward pink part; if G<C, the GC-skew value is negative, represented by the outward light green part)

图3 菌株CCBC3-3-1的质粒基因组图谱 从外向内依次为: 1: COG数据库注释结果(顺时针箭头表示正链基因); 2: 基因组的刻度, 大小为K(kilo bp); 3: GC含量(向内的红色部分表示该区域GC含量低于全基因组平均GC含量,向外的绿色部分与之相反,且峰值越高表示与平均GC含量差值越大); 4: GC偏移(具体算法为G-C/G+C,用来衡量G和C的相对含量,如果G>C,则GC偏移值为正值,用向内的粉色部分表示;G<C,则为负值,用向外的浅绿色部分表示)
Fig. 3 Plasmid genome map of strain CCBC3-3-1 From outer to inner: 1: Gene function annotation in the COG database(Clockwise arrows indicate positive chain gene); 2: genome Size(kilo bp); 3: GC content(The inward red part indicates that the GC content in this region is lower than the average GC content of the entire genome, while the outward green part is the opposite. The higher the peak, the greater the difference between the GC content and the average GC content); 4: GC-skew(The algorithm is G-C/G+C, which is used to measure the relative content of G and C. If G>C, the GC-skew value is positive, represented by the inward pink part; if G<C, the GC-skew value is negative, represented by the outward light green part)
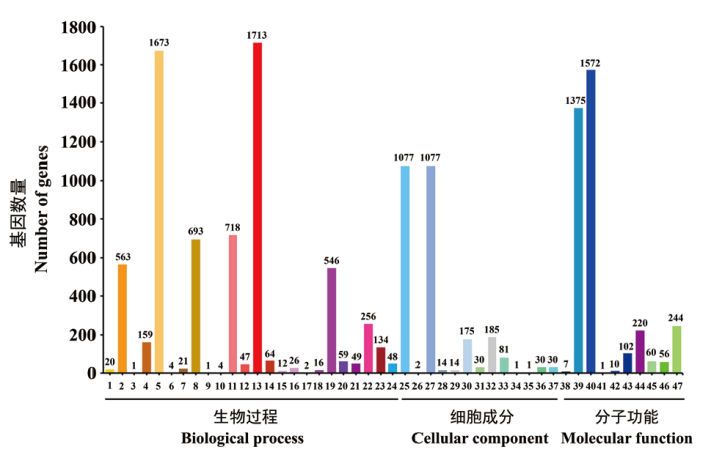
图5 CCBC3-3-1的GO功能注释分类 1:生物黏附;2:生物学调控;3:细胞杀伤;4:细胞成分的组织或生源;5:细胞过程;6:死亡;7:发育过程;8:本地化构建;9:生长;10:免疫系统过程;11:本地化;12:运输;13:代谢过程;14:多生物体过程;15:多细胞机体过程;16:生物过程的负调控;17:氮素利用;18:生物过程的正调控;19:生物过程的调控;20:繁殖;21:繁殖过程;22:刺激反应;23:信号;24:病毒繁殖;25:细胞;26:细胞连接;27:细胞部分;28:细胞外区域;29:细胞外区域部分;30:大分子复合物;31:膜封闭腔;32:细胞器;33:细胞器部分;34:突触;35:突触部分;36:病毒体;37:病毒体部分;38:抗氧化活性;39:结合;40:催化活性;41:电子载体活性;42:酶调节活性;43:分子转导活性;44:核酸结合的转录因子活性;45:蛋白质结合的转录因子活性;46:结构分子活性;47:转运活性
Fig. 5 GO function classification of strain CCBC3-3-1 1: Biological adhesion. 2: Biological regulation. 3: Cell killing. 4: Cellular component organization or biogenesis. 5: Cellular process. 6: Death. 7: Developmental process. 8: Establishment of localization. 9: Growth. 10: Immune system process. 11: Localization. 12: Locomotion. 13: Metabolic process. 14: Multi-organism process. 15: Multi cellular organismal process. 16: Negative regulation of biological process. 17: Nitrogen utilization. 18: Positive regulation of biological process. 19: Regulation of biological process. 20: Reproduction. 21: Reproductive process. 22: Response to stimulus. 23: Signaling. 24: Viral reproduction. 25: Cell. 26: Cell junction. 27: Cell part. 28: Extracellular region. 29: Extracellular region part. 30: Macromolecular complex. 31: Membrane-enclosed lumen. 32: Organelle. 33: Organelle part. 34: Synapse. 35: Synapse part. 36: Virion. 37: Virion part. 38: Antioxidant activity. 39: Binding. 40: Catalytic activity. 41: Electron carrier activity. 42: Enzyme regulator activity. 43: Molecular transducer activity. 44: Nucleic acid binding transcription factor activity. 45: Protein binding transcription factor activity. 46: Structural molecule activity. 47: Transporter activity
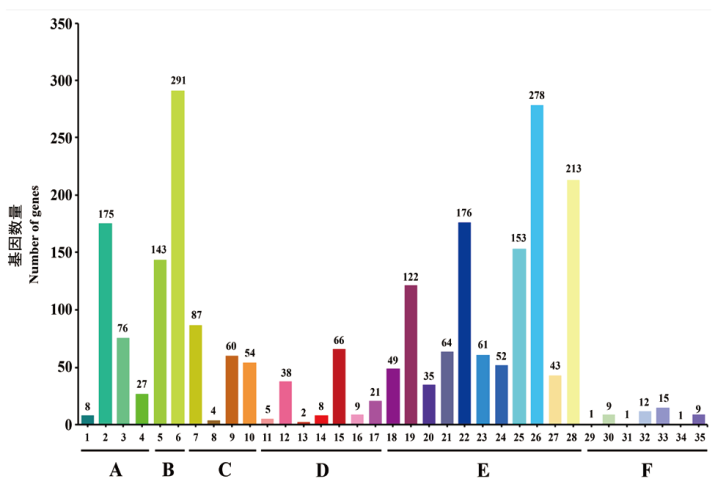
图6 CCBC3-3-1的KEEG功能注释分类 A:细胞过程(1:运输和分解代谢;2:细胞群落-原核生物;3:细胞的运动性;4:细胞生长与死亡);B:环境信息处理(5:信号转导;6:膜运输);C:遗传信息处理(7:翻译;8:转录;9:复制和修复;10:折叠、分类和降解);D:人类疾病(11:神经退行性疾病;12:侵染性疾病;13:免疫性疾病;14:内分泌和代谢疾病;15:抗药性;16:心血管疾病;17:癌症);E:新陈代谢(18:外源物质生物降解和代谢;19:核苷酸代谢;20:萜类和聚酮类代谢; 21:其他氨基酸代谢;22:辅助因子和维生素代谢;23:脂类代谢;24:多糖的生物合成与代谢;25:能量代谢;26:糖代谢;27:其他次生代谢物的生物合成;28:氨基酸代谢);F:有机体系统(29:神经系统;30:免疫系统;31:排泄系统;32:环境适应;33:内分泌系统;34:消化系统;35:衰老)
Fig. 6 KEEG function classification of strain CCBC3-3-1 A: Cellular processes(1: Transport and catabolism. 2: Cellular community-prokaryotes. 3: Cell motility. 4: Cell growth and death). B: Environmental information processing(5: Signal transduction. 6: Membrane transport). C: Genetic information processing(7: Translation. 8: Transcription. 9: Replication and repair. 10: Folding, sorting and degradation). D: Human diseases(11: Neurodegenerative diseases. 12: Infectious diseases. 13: Immune diseases. 14: Endocrine and metabolic diseases. 15: Drug resistance. 16: Cardiovascular diseases. 17: Cancers). E: Metabolism(18: Xenobiotics biodegradation and metabolism. 19: Nucleotide metabolism. 20: Metabolism of terpenoids and polyketides. 21: Metabolism of other amino acids. 22: Metabolism of cofactors and vitamins. 23: Lipid metabolism. 24: Glycan biosynthesis and metabolism. 25: Energy metabolism. 26: Carbohydrate metabolism. 27: Biosynthesis of other secondary metabolites. 28: Amino acid metabolism).F: Organismal systems(29: Nervous system. 30: Immune system. 31: Excretory system. 32: Environmental adaptation. 33: Endocrine system. 34: Digestive system. 35: Aging)
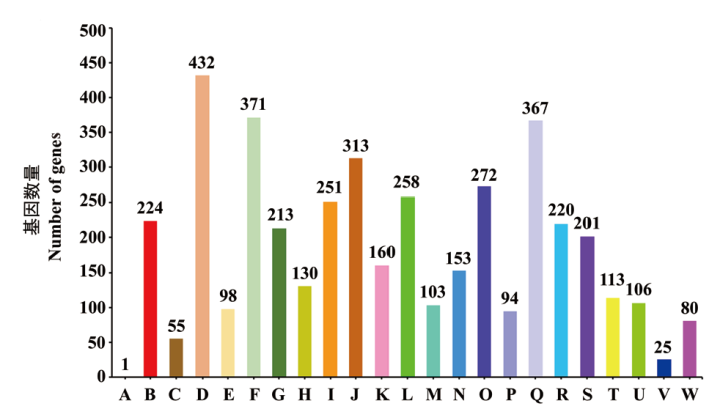
图7 CCBC3-3-1的COG功能注释分类 A:RNA加工和修饰;B:能量产生与转换;C:细胞周期控制, 细胞分裂和染色体分裂;D:氨基酸转运与代谢;E:核苷酸转运与代谢;F:碳水化合物转运与代谢;G:辅酶转运与代谢;H:脂质转运与代谢;I:翻译、核糖体结构和生物合成;J:转录;K:复制, 重组与修复;L:细胞壁/膜/包膜的生物合成;M:细胞运动:N:翻译后修饰,蛋白质转化与分子伴侣;O:无机离子的转运与代谢;P:次生代谢产物的生物合成、运输与分解代谢;Q:一般功能预测;R:功能未知;S:信号转导机制;T:细胞内运输,分泌和囊泡;U:防御机制;V:细胞外结构;W:移动基因组:原噬菌体、转座子
Fig. 7 COG function classification of strain CCBC3-3-1 A: RNA processing and modification. B: Energy production and conversion. C: Cell cycle control, cell division, chromosome partitioning. D: Amino acid transport and metabolism. E: Nucleotide transport and metabolism. F: Carbohydrate transport and metabolism. G: Coenzyme transport and metabolism. H: Lipid transport and metabolism. I: Translation, ribosomal structure and biogenesis. J: Transcription. K: Replication, recombination and repair. L: Cell wall/membrane/envelope biogenesis. M: Cell motility. N: Posttranslational modification, protein turnover, chaperones. O: Inorganic ion transport and metabolism. P: Secondary metabolites biosynthesis, transport and catabolism. Q: General function prediction only. R: Function unknown. S: Signal transduction mechanisms. T: Intracellular trafficking, secretion, and vesicular. U: Defense mechanisms. V: Extracellular structures. W: Mobilome: prophages, transposons

图8 CCBC3-3-1的CAZy功能注释分类 AAs:辅助功能的酶基因;CBMs:碳水化合物结合模块基因;CEs:糖酯酶基因;GHs:糖苷水解酶基因;GTs:糖基转移酶基因;PLs:多糖裂解酶基因
Fig. 8 CAZy function classification of strain CCBC3-3-1 AAs: Auxiliary activities. CBMs: Carbohydrate-binding modules. CEs: Carbohydrate esterases. GHs: Glycoside hydrolases.GTs: Glycosyl transferases. PLs: Polysaccharide lyases
| 项目Item | CCBC3-3-1 | P. vagans C9-1 | P. agglomerans TH81 | P. ananatis PA13 | P. eucalypti LMG24197 |
|---|---|---|---|---|---|
| GeneBank number | CP034363.1 | CP002206.1 | CP031649.1 | CP003085.1 | CP045720.1 |
| Length of chromosome /bp | 5 008 525 | 4 024 986 | 4 128 817 | 4 586 378 | 4 035 995 |
| GC content/% | 52.9 | 55.5 | 55.2 | 53.7 | 54.6 |
| Number of CDSs | 4 610 | 3 707 | 3 766 | 4 254 | 3 692 |
| Number of rRNAs | 22 | 22 | 21 | 22 | 21 |
| Number of tRNAs | 87 | 78 | 72 | 86 | 76 |
| Number of CRISPRS | 1 | 0 | 0 | 0 | 0 |
表1 菌株CCBC3-3-1与Pantoea属其他4个种的染色体序列特征比较
Table 1 Comparison of chromosome sequence feature between strain CCBC3-3-1 with other 4 species of Pantoea
| 项目Item | CCBC3-3-1 | P. vagans C9-1 | P. agglomerans TH81 | P. ananatis PA13 | P. eucalypti LMG24197 |
|---|---|---|---|---|---|
| GeneBank number | CP034363.1 | CP002206.1 | CP031649.1 | CP003085.1 | CP045720.1 |
| Length of chromosome /bp | 5 008 525 | 4 024 986 | 4 128 817 | 4 586 378 | 4 035 995 |
| GC content/% | 52.9 | 55.5 | 55.2 | 53.7 | 54.6 |
| Number of CDSs | 4 610 | 3 707 | 3 766 | 4 254 | 3 692 |
| Number of rRNAs | 22 | 22 | 21 | 22 | 21 |
| Number of tRNAs | 87 | 78 | 72 | 86 | 76 |
| Number of CRISPRS | 1 | 0 | 0 | 0 | 0 |
| 质粒 Plasmid | 数量 Number | GenBank登录号 GenBank number | 长度 Length/bp | GC含量 GC Content /% | CDSs数量 CDSs number | rRNA数量 rRNA number | tRNA数量 tRNA number | CRISPRS数量 CRISPRS number |
|---|---|---|---|---|---|---|---|---|
| CCBC3-3-1 | 3 | CP034364.1 CP034365.1 CP034366.1 | 25 471 29 467 96 304 | 46.7 42.7 49.9 | 38 37 114 | 0 0 0 | 0 0 0 | 0 0 0 |
| P. vagans C9-1 | 3 | CP001893.1 CP001894.1 CP001895.1 | 167 983 165 693 529 676 | 53.0 51.1 53.9 | 144 183 508 | 0 0 0 | 0 0 0 | 0 0 0 |
| P. agglomerans TH81 | 3 | CP031650.1 CP031651.1 CP031652.1 | 520 959 182 169 152 523 | 53.7 52.3 48.9 | 517 161 160 | 0 0 0 | 0 0 0 | 0 0 0 |
| P. ananatis PA13 | 1 | CP003086.1 | 280 753 | 52.3 | 257 | 0 | 0 | 0 |
| P. eucalypti LMG24197 | 3 | CP045721.1 CP045722.1 CP045723.1 | 529 303 138 725 94 967 | 52.2 50.9 52.2 | 528 114 99 | 0 0 0 | 0 0 0 | 0 0 0 |
表2 菌株CCBC3-3-1与Pantoea属其他4个种的质粒序列特征比较
Table 2 Comparison of strain CCBC3-3-1 plasmid sequence with other 4 species of Pantoea
| 质粒 Plasmid | 数量 Number | GenBank登录号 GenBank number | 长度 Length/bp | GC含量 GC Content /% | CDSs数量 CDSs number | rRNA数量 rRNA number | tRNA数量 tRNA number | CRISPRS数量 CRISPRS number |
|---|---|---|---|---|---|---|---|---|
| CCBC3-3-1 | 3 | CP034364.1 CP034365.1 CP034366.1 | 25 471 29 467 96 304 | 46.7 42.7 49.9 | 38 37 114 | 0 0 0 | 0 0 0 | 0 0 0 |
| P. vagans C9-1 | 3 | CP001893.1 CP001894.1 CP001895.1 | 167 983 165 693 529 676 | 53.0 51.1 53.9 | 144 183 508 | 0 0 0 | 0 0 0 | 0 0 0 |
| P. agglomerans TH81 | 3 | CP031650.1 CP031651.1 CP031652.1 | 520 959 182 169 152 523 | 53.7 52.3 48.9 | 517 161 160 | 0 0 0 | 0 0 0 | 0 0 0 |
| P. ananatis PA13 | 1 | CP003086.1 | 280 753 | 52.3 | 257 | 0 | 0 | 0 |
| P. eucalypti LMG24197 | 3 | CP045721.1 CP045722.1 CP045723.1 | 529 303 138 725 94 967 | 52.2 50.9 52.2 | 528 114 99 | 0 0 0 | 0 0 0 | 0 0 0 |
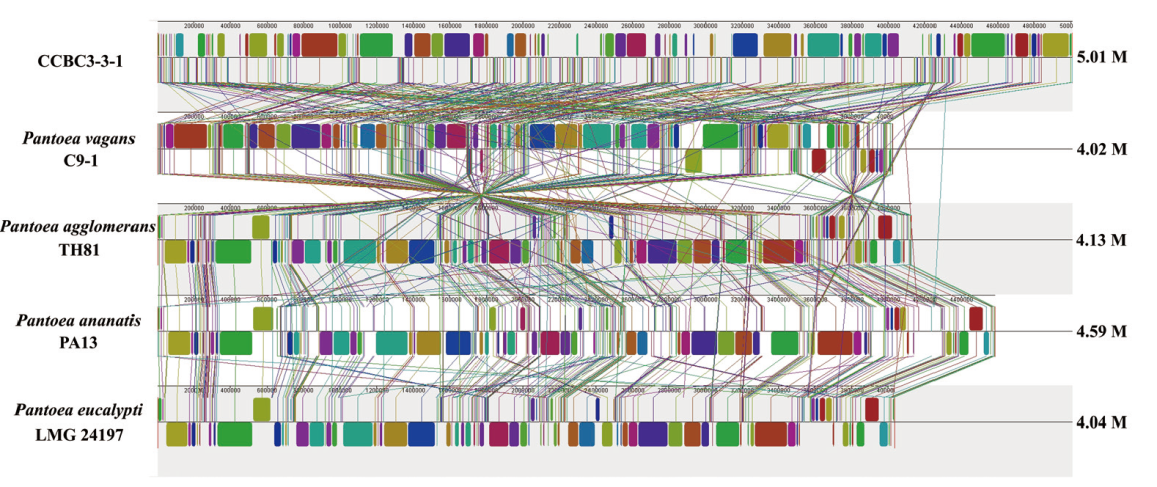
图9 CCBC3-3-1与P. vagans C9-1、P. ananatis PA13、P. agglomerans TH81和P. eucalypti LMG24197的基因组共线性分析 同种颜色区域部分代表基因组间共线性部分
Fig. 9 Genomic collinearity analysis of CCBC3-3-1 with P. vagans C9-1, P. ananatis PA13, P. agglomerans TH81 and P. eu-calypti LMG24197 The same color modules joined by linear indicate collinear region
| 物种 Species | CCBC3-3-1 | |
|---|---|---|
| OrthoANIu/% | dDDH/% | |
| P. agglomerans TH81 | 75.95 | 20.90 |
| P. agglomerans C410P1 | 75.66 | 21.00 |
| P. agglomerans CHTF15 | 75.93 | 20.80 |
| P. agglomerans CQ10 | 76.03 | 20.80 |
| P. ananatis PA13 | 74.99 | 20.60 |
| P. ananatis VY148 | 74.99 | 20.60 |
| P. ananatis LMG20103 | 74.96 | 20.70 |
| P. eucalypti LMG24197 | 75.89 | 20.90 |
| P. jilinensis D25 | 75.49 | 20.50 |
| P. vagans C9-1 | 75.91 | 20.90 |
| P. vagans FAAARGOS160 | 75.67 | 20.60 |
| P. vagans LMG24199 | 76.02 | 20.90 |
表3 菌株CCBC3-3-1与Pantoea 属12个菌株的OrthoANIu和dDDH值
Table 3 OrthoANIu and dDDH scores between strain CCBC3-3-1 and 12 strains of Pantoea
| 物种 Species | CCBC3-3-1 | |
|---|---|---|
| OrthoANIu/% | dDDH/% | |
| P. agglomerans TH81 | 75.95 | 20.90 |
| P. agglomerans C410P1 | 75.66 | 21.00 |
| P. agglomerans CHTF15 | 75.93 | 20.80 |
| P. agglomerans CQ10 | 76.03 | 20.80 |
| P. ananatis PA13 | 74.99 | 20.60 |
| P. ananatis VY148 | 74.99 | 20.60 |
| P. ananatis LMG20103 | 74.96 | 20.70 |
| P. eucalypti LMG24197 | 75.89 | 20.90 |
| P. jilinensis D25 | 75.49 | 20.50 |
| P. vagans C9-1 | 75.91 | 20.90 |
| P. vagans FAAARGOS160 | 75.67 | 20.60 |
| P. vagans LMG24199 | 76.02 | 20.90 |
| 编号 Cluster ID | 类型 Cluster type | 起始-终止位点 Start-stop site | 已知基因簇 Known cluster | 相似度 Similarity/% | 来源 Resources |
|---|---|---|---|---|---|
| 基因簇1 Cluster 1 | 其他(磷酸盐) Other(Phosphonate) | 1 233863 - 1 251863 | - | - | - |
| 基因簇2 Cluster 2 | 糖化物 Saccharide | 1 593 081 - 1 618 729 | O-抗原 O-antigen | 14 | Pseudomonas aeruginosa |
| 基因簇3 Cluster 3 | 核糖体肽类似酶 RiPP-like | 1 674 964 - 1 735 767 | - | - | - |
| 基因簇4 Cluster 4 | 萜烯 Terpene | 2 149 356 - 2 241 512 | 类胡萝卜素 Carotenoid | 100 | Enterobacteriaceae bacterium DC413 |
| 基因簇5 Cluster 5 | 非核糖体多肽合成酶 NRPS | 2 335 269 - 2 383 545 | - | - | - |
| 基因簇6 Cluster 6 | 非核糖体多肽合成酶 NRPS | 3 112 137 - 3 156 045 | Amonabactin | 57 | Aeromonas hydrophila subsp. hydrophila ATCC 7966 |
| 基因簇7 Cluster 7 | 核糖体肽类似酶 RiPP-like | 3 734 150 - 3 762 973 | - | - | - |
| 基因簇8 Cluster 8 | 核糖体肽类似酶 RiPP-like | 4 182 791 - 4 203 075 | - | - | - |
表4 菌株CCBC3-3-1抗生素及次生代谢产物合成相关基因簇分析
Table 4 Analysis of antibiotic and secondary metabolite gene clusters of strain CCBC3-3-1
| 编号 Cluster ID | 类型 Cluster type | 起始-终止位点 Start-stop site | 已知基因簇 Known cluster | 相似度 Similarity/% | 来源 Resources |
|---|---|---|---|---|---|
| 基因簇1 Cluster 1 | 其他(磷酸盐) Other(Phosphonate) | 1 233863 - 1 251863 | - | - | - |
| 基因簇2 Cluster 2 | 糖化物 Saccharide | 1 593 081 - 1 618 729 | O-抗原 O-antigen | 14 | Pseudomonas aeruginosa |
| 基因簇3 Cluster 3 | 核糖体肽类似酶 RiPP-like | 1 674 964 - 1 735 767 | - | - | - |
| 基因簇4 Cluster 4 | 萜烯 Terpene | 2 149 356 - 2 241 512 | 类胡萝卜素 Carotenoid | 100 | Enterobacteriaceae bacterium DC413 |
| 基因簇5 Cluster 5 | 非核糖体多肽合成酶 NRPS | 2 335 269 - 2 383 545 | - | - | - |
| 基因簇6 Cluster 6 | 非核糖体多肽合成酶 NRPS | 3 112 137 - 3 156 045 | Amonabactin | 57 | Aeromonas hydrophila subsp. hydrophila ATCC 7966 |
| 基因簇7 Cluster 7 | 核糖体肽类似酶 RiPP-like | 3 734 150 - 3 762 973 | - | - | - |
| 基因簇8 Cluster 8 | 核糖体肽类似酶 RiPP-like | 4 182 791 - 4 203 075 | - | - | - |
| 序号No. | 名称Name | MS2分值MS2 score |
|---|---|---|
| 1 | 依诺沙星Enoxacin | 0.99 |
| 2 | 新生霉素Novobiocin | 0.94 |
| 3 | 链脲霉素Streptozocin | 0.85 |
| 4 | 手霉素A Manumycin A | 0.68 |
| 5 | 司帕沙星Sparfloxacin | 0.67 |
| 6 | 阿克拉霉素Aclarubicin | 0.65 |
表5 CCBC3-3-1发酵液中鉴定出的抗生素类物质
Table 5 Antibiotics in the fermentation broth of CCBC3-3-1
| 序号No. | 名称Name | MS2分值MS2 score |
|---|---|---|
| 1 | 依诺沙星Enoxacin | 0.99 |
| 2 | 新生霉素Novobiocin | 0.94 |
| 3 | 链脲霉素Streptozocin | 0.85 |
| 4 | 手霉素A Manumycin A | 0.68 |
| 5 | 司帕沙星Sparfloxacin | 0.67 |
| 6 | 阿克拉霉素Aclarubicin | 0.65 |
| [1] | 李海龙, 李端亮. 黄栌属植物研究进展[J]. 陕西林业科技, 2009(6): 22-27. |
| Li HL, Li DL. Advances in studies on genus Cotinus(tourn.) mill[J]. Shaanxi For Sci Technol, 2009(6): 22-27. | |
| [2] | 葛雨萱, 周肖红, 刘洋, 等. 黄栌属种质资源、栽培繁殖、化学成分、叶色调控研究进展[J]. 园艺学报, 2014, 41(9): 1833-1845. |
| Ge YX, Zhou XH, et al. Recent advances in germplasm, cultivation, propagation, chemical components and leaf color regulation of Cotinus[J]. Acta Hortic Sin, 2014, 41(9): 1833-1845. | |
| [3] | 雷增普. 北京地区黄栌黄萎病研究初报[J]. 森林病虫通讯, 1991, 10(3): 12. |
| Lei ZP. Preliminary report on Verticillium wilt of cotinum coggygria in Beijing area[J]. For Pest Dis, 1991, 10(3): 12. | |
| [4] | 雷增普. 北京地区黄栌黄萎病病原菌的研究[J]. 北京林业大学学报, 1993, 15(3): 88-93. |
| Lei ZP. Study of Verticillium causing Cotinus coggygria wilt in the Beijing area[J]. J Beijing For Univ, 1993, 15(3): 88-93. | |
| [5] | Dung JKS, Hamm PB, Eggers JE, et al. Incidence and impact of Verticillium dahliae in soil associated with certified potato seed lots[J]. Phytopathology, 2013, 103(1): 55-63. |
| [6] | 张芸. 棉花内生真菌对棉花黄萎病的控制作用及机理研究[D]. 杨凌: 西北农林科技大学, 2016. |
| Zhang Y. Control effects and mechanism research of cotton endophytic fungi against Verticillium wilt in Gossypium hirsutum[D]. Yangling: Northwest A & F University, 2016. | |
| [7] | 刘璐. 对节白蜡内生细菌YZU-SG146对棉花黄萎病生防作用机制的研究[D]. 荆州: 长江大学, 2021. |
| Liu L. Study on the biological control mechanism of endophytic bacteria YZU-SG146 from Fraxinus hupehensis against Verticillium wilt of cotton[D]. Jingzhou: Yangtze University, 2021. | |
| [8] | 王建美, 田呈明, 过颂新, 等. 黄栌根内生真菌分离鉴定及拮抗真菌筛选[J]. 菌物研究, 2008, 6(1): 35-39. |
| Wang JM, Tian CM, Guo SX, et al. Isolation and screening of endophytic antifungal fungi from Cotinus coggygria roots[J]. J Fungal Res, 2008, 6(1): 35-39. | |
| [9] | 孙妍, 于地美, 等. 黄栌枯萎病菌拮抗细菌的分离与鉴定[J]. 南京林业大学学报: 自然科学版, 2015, 39(6): 17-23. |
| Sun Y, Yu DM, et al. Isolation and identification of an antagonistic bacterium against smoke tree wilt fungus Verticillium dahliae[J]. J Nanjing For Univ Nat Sci Ed, 2015, 39(6): 17-23. | |
| [10] | 许芳. 植物内生细菌对大丽轮枝菌毒素减毒作用的研究[D]. 南京: 南京师范大学, 2012. |
| Xu F. Study on the attenuation of Verticillium dahliae toxin by endophytic bacteria in plants[D]. Nanjing: Nanjing Normal University, 2012. | |
| [11] | Tanizawa Y, Fujisawa T, Kaminuma E, et al. DFAST and DAGA: web-based integrated genome annotation tools and resources[J]. Biosci Microbiota Food Health, 2016, 35(4): 173-184. |
| [12] |
Tanizawa Y, Fujisawa T, Nakamura Y. DFAST: a flexible prokaryotic genome annotation pipeline for faster genome publication[J]. Bioinformatics, 2018, 34(6): 1037-1039.
doi: 10.1093/bioinformatics/btx713 pmid: 29106469 |
| [13] |
Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition[J]. Proc Natl Acad Sci USA, 2009, 106(45): 19126-19131.
doi: 10.1073/pnas.0906412106 pmid: 19855009 |
| [14] | Wayne LG, Moore WEC, et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics[J]. Int J Syst Evol Microbiol, 1987, 37(4): 463-464. |
| [15] | Yoon SH, Ha SM, Lim J, et al. A large-scale evaluation of algorithms to calculate average nucleotide identity[J]. Antonie Van Leeuwenhoek, 2017, 110(10): 1281-1286. |
| [16] | Meier-Kolthoff JP, Carbasse JS, Peinado-Olarte RL, et al. TYGS and LPSN: a database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes[J]. Nucleic Acids Res, 2022, 50(D1): D801-D807. |
| [17] |
Darling ACE, Mau B, Blattner FR, et al. Mauve: multiple alignment of conserved genomic sequence with rearrangements[J]. Genome Res, 2004, 14(7): 1394-1403.
doi: 10.1101/gr.2289704 pmid: 15231754 |
| [18] | Blin K, Shaw S, Kloosterman AM, et al. antiSMASH 6.0: improving cluster detection and comparison capabilities[J]. Nucleic Acids Res, 2021, 49(W1): W29-W35. |
| [19] | Reddy BVB, Kallifidas D, Kim JH, et al. Natural product biosynthetic gene diversity in geographically distinct soil microbiomes[J]. Appl Environ Microbiol, 2012, 78(10): 3744-3752. |
| [20] | Gontang EA, Gaudêncio SP, Fenical W, et al. Sequence-based analysis of secondary-metabolite biosynthesis in marine Actinobacteria[J]. Appl Environ Microbiol, 2010, 76(8): 2487-2499. |
| [21] |
Dunn WB, Broadhurst D, Begley P, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry[J]. Nat Protoc, 2011, 6(7): 1060-1083.
doi: 10.1038/nprot.2011.335 pmid: 21720319 |
| [22] |
Kuhl C, Tautenhahn R, Böttcher C, et al. CAMERA: an integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets[J]. Anal Chem, 2012, 84(1): 283-289.
doi: 10.1021/ac202450g pmid: 22111785 |
| [23] | Zhou JH, Xia F, Che SC, et al. Complete genome sequence of Pantoea sp. strain CCBC3-3-1, an antagonistic endophytic bacterium isolated from a Cotinus coggygria branch[J]. Microbiol Resour Announc, 2019, 8(38):e01004-19. |
| [24] |
Walterson AM, Stavrinides J. Pantoea: insights into a highly versatile and diverse genus within the Enterobacteriaceae[J]. FEMS Microbiol Rev, 2015, 39(6): 968-984.
doi: 10.1093/femsre/fuv027 pmid: 26109597 |
| [25] | Jiang LM, Jeong JC, Lee JS, et al. Potential of Pantoea dispersa as an effective biocontrol agent for black rot in sweet potato[J]. Sci Rep, 2019, 9(1): 16354. |
| [26] |
Smits THM, Duffy B, Blom J, et al. Pantocin A, a peptide-derived antibiotic involved in biological control by plant-associated Pantoea species[J]. Arch Microbiol, 2019, 201(6): 713-722.
doi: 10.1007/s00203-019-01647-7 pmid: 30868174 |
| [27] | Giddens SR, Feng YJ, Mahanty HK. Characterization of a novel phenazine antibiotic gene cluster in Erwinia herbicola Eh1087[J]. Mol Microbiol, 2002, 45(3): 769-783. |
| [28] |
Pusey PL, Stockwell VO, Reardon CL, et al. Antibiosis activity of Pantoea agglomerans biocontrol strain E325 against Erwinia amylovora on apple flower stigmas[J]. Phytopathology, 2011, 101(10): 1234-1241.
doi: 10.1094/PHYTO-09-10-0253 pmid: 21679036 |
| [29] | Wright SA, Zumoff CH, Schneider L, et al. Pantoea agglomerans strain EH318 produces two antibiotics that inhibit Erwinia amylovora in vitro[J]. Appl Environ Microbiol, 2001, 67(1): 284-292. |
| [30] | Aydin M, Lucht N, König WA, et al. Structure elucidation of the peptide antibiotics herbicolin A and B[J]. Liebigs Ann Chem, 1985(11): 2285-2300. |
| [31] | Vanneste JL, Cornish DA, Yu J, et al. The peptide antibiotic produced by Pantoea agglomerans eh252 is a microcin[J]. Acta Hortic, 2002(590): 285-290. |
| [32] | Vanneste JL, Yu J, Cornish DA. Presence of genes homologous to those necessary for synthesis of microcin mcceh252 in strains of Pantoea agglomerans[J]. Acta Hortic, 2008(793): 391-396. |
| [33] | Xu SD, Liu YX, Cernava T, et al. Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts[J]. Nat Microbiol, 2022, 7(6): 831-843. |
| [1] | 田彤彤, 葛家振, 高鹏程, 李学瑞, 宋国栋, 郑福英, 储岳峰. 绵羊肺炎支原体GH3-3株全基因组测序及生物信息学分析[J]. 生物技术通报, 2024, 40(7): 323-334. |
| [2] | 孙亚楠, 王春雪, 王欣, 杜秉海, 刘凯, 汪城墙. 萎缩芽孢杆菌CNY01的生防特性及其对玉米的抗盐促生作用[J]. 生物技术通报, 2024, 40(5): 248-260. |
| [3] | 王腾辉, 葛雯冬, 罗雅方, 范震宇, 王玉书. 基于极端混合池(BSA)全基因组重测序的羽衣甘蓝白色叶基因定位[J]. 生物技术通报, 2023, 39(9): 176-182. |
| [4] | 方澜, 黎妍妍, 江健伟, 成胜, 孙正祥, 周燚. 盘龙参内生真菌胞内细菌7-2H的分离鉴定和促生特性研究[J]. 生物技术通报, 2023, 39(8): 272-282. |
| [5] | 郭少华, 毛会丽, 刘征权, 付美媛, 赵平原, 马文博, 李旭东, 关建义. 一株鱼源致病性嗜水气单胞菌XDMG的全基因组测序及比较基因组分析[J]. 生物技术通报, 2023, 39(8): 291-306. |
| [6] | 潘国强, 吴思源, 刘璐, 郭惠明, 程红梅, 苏晓峰. 大丽轮枝菌(Verticillim dahliae)突变体库的构建与分析[J]. 生物技术通报, 2023, 39(5): 112-119. |
| [7] | 张志霞, 李天培, 曾虹, 朱稀贤, 杨天雄, 马斯楠, 黄磊. 冰冷杆菌PG-2的基因组测序及生物信息学分析[J]. 生物技术通报, 2023, 39(3): 290-300. |
| [8] | 和梦颖, 刘文彬, 林震鸣, 黎尔彤, 汪洁, 金小宝. 一株抗革兰阳性菌的戈登氏菌WA4-43全基因组测序与分析[J]. 生物技术通报, 2023, 39(2): 232-242. |
| [9] | 张傲洁, 李青云, 宋文红, 颜少慧, 唐爱星, 刘幽燕. 基于苯酚降解的粪产碱杆菌Alcaligenes faecalis JF101的全基因组分析[J]. 生物技术通报, 2023, 39(10): 292-303. |
| [10] | 王帅, 吕鸿睿, 张昊, 吴占文, 肖翠红, 孙冬梅. 解磷菌PSB-R全基因组测序鉴定及其解磷特性分析[J]. 生物技术通报, 2023, 39(1): 274-283. |
| [11] | 张泽颖, 范清锋, 邓云峰, 韦廷舟, 周正富, 周建, 王劲, 江世杰. 一株高产脂肪酶菌株WCO-9全基因组测序及比较基因组分析[J]. 生物技术通报, 2022, 38(10): 216-225. |
| [12] | 田李, 李俊娇, 戴小枫, 张丹丹, 陈捷胤. 从功能基因到生物学性状:大丽轮枝菌致病性形成的分子基础[J]. 生物技术通报, 2022, 38(1): 51-69. |
| [13] | 薛清, 杜虹锐, 薛会英, 王译浩, 王暄, 李红梅. 苜蓿滑刃线虫线粒体基因组及其系统发育研究[J]. 生物技术通报, 2021, 37(7): 98-106. |
| [14] | 陈体强, 徐晓兰, 石林春, 钟礼义. 紫芝栽培品种‘武芝2号’(‘紫芝S2’)全基因组测序及分析[J]. 生物技术通报, 2021, 37(11): 42-56. |
| [15] | 郭鹤宝, 王星, 何山文, 张晓霞. 表型特征结合基因组分析鉴定不同菌落形态Bacillus velezensis ACCC 19742[J]. 生物技术通报, 2020, 36(2): 142-148. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||