








生物技术通报 ›› 2024, Vol. 40 ›› Issue (8): 212-220.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0229
许雪飞1,2( ), 杨盼盼2, 张文亮3, 边光亚3, 徐雷锋2, 刘会超1(
), 杨盼盼2, 张文亮3, 边光亚3, 徐雷锋2, 刘会超1( ), 明军2(
), 明军2( )
)
收稿日期:2024-03-08
出版日期:2024-08-26
发布日期:2024-06-27
通讯作者:
明军,男,博士,研究员,研究方向:观赏植物资源与育种;E-mail: mingjun@caas.cn;作者简介:许雪飞,女,硕士研究生,研究方向:园林植物与应用;E-mail: 2378472302@qq.com基金资助:
XU Xue-fei1,2( ), YANG Pan-pan2, ZHANG Wen-liang3, BIAN Guang-ya3, XU Lei-feng2, LIU Hui-chao1(
), YANG Pan-pan2, ZHANG Wen-liang3, BIAN Guang-ya3, XU Lei-feng2, LIU Hui-chao1( ), MING Jun2(
), MING Jun2( )
)
Received:2024-03-08
Published:2024-08-26
Online:2024-06-27
摘要:
【目的】百合斑驳病毒(lily mottle virus, LMoV)是危害重要食药用百合卷丹的主要病毒之一,每年给百合种植业造成数十亿的损失。通过原位杂交技术明确LMoV在卷丹顶端分生组织中的分布,为百合脱毒培养提供支撑。【方法】采用种植于贵州省清镇市的栽培卷丹作为试验材料,利用RT-PCR并于NCBI网站下载不同地方LMoV分离物构建进化树鉴定卷丹所携带的病毒种类,据检测结果选取LMoV为对象,基于LMoV基因组序列设计并制备RNA荧光探针,开展卷丹茎尖和根尖石蜡切片组织的RNA原位杂交,观察卷丹茎尖和根尖中LMoV的分布。【结果】RT-PCR检测表明卷丹材料侵染了LMoV,构建进化树发现本实验分离物与吉林和辽宁的LMoV分离物关系最近,原位杂交进而表明卷丹茎尖中LMoV杂交信号主要分布在顶端分生组织下方0.15-0.2 mm的组织中,靠近紧挨分生组织叶原基的初生叶基部有较弱的病毒杂交信号,靠近初生叶的成熟叶顶端中病毒荧光信号强烈,感染LMoV的卷丹分生组织中心纵切面无毒区大小为(0.4-0.6)mm ×(0.15-0.2)mm;在根尖0.25 mm × 0.2 mm分生组织中无杂交信号分布,在皮层细胞中信号最强烈,在根冠和根伸长区信号较弱。【结论】卷丹顶端分生组织及最接近顶端的一个叶原基[大小约(0.4-0.6)mm×0.2 mm]未发现病毒信号,可作为茎尖脱毒的起始材料。根顶端分生组织外侧根冠有较强病毒信号,不适合作为卷丹脱毒培养的外植体。
许雪飞, 杨盼盼, 张文亮, 边光亚, 徐雷锋, 刘会超, 明军. 百合斑驳病毒在卷丹顶端分生组织中的分布[J]. 生物技术通报, 2024, 40(8): 212-220.
XU Xue-fei, YANG Pan-pan, ZHANG Wen-liang, BIAN Guang-ya, XU Lei-feng, LIU Hui-chao, MING Jun. Distribution of Lily Mottle Virus in the Shoot Tips and Root Tips of Lilium lancifolium[J]. Biotechnology Bulletin, 2024, 40(8): 212-220.
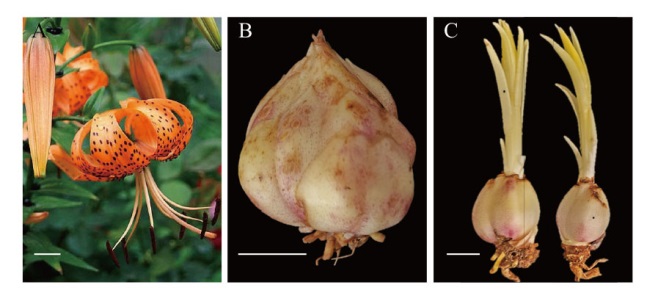
图1 卷丹原位杂交试验材料 A:花;B:采收后的鳞茎;C:包埋后抽茎的鳞茎。标尺=1 cm
Fig. 1 In situ hybridization material of L. lancifolium A: Flower; B: bulbs after harvesting; C: bulbs after embedding. Bar = 1 cm
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 产物大小Product size/bp | 参考文献Reference |
|---|---|---|---|
| CMV- Forward primer | ATGGACAAATCTGAATCAACCAG | 657 | [ |
| CMV- Reverse primers | TCAGACTGGGAGCACTCCAG | ||
| LSV- Forward primer | GAAGAAGCACGCTGGACTG | 171 | |
| LSV- Reverse primers | CGCCTGATGATCCCCTC | ||
| LMoV- Forward primer | GAAGAAGCACGCTGGACTG | 428 | |
| LMoV - Reverse primers | CGCCTGATGATCCCCTC | ||
| Lily Actin- Forward primer | GCATCACACCTTCTACAACG | 257 | [ |
| Lily Actin - Reverse primers | GAAGAGCATAACCCTCATAGA |
表1 病毒检测引物序列
Table 1 Primer sequences for virus detection
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 产物大小Product size/bp | 参考文献Reference |
|---|---|---|---|
| CMV- Forward primer | ATGGACAAATCTGAATCAACCAG | 657 | [ |
| CMV- Reverse primers | TCAGACTGGGAGCACTCCAG | ||
| LSV- Forward primer | GAAGAAGCACGCTGGACTG | 171 | |
| LSV- Reverse primers | CGCCTGATGATCCCCTC | ||
| LMoV- Forward primer | GAAGAAGCACGCTGGACTG | 428 | |
| LMoV - Reverse primers | CGCCTGATGATCCCCTC | ||
| Lily Actin- Forward primer | GCATCACACCTTCTACAACG | 257 | [ |
| Lily Actin - Reverse primers | GAAGAGCATAACCCTCATAGA |
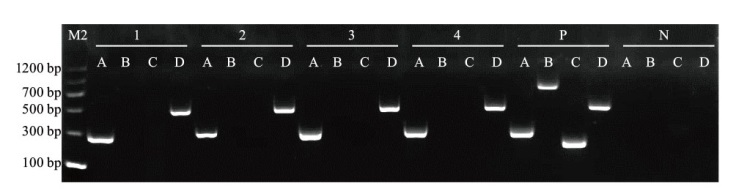
图2 三种病毒RT-PCR检测电泳图 1-4分别为4株卷丹百合样品; P:阳性对照;N:阴性对照;A:actin(257 bp);B:CMV(657 bp);C:LSV(171 bp);D:LMoV(428 bp);M2:DNA marker
Fig. 2 Electrophoresis diagram of three viruses via RT-PCR 1-4 are 4 lily samples; P: positive control; N: negative control
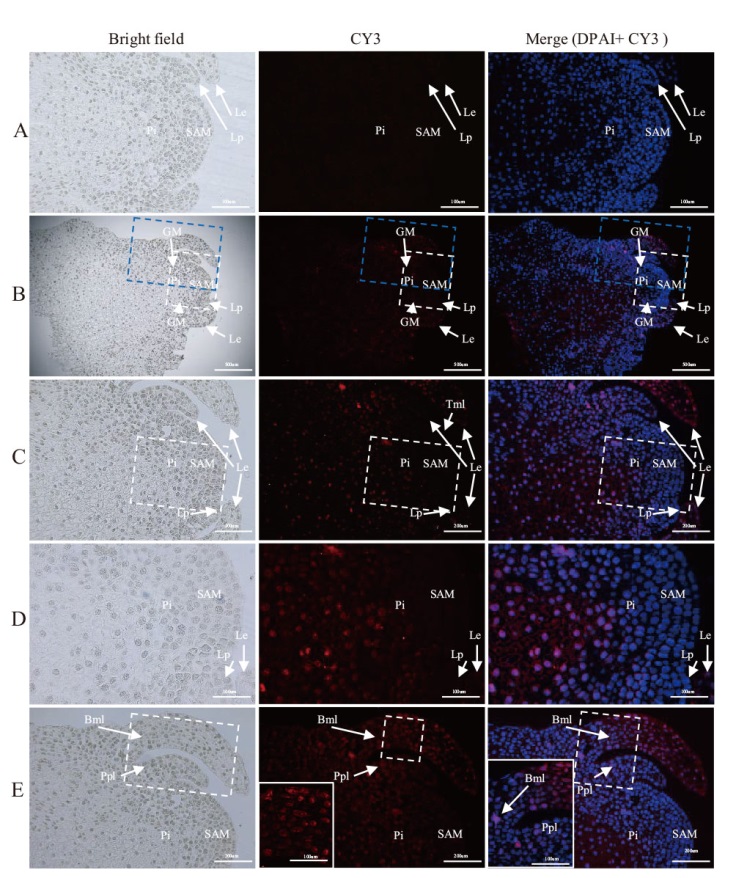
图4 LMoV在卷丹茎尖中的原位杂交结果 A:阴性对照(未感染LMoV 的卷丹根尖);B:茎尖中LMoV信号;C:茎尖分生组织及其周围组织中病毒分布(为B中白色虚线框放大图);D:分生组织及其最靠近顶端的一个叶原基无病毒信号(为C中白色虚线框放大图);E:顶端分化新的叶原基图,原先第一片靠近顶端的叶原基发育来的幼叶基部开始感染(为B中蓝色虚线框放大图);SAM:顶端分生组织;Le:叶片;Lp:叶原基;Bpl:初生叶基部;Bml:成熟叶基部;Tml:成熟叶顶端;Pi:髓细胞。B比例尺=500 μm;C和E比例尺=200 μm,A和D标尺=100 μm。蓝色为细胞核染色,红色为LMoV阳性信号
Fig. 4 In situ hybridization of LMoV in the shoot tips of L. lancifolium A: Negative control(LMoV-uninfected stem tips of L. lancifolium); B: LMoV signal in the stem tip; C: virus distribution in the apical meristem and its surrounding tissues(enlarged white dashed box in B); D: non-virus signal in the meristem and its closest apical leaf primordium(enlarged white dashed box in C); E: image of the apical meristem dividing into new leaf primordia, where the base of the young leaves developed from the original first near-apical leaf primordium began to be infected(enlarged blue dashed box in B); SAM: apical meristematic tissue; Le: leaf blade; Lp: leaf primordium; Bpl: the base of the primary leaf; Bml: the base of the mature leaf; Tml: the tip of a mature leaf; Pi: pith cell. B scale bar = 500 μm; C and E scale bar = 200 μm; A and D scale bar = 100 μm. Blue is nuclear staining and red is LMoV positive signal
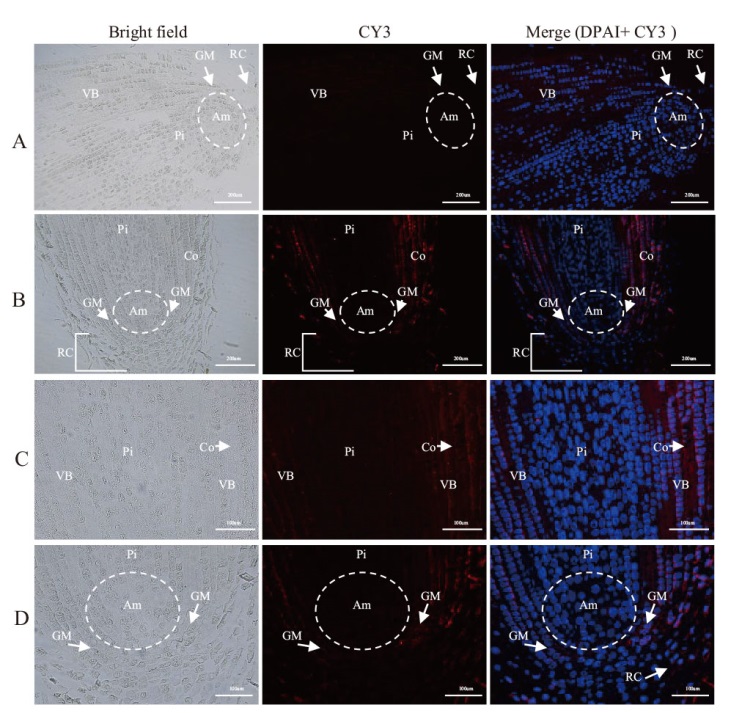
图5 LMoV在卷丹根尖中的原位杂交结果 A:阴性对照(未感染LMoV的卷丹根尖);B:根尖中心纵剖面中LMoV分布图;C:LMoV在根尖皮层细胞和维管束中分布;D:LMoV在根顶端组织中的分布;VB:维管束;Pi:髓细胞;Co:皮层细胞;Ez:伸长区。蓝色为细胞核染色,红色为LMoV阳性信号。A, B标尺=200 μm;C、D标尺=100 μm
Fig. 5 In situ hybridization of LMoV in the root tip of L. lancifolium A: Negative control(LMoV-uninfected stem tips of L. lancifolium); B: LMoV distribution in the longitudinal profile of the apical center; C: distribution of LMoV in root tip cortical cells and vascular bundles; D: distribution of LMoV in root apical meristem(Am)and a small amount invades the root cap(RC); VB: vascular bundles; Pi: pith cells; Co: cortex cells; Ez: elongation zone. The blue color is the nuclei staining, and the red color is the LMoV positive signal. A, B bars = 200 μm, C, D bars = 100 μm
| [1] | Asjes CJ. Control of aphid-borne Lily symptomless virus and Lily mottle virus in Lilium in the Netherlands[J]. Virus Res, 2000, 71(1/2): 23-32. |
| [2] |
Chinestra SC, Facchinetti C, Curvetto NR, et al. Detection and frequency of lily viruses in Argentina[J]. Plant Dis, 2010, 94(10): 1188-1194.
doi: 10.1094/PDIS-07-09-0419 pmid: 30743618 |
| [3] | 廖天蓝, 罗金燕, 陈磊, 等. 郁金香和百合主要病害、病原及鉴定方法[J]. 植物保护, 2023, 49(6): 1-9. |
| Liao TL, Luo JY, Chen L, et al. Major diseases and pathogens of tulip and lily and the methods for pathogen detection and identification[J]. Plant Prot, 2023, 49(6): 1-9. | |
| [4] | Ogilvie L. A transmissible virus disease of the Easter lily[J]. Ann Appl Biol, 1928, 15(4): 540-562. |
| [5] | 柳玉晶. 百合植物组织培养脱毒技术研究进展[J]. 现代园艺, 2023, 46(13): 12-14. |
| Liu YJ. Research progress on virus-free technology of lily plant tissue culture[J]. Contemp Hortic, 2023, 46(13): 12-14. | |
| [6] | Chinestra SC, Curvetto NR, Marinangeli PA. Production of virus-free plants of Lilium spp. from bulbs obtained in vitro and ex vitro[J]. Sci Hortic, 2015, 194: 304-312. |
| [7] |
Lim MS, Kim SM, Choi SH. Simultaneous detection of three lily-infecting viruses using a multiplex Luminex bead array[J]. J Virol Methods, 2016, 231: 34-37.
doi: 10.1016/j.jviromet.2016.02.007 pmid: 26898956 |
| [8] | 源朝政. 卷丹百合病毒检测及离体培养研究[D]. 长沙: 湖南农业大学, 2014. |
| Yuan CZ. The study of virus detection and isolated culture on lanceleaf lily bulb[D]. Changsha: Hunan Agricultural University, 2014. | |
| [9] | 周晓波, 吴艺飞, 丁茁荑, 等. 卷丹百合3种主要病毒脱毒方法研究[J]. 湖南农业科学, 2016(10): 7-10. |
| Zhou XB, Wu YF, Ding ZY, et al. Detoxification methods of three viruses of Lilium lancifolium[J]. Hunan Agric Sci, 2016(10): 7-10. | |
| [10] | 符勇耀, 杨利平, 高海洪, 等. 重庆卷丹病毒检测与脱毒方法研究[J]. 西北农业学报, 2020, 29(7): 1068-1077. |
| Fu YY, Yang LP, Gao HH, et al. Virus detection of Lilium lancifolium thunb. in Chongqing and study on its detoxification technology[J]. Acta Agric Boreali Occidentalis Sin, 2020, 29(7): 1068-1077. | |
| [11] | 尹计成. 兰州百合主要病毒检测与脱毒体系构建[D]. 西宁: 青海大学, 2022. |
| Yin JC. Virus detection and system construction to obtain virus-free Lilium davidii var. unicolor[D]. Xining: Qinghai University, 2022. | |
| [12] | 江明殊, 王跃华, 刘涛, 等. 多倍体川贝母脱毒苗的诱导[J]. 江苏农业科学, 2015, 43(3): 41-43. |
| Jiang MS, Wang YH, Liu T, et al. Induction of virus-free seedlings of polyploid Fritillaria cirrhosa[J]. Jiangsu Agric Sci, 2015, 43(3): 41-43. | |
| [13] | Gong HL, Dusengemungu L, Lv P, et al. Advancements in lily viruses management: challenges and solutions in elimination and detection[J]. Horticulturae, 2023, 9(7): 790. |
| [14] |
Zhang YB, Wang YJ, Meng J, et al. Development of an immunochromatographic strip test for rapid detection of lily symptomless virus[J]. J Virol Methods, 2015, 220: 13-17.
doi: 10.1016/j.jviromet.2015.03.021 pmid: 25845624 |
| [15] | 徐榕雪, 明军, 穆鼎, 等. 百合三种病毒的多重RT-PCR检测[J]. 园艺学报, 2007, 34(2): 443-448. |
| Xu RX, Ming J, Mu D, et al. Detection of three lily viruses by multiplex RT-PCR[J]. Acta Hortic Sin, 2007, 34(2): 443-448. | |
| [16] | Xu LF, Ming J. Development of a multiplex RT-PCR assay for simultaneous detection of Lily symptomless virus, Lily mottle virus, Cucumber mosaic virus, and Plantago asiatica mosaic virus in Lilies[J]. Virol J, 2022, 19(1): 219. |
| [17] | Xu LF, Song M, Ming J. Application of multiplex TaqMan real-time PCR assay in survey of five lily viruses infecting Lilium spp[J]. Agronomy, 2021, 12(1): 47. |
| [18] | Lee HJ, Choi S, Cho IS, et al. Development and application of a reverse transcription droplet digital PCR assay for detection and quantification of Plantago asiatica mosaic virus[J]. Crop Prot, 2023, 169: 106255. |
| [19] | Zhang YB, Wang YJ, Xie ZK, et al. Rapid Detection of Lily mottle virus and Arabis mosaic virus infecting lily(Lilium spp.)using reverse transcription loop-mediated isothermal amplification[J]. Plant Pathol J, 2020, 36(2): 170-178. |
| [20] |
Zhang YB, Wang YJ, Xie ZK, et al. Simultaneous detection of three lily viruses using Triplex IC-RT-PCR[J]. J Virol Methods, 2017, 249: 69-75.
doi: S0166-0934(17)30463-9 pmid: 28847563 |
| [21] |
Munganyinka E, Margaria P, Sheat S, et al. Localization of cassava brown streak virus in Nicotiana rustica and cassava Manihot esculenta(Crantz)using RNAscope® in situ hybridization[J]. Virol J, 2018, 15: 128.
doi: 10.1186/s12985-018-1038-z pmid: 30107851 |
| [22] |
Ebata M, Matsushita Y, Morimoto M, et al. Distribution of chrysanthemum chlorotic mottle viroid in shoot meristem and flower buds of chrysanthemum[J]. Eur J Plant Pathol, 2019, 154(3): 555-563.
doi: 10.1007/s10658-019-01679-1 |
| [23] | Wang C, Sun JJ, Yang XY, et al. An optimized protocol using Steedman's wax for high-sensitivity RNA in situ hybridization in shoot apical meristems and flower buds of cucumber[J]. J Integr Agric, 2023, 22(2): 464-470. |
| [24] |
张少然, 宗琪, 刘阳, 等. 柑橘黄龙病病原菌nrdB基因的原位杂交分析[J]. 园艺学报, 2023, 50(12): 2689-2700.
doi: 10.16420/j.issn.0513-353x.2022-1095 |
| Zhang SR, Zong Q, Liu Y, et al. In situ hybridization analysis of distinctive ribonucleotide reductase β subunit gene from candidatus Liberibacter asiaticus of Citrus[J]. Acta Hortic Sin, 2023, 50(12): 2689-2700. | |
| [25] | Gosalvez-Bernal B, Garcia-Castillo S, Pallas V, et al. Distribution of carnation viruses in the shoot tip: exclusion from the shoot apical meristem[J]. Physiol Mol Plant Pathol, 2006, 69(1/2/3): 43-51. |
| [26] | 赵英, 牛建新. 利用原位杂交及原位RT-PCR技术检测梨树组织中苹果茎痘病毒的分布[J]. 中国农业科学, 2008, 41(12): 4092-4099. |
| Zhao Y, Niu JX. A study of the distribution of apple stem pitting virus in tissues of pear tree using in situ hybridization and in situ RT-PCR[J]. Sci Agric Sin, 2008, 41(12): 4092-4099. | |
| [27] | Lou H, Liu ZQ, Xu QJ. Detection and elimination of shallot latent virus and onion yellow dwarf virus for potato onion(Allium cepa L. var. aggregatum Don)[J]. Ann Appl Biol, 2023, 182(1): 112-120. |
| [28] |
Gosalvez-Bernal B, Genoves A, Antonio Navarro J, et al. Distribution and pathway for phloem-dependent movement of Melon necrot-ic spot virus in melon plants[J]. Mol Plant Pathol, 2008, 9(4): 447-461.
doi: 10.1111/j.1364-3703.2008.00474.x pmid: 18705860 |
| [29] | Shemesh-Mayer E, Gelbart D, Belausov E, et al. Garlic potyviruses are translocated to the true seeds through the vegetative and reproductive systems of the mother plant[J]. Viruses, 2022, 14(10): 2092. |
| [30] | Zhang ZB, Lee Y, Sivertsen A, et al. Low temperature treatment affects concentration and distribution of Chrysanthemum stunt viroid in Argyranthemum[J]. Front Microbiol, 2016, 7: 224. |
| [31] | 梁云, 袁素霞, 冯慧颖, 等. 百合肌动蛋白基因lilyActin的克隆与表达分析[J]. 园艺学报, 2013, 40(7): 1318-1326. |
| Liang Y, Yuan SX, Feng HY, et al. Cloning and expression analysis of actin gene(lilyActin)from lily[J]. Acta Hortic Sin, 2013, 40(7): 1318-1326. | |
| [32] | 韦伟, 单守明. 葡萄脱毒与快繁技术研究进展[J]. 分子植物育种, 2022, 20(1): 259-265. |
| Wei W, Shan SM. Research progress on virus-free grape and rapid propagation technology[J]. Mol Plant Breed, 2022, 20(1): 259-265. | |
| [33] | Bettoni JC, Mathew L, Pathirana R, et al. Eradication of Potato virus S, Potato virus a, and Potato virus m from infected in vitro-grown potato shoots using in vitro therapies[J]. Front Plant Sci, 2022, 13: 878733. |
| [34] | 杜易静, 刘文林, 乔月莲, 等. ‘阳光玫瑰’葡萄试管苗热处理结合茎尖和腋芽培养脱毒技术研究[J]. 园艺学报, 2024, 51(4): 893-902. |
| Du YJ, Liu WL, Qiao YL, et al. Virus elimination from ‘Shine Muscat’ grape plants in vitro via heat treatment combined with shoot tip and Axillary bud culture[J]. Acta Horticulturae Sinica, 2024, 51(4): 893-902. | |
| [35] | 刘肇先. 柑橘主要病害分子检测、WOX转录因子家族、茎尖原位杂交与微芽嫁接研究[D]. 武汉: 华中农业大学, 2022. |
| Liu ZX. Study on molecular detection of major diseases, WOX transcription factor family, in situ hybridization and shoot-tip grafting of Citrus[D]. Wuhan: Huazhong Agricultural University, 2022. | |
| [36] |
Zhao L, Wang MR, Cui ZH, et al. Combining thermotherapy with cryotherapy for efficient Eradication of apple stem grooving virus from infected in-vitro -cultured apple shoots[J]. Plant Dis, 2018, 102(8): 1574-1580.
doi: 10.1094/PDIS-11-17-1753-RE pmid: 30673422 |
| [37] | 郝春磊. 切花乒乓菊茎尖脱毒体系建立及盆栽矮化技术研究[D]. 银川: 宁夏大学, 2021. |
| Hao CL. Establishment of detoxification system and potted dwarf technology of pompon[D]. Yinchuan: Ningxia University, 2021. | |
| [38] | 陈龙. 外施褪黑素促进苹果脱毒的技术与机理研究[D]. 杨凌: 西北农林科技大学, 2021. |
| Chen L. Exogenous melatonin for virus eradication from in vitro-cultured apple shoots: application and mechanism[D]. Yangling: Northwest A & F University, 2021. | |
| [39] | Bi WL, Hao XY, Cui ZH, et al. Shoot tip cryotherapy for efficient eradication of grapevine leafroll-associated virus-3 from diseased grapevine in vitro plants[J]. Ann Appl Biol, 2018, 173(3): 261-270. |
| [40] | Baluska F. Cell-cell channels, viruses, and evolution: via infection, parasitism, and symbiosis toward higher levels of biological complexity[J]. Ann N Y Acad Sci, 2009, 1178: 106-119. |
| [41] |
Tilsner J, Nicolas W, Rosado A, et al. Staying tight: plasmodesmal membrane contact sites and the control of cell-to-cell connectivity in plants[J]. Annu Rev Plant Biol, 2016, 67: 337-364.
doi: 10.1146/annurev-arplant-043015-111840 pmid: 26905652 |
| [42] | Hao XJ, Zheng YY, Cui BM, et al. Localization of southern tomato virus(STV)in tomato tissues[J]. J Plant Dis Prot, 2023, 130(5): 1143-1147. |
| [43] | Csorba T, Kontra L, Burgyán J. Viral silencing suppressors: tools forged to fine-tune host-pathogen coexistence[J]. Virology, 2015, 479/480: 85-103. |
| [44] | 刘德水. 茎尖脱毒过程中黄瓜花叶病毒自身调控的分子机制[D]. 北京: 中国农业大学, 2016. |
| Liu DS. Molecular mechanisms underlying cucumber mosaic virus self-regulation during viral exclusion from shoot apical meristem[D]. Beijing: China Agricultural University, 2016. | |
| [45] | Claßen-Bockhoff R, Franke D, Krähmer H. Early ontogeny defines the diversification of primary vascular bundle systems in angiosperms[J]. Bot J Linn Soc, 2021, 195(3): 281-307. |
| [46] | Sharman BC, Hitch PA. Initiation of procambial strands in leaf primordia of bread wheat, Triticum aestivum L[J]. Ann Bot, 1967, 31(2): 229-243. |
| [47] |
Lefeuvre P, Martin DP, Elena SF, et al. Evolution and ecology of plant viruses[J]. Nat Rev Microbiol, 2019, 17(10): 632-644.
doi: 10.1038/s41579-019-0232-3 pmid: 31312033 |
| [48] | Dong F, Mochizuki T, Ohki ST. Tobacco ringspot virus persists in the shoot apical meristem but not in the root apical meristem of infected tobacco[J]. Eur J Plant Pathol, 2010, 126(1): 117-122. |
| [49] | Mochizuki T, Ohki ST. Shoot meristem tissue of tobacco inoculated with Cucumber mosaic virus is infected with the virus and subsequently recovers from infection by RNA silencing[J]. J Gen Plant Pathol, 2004, 70(6): 363-366. |
| [50] |
Martín-Hernández AM, Baulcombe DC. Tobacco rattle virus 16-kilodalton protein encodes a suppressor of RNA silencing that allows transient viral entry in meristems[J]. J Virol, 2008, 82(8): 4064-4071.
doi: 10.1128/JVI.02438-07 pmid: 18272576 |
| [51] | Incarbone M, Bradamante G, Pruckner F, et al. Salicylic acid and RNA interference mediate antiviral immunity of plant stem cells[J]. Proc Natl Acad Sci USA, 2023, 120(42): e2302069120. |
| [1] | 李心怡, 姜春秀, 薛丽, 蒋洪涛, 姚伟, 邓祖湖, 张木清, 余凡. 多荧光标记引物增强甘蔗染色体寡聚核苷酸探针杂交信号[J]. 生物技术通报, 2023, 39(5): 103-111. |
| [2] | 蔡梦鲜, 高作敏, 胡利娟, 冯群, 王洪程, 朱斌. 天然甘蓝型油菜C染色体组C1,C2缺体的创建及遗传分析[J]. 生物技术通报, 2023, 39(3): 81-88. |
| [3] | 汪格格, 邱诗蕊, 张琳晗, 杨国伟, 徐小云, 汪爱羚, 曾淑华, 刘雅洁. 异源三倍体普通烟草(SST)减数分裂期的分子细胞学研究[J]. 生物技术通报, 2023, 39(2): 183-192. |
| [4] | 孙宝箴, 全龙萍, 康慧, 姚玉新, 沈甜, 陈卫平, 杜远鹏, 高振. 基于跨反向剪接位点引物特异性检测circRNA的PCR方法[J]. 生物技术通报, 2022, 38(5): 279-285. |
| [5] | 符勇耀, 易德燕, 杨先茂, 蔡莉, 梁渝华, 雷美艳, 杨利平. 卷丹新种质JD-h-15的形态特征与遗传变异分析[J]. 生物技术通报, 2022, 38(11): 140-150. |
| [6] | 范亚朋, 芮存, 张悦新, 陈修贵, 陆许可, 王帅, 张红, 徐楠, 王晶, 陈超, 叶武威. 陆地棉耐碱基因GHZAT12的克隆、表达及生物信息学分析[J]. 生物技术通报, 2021, 37(8): 121-130. |
| [7] | 张静, 熊燕, 华永琳, 郭玉, 熊显荣, 字向东, 李键. 小鼠骨骼肌纤维类型定量PCR内参基因的筛选[J]. 生物技术通报, 2021, 37(2): 71-79. |
| [8] | 王新艳, 张俊玲, 施志仪. 牙鲆cbx2基因的分子特征与组织表达[J]. 生物技术通报, 2019, 35(4): 69-75. |
| [9] | 何硕康, 罗泽伟. QUARTET突变四倍体拟南芥的获得与表型分析[J]. 生物技术通报, 2018, 34(7): 119-125. |
| [10] | 耿龙泼, 王鑫旺, 黄路华, 邓基利, 汪世华, 张峰. 黄曲霉核糖体蛋白基因在不同生长时期的表达分析[J]. 生物技术通报, 2018, 34(4): 194-200. |
| [11] | 卢佳, 邓秋萍, 任文华. 少棘蜈蚣抗菌肽Scolopin 2-NH2的抗菌作用机制研究[J]. 生物技术通报, 2018, 34(11): 179-190. |
| [12] | 孔维欢, 王晶晶, 万鹏程, 石国庆. 猪PEDV与PDCoV二重RT-PCR检测方法的建立[J]. 生物技术通报, 2018, 34(11): 205-209. |
| [13] | 唐辉蓉,崔进,张家骅,代佑果,李为明,廖陈. PDSW联合热疗对胃癌细胞和组织中COX-2、Bcl-2表达的影响[J]. 生物技术通报, 2017, 33(7): 224-230. |
| [14] | 李京, 吴奇, 张琳婕, 李旭婷, 周敏琪, 韦善君. 结缕草转录因子基因ZjDREB4.1克隆和逆境表达模式[J]. 生物技术通报, 2017, 33(2): 80-88. |
| [15] | 宋伟凤, 李明聪, 高峥. 环境中微生物原位检测方法研究进展[J]. 生物技术通报, 2017, 33(10): 26-32. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||