








生物技术通报 ›› 2024, Vol. 40 ›› Issue (8): 24-38.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0183
收稿日期:2024-02-28
出版日期:2024-08-26
发布日期:2024-09-05
通讯作者:
郭淑元, 女, 博士, 教授, 研究方向:基因编辑和蛋白质工程;E-mail: guosy@bit.edu.cn作者简介:韩钟娆, 女, 硕士研究生, 研究方向:微生物和基因编辑;E-mail: hanzhongrao@126.com
基金资助:
HAN Zhong-rao( ), HUO Yi-xin, GUO Shu-yuan(
), HUO Yi-xin, GUO Shu-yuan( )
)
Received:2024-02-28
Published:2024-08-26
Online:2024-09-05
摘要:
芽胞杆菌作为一种极具潜力的底盘菌株,能够在多种工农业废物和极端环境中生长,也能生产出多种工业化产品,如食品、饲料、益生菌、植物生长促进剂、酶和生物活性化合物等。然而,尽管具有生产高效、成本低廉等优点,芽胞杆菌在发酵生产中依然存在几个瓶颈问题,导致其工业生产的巨大潜力难以得到充分利用。其中一个关键问题在于,发酵生产过程中的生长以及生产效率较易受到多种胁迫条件的限制,进而导致发酵生产的不彻底、不完全。因此,探究芽胞杆菌胁迫响应的影响因子及改造策略,发掘其多种代谢活动与生长性状间的联系,就可以增强芽胞杆菌的抗胁迫能力,进而提高芽胞杆菌在工业应用中的质与量。本文首先分析了芽胞杆菌的多种应激反应机制,为进一步提高芽胞杆菌胁迫耐受能力、构建一个高效生产且具有良好抗逆性能的底盘菌株,总结阐述了非理性设计筛选抗性菌株的多种策略,以及抗逆基因线路和高耐受性微生物底盘的多种构建方法。为推动芽胞杆菌胁迫耐受机制的研究及工业应用领域的拓展提供策略和思路。
韩钟娆, 霍毅欣, 郭淑元. 芽胞杆菌耐受胁迫条件的机制及工业应用[J]. 生物技术通报, 2024, 40(8): 24-38.
HAN Zhong-rao, HUO Yi-xin, GUO Shu-yuan. Mechanism and Industrial Application of Bacillus Tolerance to Stress Conditions[J]. Biotechnology Bulletin, 2024, 40(8): 24-38.
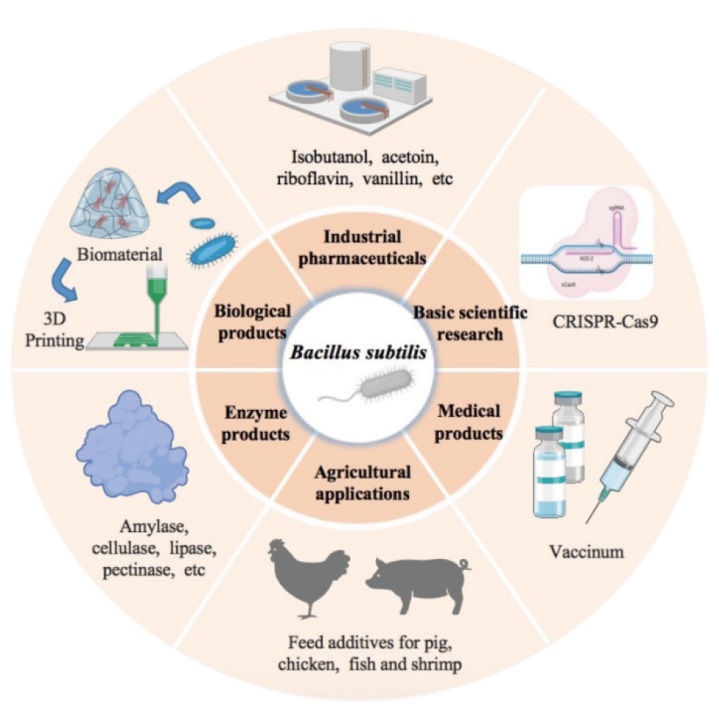
图1 芽胞杆菌在基因工程、工业化学品或酶生产、农业、医药和生物材料中的应用
Fig. 1 Applications of Bacillus in genetic engineering, industrial chemicals or enzyme production, agriculture, medicine, and biomaterials
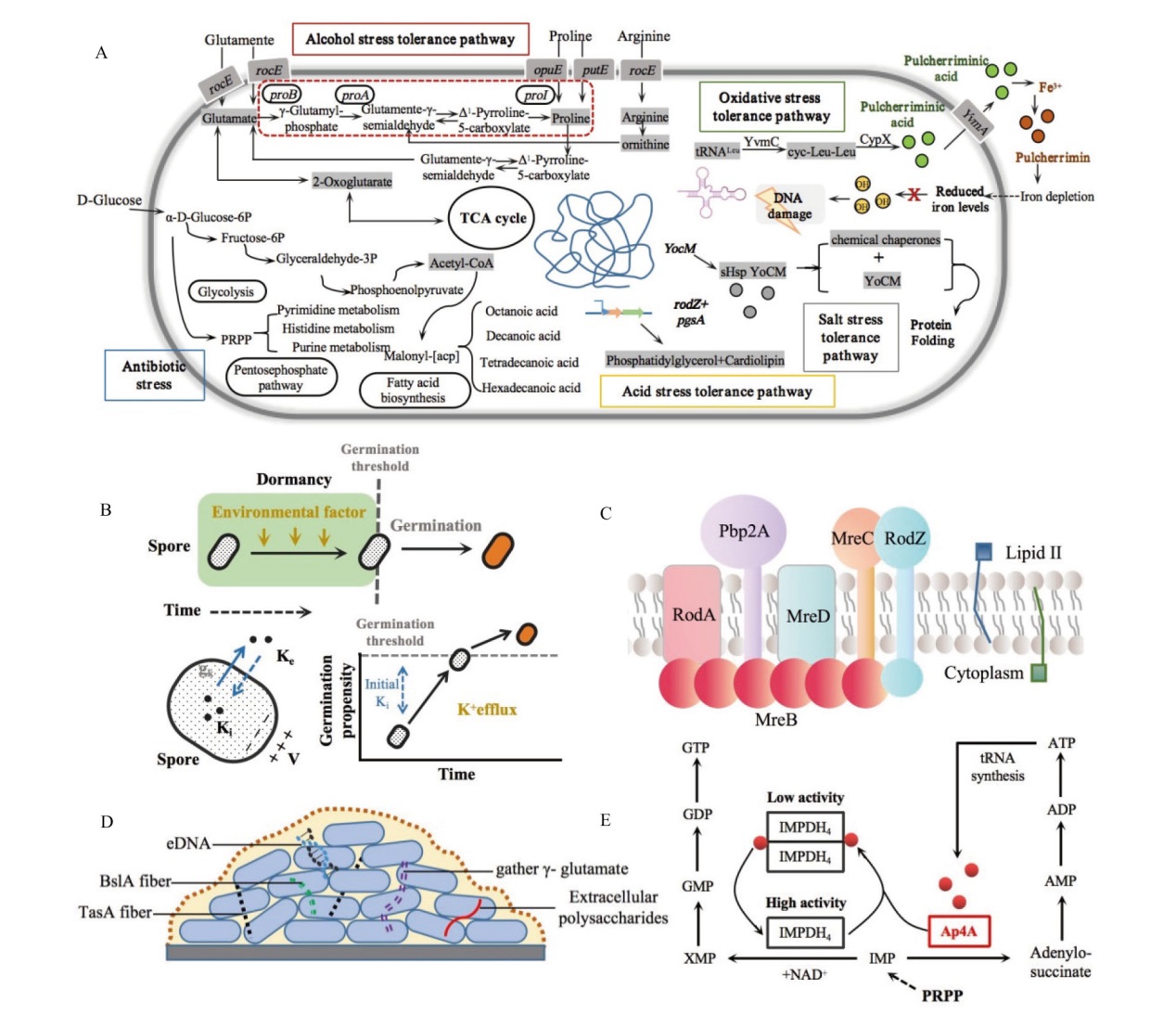
图2 芽胞杆菌胁迫响应机制 A:芽胞杆菌在多种胁迫环境下响应机制;B:芽胞杆菌感知外部环境机制[30];C:膜上细胞壁前体示意图[31];D:细胞包膜应激反应中的膜成分示意图[32]; E:四磷酸二腺苷调节耐热性能原理图[33]
Fig. 2 Bacillus stress response mechanisms A: Bacillus response mechanisms under various stress environments. B: Bacillus perception of the external environment mechanisms[30]. C: Schematic representation of membrane-bound cell wall precursors[31]. D: Schematic representation of membrane components in cell envelope stress response[32]. E: Principles of heat resistance regulation by tetraphosphoadenosine[33]
| 工业产品 Industrial application | 菌株 Strain | 菌株特性 Characteristics | 产量 Titer | 参考文献 Reference |
|---|---|---|---|---|
| 乙偶姻 | B. subtilis 168-phaCBA | 将聚羟基丁酸合成途径引入B. subtilis 168,显著提升菌株乙偶姻耐受能力,进而提升乙偶姻工业生产产量 | 70.14 g/L | [ |
| 甲萘醌-7 | B. subtilis 20-QT | 枯草芽胞杆菌中共过表达基因tatAD-CD和qcrA-C | 410 mg/L | [ |
| 鲨肌醇 | B. subtilis KW018 | 敲除基因iolHIJ、iolX 和iolR且过表达基因amyE | 22 g/L | [ |
| 透明质酸 | B. subtilis RBSTr3 | 通过改造透明质酸合酶(HAS)获得高产透明质酸突变菌株 | 728 mg/L | [ |
| 核黄素 | B. subtilis U3 | 利用基于液滴的微流体技术筛选分离枯草芽胞杆菌高产核黄素突变体 | 24.3 g/L | [ |
| 2,3-丁二醇 | B. subtilis BS-ppb11 | 枯草芽胞杆菌中过表达2,3-丁二醇脱氢酶 | 96.5 g/L | [ |
| 聚γ-谷氨酸 | B. subtilis GXC-36 | 使用阶段控制发酵和黏度降低策略优化发酵流程,提升聚γ-谷氨酸工业发酵产量 | 22.17 g/L | [ |
| N-乙酰葡糖胺 | B. subtilis 168 BNDR122 | 敲除基因nagP、gamP、gamA、gamR、nagA、nagB、ldh、alsRSD、pta、ackA、glcK、pckA、pyk、lacA和amyE | 131.6 g/L | [ |
| 异丁醇 | B. subtilis GI10 | 敲除非必须基因134.3 kb后获得异丁醇高产菌株 | 201.7 mg/L | [ |
表1 枯草芽胞杆菌的代表性工业产品
Table 1 Representative industrial applications of B. subtilis
| 工业产品 Industrial application | 菌株 Strain | 菌株特性 Characteristics | 产量 Titer | 参考文献 Reference |
|---|---|---|---|---|
| 乙偶姻 | B. subtilis 168-phaCBA | 将聚羟基丁酸合成途径引入B. subtilis 168,显著提升菌株乙偶姻耐受能力,进而提升乙偶姻工业生产产量 | 70.14 g/L | [ |
| 甲萘醌-7 | B. subtilis 20-QT | 枯草芽胞杆菌中共过表达基因tatAD-CD和qcrA-C | 410 mg/L | [ |
| 鲨肌醇 | B. subtilis KW018 | 敲除基因iolHIJ、iolX 和iolR且过表达基因amyE | 22 g/L | [ |
| 透明质酸 | B. subtilis RBSTr3 | 通过改造透明质酸合酶(HAS)获得高产透明质酸突变菌株 | 728 mg/L | [ |
| 核黄素 | B. subtilis U3 | 利用基于液滴的微流体技术筛选分离枯草芽胞杆菌高产核黄素突变体 | 24.3 g/L | [ |
| 2,3-丁二醇 | B. subtilis BS-ppb11 | 枯草芽胞杆菌中过表达2,3-丁二醇脱氢酶 | 96.5 g/L | [ |
| 聚γ-谷氨酸 | B. subtilis GXC-36 | 使用阶段控制发酵和黏度降低策略优化发酵流程,提升聚γ-谷氨酸工业发酵产量 | 22.17 g/L | [ |
| N-乙酰葡糖胺 | B. subtilis 168 BNDR122 | 敲除基因nagP、gamP、gamA、gamR、nagA、nagB、ldh、alsRSD、pta、ackA、glcK、pckA、pyk、lacA和amyE | 131.6 g/L | [ |
| 异丁醇 | B. subtilis GI10 | 敲除非必须基因134.3 kb后获得异丁醇高产菌株 | 201.7 mg/L | [ |
| 工业产品 Industrial application | 菌株 Strain | 菌株特性 Characteristics | 产量或酶活性 Titer or enzymatic activity | 参考文献 Reference |
|---|---|---|---|---|
| 聚-γ-谷氨酸 | B. licheniformis CGMCC NO. 23967 | 发酵体系中添加FeSO4·7H2O可提高地衣芽胞杆菌聚-γ-谷氨酸的生产效率,这种添加导致细胞内代谢物丰度增加,包括氨基酸,有机酸和关键的TCA循环中间体等含量的上调 | 70.436 g/L | [ |
| 碱性蛋白酶 | B. licheniformis BL10 | 利用单因素实验和响应面法开发和优化发酵条件,提升生产效率 | 39 233.6 U/mL | [ |
| 淀粉酶 | B. licheniformis XS-4 | 采用常压室温等离子体(ARTP)诱导突变,获得地衣芽胞杆菌高产淀粉酶突变株 | 15 U/mL | [ |
| 几丁质酶 | B. licheniformis PR2 | 地衣芽胞杆菌PR2可以分泌几丁质酶用于工蚁生物防治 | 82.3 U/mL | [ |
| 角质蛋白酶 | B. licheniformis | 使用Ni-NTA色谱法纯化后获得较高浓度角质蛋白酶 | 222.01 U/mL | [ |
表2 地衣芽胞杆菌的代表性工业产品
Table 2 Representative industrial applications of B. licheniformis
| 工业产品 Industrial application | 菌株 Strain | 菌株特性 Characteristics | 产量或酶活性 Titer or enzymatic activity | 参考文献 Reference |
|---|---|---|---|---|
| 聚-γ-谷氨酸 | B. licheniformis CGMCC NO. 23967 | 发酵体系中添加FeSO4·7H2O可提高地衣芽胞杆菌聚-γ-谷氨酸的生产效率,这种添加导致细胞内代谢物丰度增加,包括氨基酸,有机酸和关键的TCA循环中间体等含量的上调 | 70.436 g/L | [ |
| 碱性蛋白酶 | B. licheniformis BL10 | 利用单因素实验和响应面法开发和优化发酵条件,提升生产效率 | 39 233.6 U/mL | [ |
| 淀粉酶 | B. licheniformis XS-4 | 采用常压室温等离子体(ARTP)诱导突变,获得地衣芽胞杆菌高产淀粉酶突变株 | 15 U/mL | [ |
| 几丁质酶 | B. licheniformis PR2 | 地衣芽胞杆菌PR2可以分泌几丁质酶用于工蚁生物防治 | 82.3 U/mL | [ |
| 角质蛋白酶 | B. licheniformis | 使用Ni-NTA色谱法纯化后获得较高浓度角质蛋白酶 | 222.01 U/mL | [ |
| 工业产品 Industrial application | 菌株 Strain | 菌株特性 Characteristics | 产量或酶活性 Titer or enzymatic activity | 参考文献 Reference |
|---|---|---|---|---|
| 碱性蛋白酶 | B. cereus S8 | 由于其热稳定性和在碱性pH值下的活性较好,蜡样芽胞杆菌菌株S8表达的蛋白酶降解蛋白效率较高 | 20 U/mg | [ |
| 细菌纤维素 | B. cereus | 共培养蜡样芽胞杆菌和木糖驹形氏杆菌(Komagataeibacter xylinus)提高细菌生产纤维素的效率 | 4.4 g/L | [ |
| 反式-4-羟基-l-脯氨酸 | B. cereus HBL-AI | 从空气中分离出生产反式-4-羟基-l-脯氨酸(trans-Hyp)的蜡状芽胞杆菌HBL-AI,仅使用l-脯氨酸作为碳源进行筛选分离培养 | 46.2 g/L | [ |
| 角蛋白酶 | B. cereus IIPK35 | 通过固态发酵(SSF)技术提升了蜡状芽胞杆菌IIPK35的角蛋白酶生产效率 | 648.28 U/gds | [ |
| 磷脂酶C | B. cereus PLCBc | 利用响应曲面法(RMS)和效应面法设计优化蜡样芽胞杆菌的磷脂酶C 生产效率 | 51 U/mL | [ |
表3 蜡样芽胞杆菌的代表性工业产品
Table 3 Representative industrial applications of B. cereus
| 工业产品 Industrial application | 菌株 Strain | 菌株特性 Characteristics | 产量或酶活性 Titer or enzymatic activity | 参考文献 Reference |
|---|---|---|---|---|
| 碱性蛋白酶 | B. cereus S8 | 由于其热稳定性和在碱性pH值下的活性较好,蜡样芽胞杆菌菌株S8表达的蛋白酶降解蛋白效率较高 | 20 U/mg | [ |
| 细菌纤维素 | B. cereus | 共培养蜡样芽胞杆菌和木糖驹形氏杆菌(Komagataeibacter xylinus)提高细菌生产纤维素的效率 | 4.4 g/L | [ |
| 反式-4-羟基-l-脯氨酸 | B. cereus HBL-AI | 从空气中分离出生产反式-4-羟基-l-脯氨酸(trans-Hyp)的蜡状芽胞杆菌HBL-AI,仅使用l-脯氨酸作为碳源进行筛选分离培养 | 46.2 g/L | [ |
| 角蛋白酶 | B. cereus IIPK35 | 通过固态发酵(SSF)技术提升了蜡状芽胞杆菌IIPK35的角蛋白酶生产效率 | 648.28 U/gds | [ |
| 磷脂酶C | B. cereus PLCBc | 利用响应曲面法(RMS)和效应面法设计优化蜡样芽胞杆菌的磷脂酶C 生产效率 | 51 U/mL | [ |
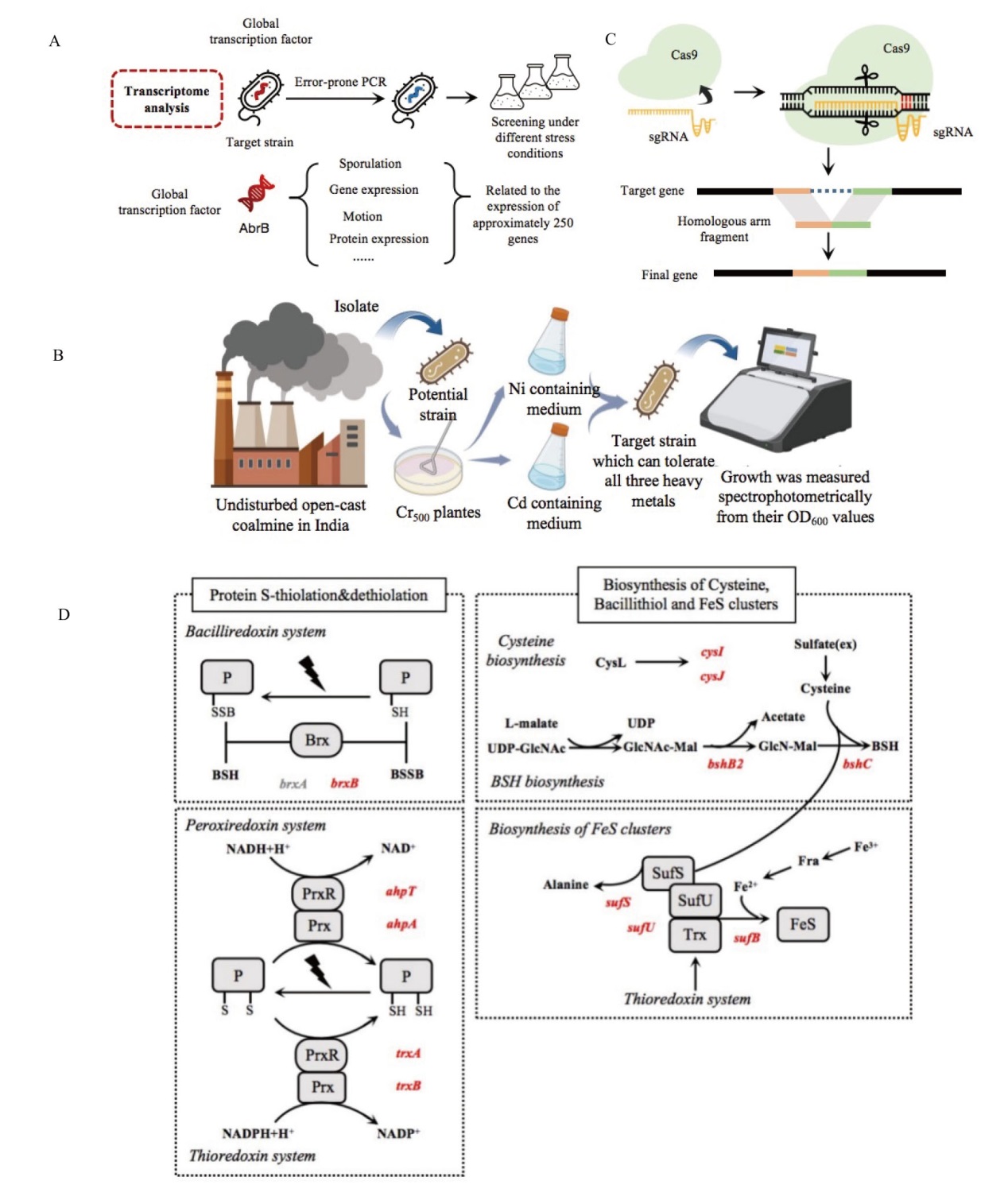
图3 非理性设计筛选抗性菌株方法示意图 A:非理性改造结合反向代谢工程策略;B:基于CRISPR技术实现微生物基因组的非理性改造;C:非理性设计提升蜡样芽胞杆菌对重金属的耐受能力; D:基因组精简后的枯草芽胞杆菌突变菌株在氧化硫胁迫下的差异代谢[43](红色字体表示蛋白质上调,灰色表示下调)
Fig. 3 Schematic representation of irrational design for screening resistant strains A: Irrational modification combined with reverse metabolic engineering strategy. B: Implementing irrational modification of microbial genomes based on CRISPR technology(Created with BioRender.com). C: Enhancement of Bacillus cereus tolerance to heavy metals through irrational design. D: Differential metabolism of B. subtilis mutant strains with streamlined genomes under sulfur oxidation stress[43](Red font indicates protein upregulation, gray represents downregulation)
| 胁迫耐受种类 Type of stress tolerance | 底盘菌株 Chassis strain | 年份 Year | 筛选方法 Screening method | 参考文献 Reference |
|---|---|---|---|---|
| 丁醇胁迫耐受 | B. subtilis | 2011 | 使用0.1%的丁醇浓度驯化后分离培养 | [ |
| 紫外线胁迫耐受 | B. subtilis | 2013 | 利用吲哚-3-乙酸、吲哚-3-丁酸或1-萘乙酸生长素分别预处理后分离培养 | [ |
| 热胁迫耐受 | B. subtilis | 2013 | 使用吲哚-3-乙酸或1-萘乙酸生长素预处理后分离培养 | [ |
| 溶菌酶胁迫耐受 | B. licheniformis | 2017 | 从芒果泡菜中分离获得了具有较强耐受性的菌株 | [ |
| 重金属胁迫耐受 | B. cereus | 2018 | 从废弃露天矿场中分离培养蜡样芽胞杆菌,在异养条件下,其可耐受高达2 000 mg/L的Cr500浓度 | [ |
| 酸性胁迫耐受 | B. amyloliquefaciens | 2019 | 从阿萨姆邦迪普的3个不同地点采集表层的大块土壤进行分离培养,在低pH值的培养基中筛选获得在pH 4.0下对酸胁迫更耐受的菌株 | [ |
| 胆汁耐受性 | B. pumilus | 2022 | 从西藏自治区牦牛肠道分离培养目的菌株 | [ |
| 高浓度代谢底物耐受 | B. subtilis | 2023 | 使用100%基于水解物的培养基分离培养 | [ |
表4 利用非理性设计筛选抗性菌株
Table 4 Screening resistant strains using irrational design
| 胁迫耐受种类 Type of stress tolerance | 底盘菌株 Chassis strain | 年份 Year | 筛选方法 Screening method | 参考文献 Reference |
|---|---|---|---|---|
| 丁醇胁迫耐受 | B. subtilis | 2011 | 使用0.1%的丁醇浓度驯化后分离培养 | [ |
| 紫外线胁迫耐受 | B. subtilis | 2013 | 利用吲哚-3-乙酸、吲哚-3-丁酸或1-萘乙酸生长素分别预处理后分离培养 | [ |
| 热胁迫耐受 | B. subtilis | 2013 | 使用吲哚-3-乙酸或1-萘乙酸生长素预处理后分离培养 | [ |
| 溶菌酶胁迫耐受 | B. licheniformis | 2017 | 从芒果泡菜中分离获得了具有较强耐受性的菌株 | [ |
| 重金属胁迫耐受 | B. cereus | 2018 | 从废弃露天矿场中分离培养蜡样芽胞杆菌,在异养条件下,其可耐受高达2 000 mg/L的Cr500浓度 | [ |
| 酸性胁迫耐受 | B. amyloliquefaciens | 2019 | 从阿萨姆邦迪普的3个不同地点采集表层的大块土壤进行分离培养,在低pH值的培养基中筛选获得在pH 4.0下对酸胁迫更耐受的菌株 | [ |
| 胆汁耐受性 | B. pumilus | 2022 | 从西藏自治区牦牛肠道分离培养目的菌株 | [ |
| 高浓度代谢底物耐受 | B. subtilis | 2023 | 使用100%基于水解物的培养基分离培养 | [ |
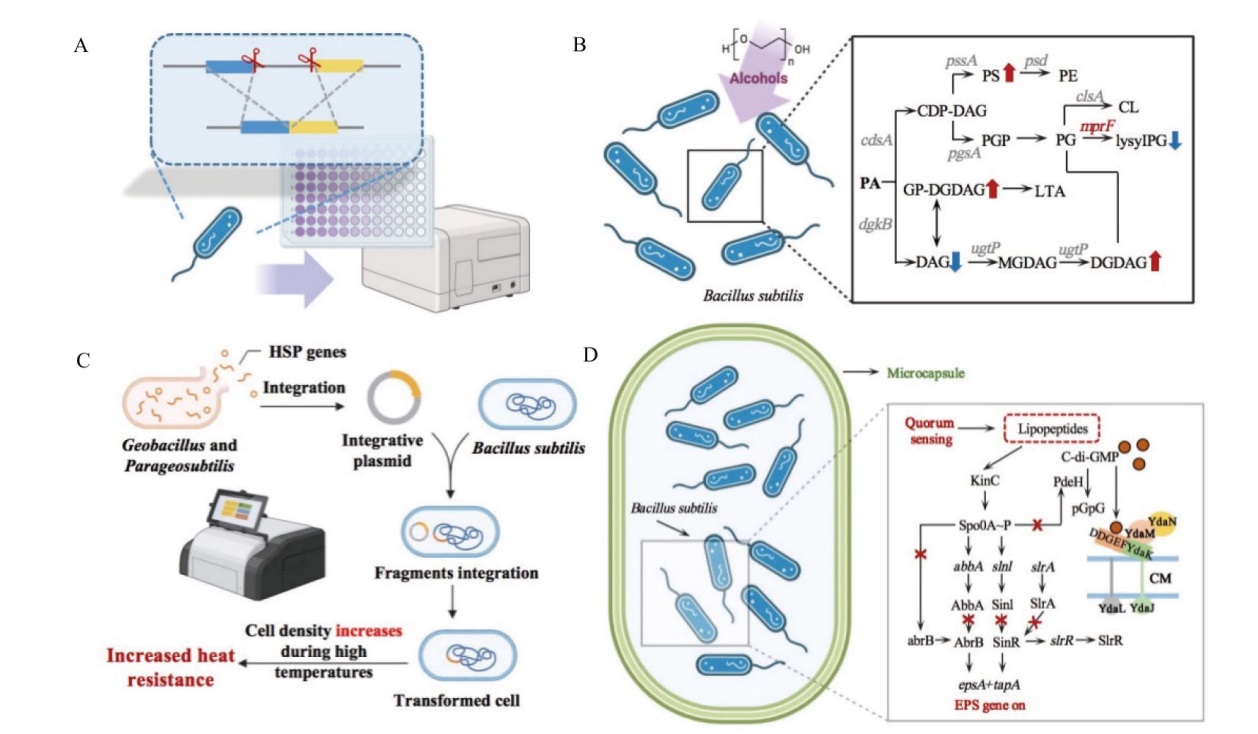
图4 抗逆基因线路及高耐受性微生物底盘的构建策略示意图 A, B:基于多基因表达调控提高芽胞杆菌醇胁迫耐受能力[93];C:芽胞杆菌特异性热量调节引擎的设计与构建;D:将芽胞杆菌包埋胶囊显著提高低pH耐受能力[94]
Fig. 4 Schematic representation of construction strategies for stress-resistant gene circuits and highly tolerant microbial chassis A, B: Improvement of B. subtilis tolerance to ethanol stress based on multi-gene expression regulation[93]; C: design and construction of Bacillus-specific heat regulation engine; D: significant improvement of B. cereus low pH tolerance through encapsulation[94]
| 胁迫种类 Classification | 底盘菌株 Chassis strain | 年份 Year | 基因名称 Gene name | 基因大小 Gene size/bp | 基因功能 Gene function | 参考文献 References |
|---|---|---|---|---|---|---|
| 盐胁迫 | B. subtilis | 2009 | ytvA | 620 | 与盐胁迫耐受相关 | [ |
| 溶菌酶胁迫 | B. subtilis | 2011 | sigV | 501 | 与ECFσ因子有关 | [ |
| 水、渗透压胁迫 | B. subtilis | 2013 | rsbR,proHJ | 300-1 500 | 与σB的表达调控相关 | [ |
| 氧化应激胁迫 | B. subtilis | 2013 | ubK | 400-3 000 | 与σB的表达调控相关 | [ |
| 山梨酸和乙酸等弱有机酸胁迫 | B. subtilis | 2016 | rodZ,pgsA | 867,582 | 维持细胞的杆状,且与细菌细胞骨架的形成有关 | [ |
| 醇耐受 | B. subtilis | 2018 | ugtP,mprF | 500-3 000 | 与膜脂生物合成途径相关 | [ |
| 热胁迫 | B. subtilis | 2019 | dnaK, dnaJ, grpE | 300-2 000 | 与σB的表达调控相关 | [ |
| 抗生素杆菌肽胁迫 | B. subtilis | 2020 | bcrC | 582 | 与脂质II循环系统有关 | [ |
| 磷酸糖胁迫 | B. subtilis | 2021 | ywpJ | 858 | 与磷酸酶的表达有关 | [ |
| 外源氧化应激 | B. cereus | 2022 | ctaA | 921 | 与细胞色素A3活性有关 | [ |
| 镉胁迫 | B. subtilis | 2022 | sigD | 765 | 与鞭毛编码和组装基因的表达相关 | [ |
| 外源蛋白分泌应激反应 | B. subtilis | 2023 | htrA,htrB | 1 350,1 377 | 与胞质蛋白酶AprX和胞外蛋白酶AprE的表达有关 | [ |
表5 影响芽胞杆菌胁迫耐受能力基因位点总结
Table 5 Summary of gene loci influencing stress tolerance in Bacillus
| 胁迫种类 Classification | 底盘菌株 Chassis strain | 年份 Year | 基因名称 Gene name | 基因大小 Gene size/bp | 基因功能 Gene function | 参考文献 References |
|---|---|---|---|---|---|---|
| 盐胁迫 | B. subtilis | 2009 | ytvA | 620 | 与盐胁迫耐受相关 | [ |
| 溶菌酶胁迫 | B. subtilis | 2011 | sigV | 501 | 与ECFσ因子有关 | [ |
| 水、渗透压胁迫 | B. subtilis | 2013 | rsbR,proHJ | 300-1 500 | 与σB的表达调控相关 | [ |
| 氧化应激胁迫 | B. subtilis | 2013 | ubK | 400-3 000 | 与σB的表达调控相关 | [ |
| 山梨酸和乙酸等弱有机酸胁迫 | B. subtilis | 2016 | rodZ,pgsA | 867,582 | 维持细胞的杆状,且与细菌细胞骨架的形成有关 | [ |
| 醇耐受 | B. subtilis | 2018 | ugtP,mprF | 500-3 000 | 与膜脂生物合成途径相关 | [ |
| 热胁迫 | B. subtilis | 2019 | dnaK, dnaJ, grpE | 300-2 000 | 与σB的表达调控相关 | [ |
| 抗生素杆菌肽胁迫 | B. subtilis | 2020 | bcrC | 582 | 与脂质II循环系统有关 | [ |
| 磷酸糖胁迫 | B. subtilis | 2021 | ywpJ | 858 | 与磷酸酶的表达有关 | [ |
| 外源氧化应激 | B. cereus | 2022 | ctaA | 921 | 与细胞色素A3活性有关 | [ |
| 镉胁迫 | B. subtilis | 2022 | sigD | 765 | 与鞭毛编码和组装基因的表达相关 | [ |
| 外源蛋白分泌应激反应 | B. subtilis | 2023 | htrA,htrB | 1 350,1 377 | 与胞质蛋白酶AprX和胞外蛋白酶AprE的表达有关 | [ |
| [1] |
Su Y, Liu C, Fang H, et al. Bacillus subtilis: a universal cell factory for industry, agriculture, biomaterials and medicine[J]. Microb Cell Fact, 2020, 19(1): 173.
doi: 10.1186/s12934-020-01436-8 pmid: 32883293 |
| [2] | Popp PF, Benjdia A, Strahl H, et al. The epipeptide YydF intrinsically triggers the cell envelope stress response of Bacillus subtilis and causes severe membrane perturbations[J]. Front Microbiol, 2020, 11: 151. |
| [3] | Herrmann LW, Letti LAJ, Penha RO, et al. Bacillus genus industrial applications and innovation: first steps towards a circular bioeconomy[J]. Biotechnol Adv, 2024, 70: 108300. |
| [4] |
Nadezhdin E, Murphy N, Dalchau N, et al. Stochastic pulsing of gene expression enables the generation of spatial patterns in Bacillus subtilis biofilms[J]. Nat Commun, 2020, 11: 950.
doi: 10.1038/s41467-020-14431-9 pmid: 32075967 |
| [5] | Łubkowska B, Jeżewska-Frąckowiak J, Sroczyński M, et al. Analysis of industrial Bacillus species as potential probiotics for dietary supplements[J]. Microorganisms, 2023, 11(2): 488. |
| [6] |
Stefanic P, Belcijan K, Kraigher B, et al. Kin discrimination promotes horizontal gene transfer between unrelated strains in Bacillus subtilis[J]. Nat Commun, 2021, 12(1): 3457.
doi: 10.1038/s41467-021-23685-w pmid: 34103505 |
| [7] | Luna-Bulbarela A, Romero-Gutiérrez MT, Tinoco-Valencia R, et al. Response of Bacillus velezensis 83 to interaction with Colletotrichum gloeosporioides resembles a Greek phalanx-style formation: a stress resistant phenotype with antibiosis capacity[J]. Microbiol Res, 2024, 280: 127592. |
| [8] |
Rodriguez Ayala F, Bartolini M, Grau R. The stress-responsive alternative Sigma factor SigB of Bacillus subtilis and its relatives: an old friend with new functions[J]. Front Microbiol, 2020, 11: 1761.
doi: 10.3389/fmicb.2020.01761 pmid: 33042030 |
| [9] | Neissi A, Majidi Zahed H, Roshan R. Probiotic performance of B. subtilis MS. 45 improves aquaculture of rainbow trout Oncorhynchus mykiss during acute hypoxia stress[J]. Sci Rep, 2024, 14: 3720. |
| [10] | Wang RF, Li HB, Qin Z, et al. Antifungal activity and application of Bacillus tequilensis A13 in biocontrol of Rehmannia glutinosa root-rot disease[J]. Chem Biol Technol Agric, 2023, 10(1): 20. |
| [11] | Xiao JC, Cao MY, Lai KY, et al. Unveiling key metabolic pathways in Bacillus subtilis-mediated salt tolerance enhancement in Glycyrrhiza uralensis Fisch. through multi-omics analysis[J]. Environ Exp Bot, 2024, 219: 105631. |
| [12] | Zhu XK, Yang BT, Hao ZP, et al. Dietary supplementation with Weissella cibaria C-10 and Bacillus amyloliquefaciens T-5 enhance immunity against Aeromonas veronii infection in crucian carp(Carassiu auratus)[J]. Microb Pathog, 2022, 167: 105559. |
| [13] | Bozsó Z, Lapat V, Ott PG, et al. Disparate effects of two clerodane diterpenes of giant goldenrod(Solidago gigantea ait.)on Bacillus spizizenii[J]. Int J Mol Sci, 2024, 25(3): 1531. |
| [14] | Odo EO, Ikwuegbu JA, Obeagu EI, et al. Analysis of the antibacterial effects of turmeric on particular bacteria[J]. Medicine, 2023, 102(48): e36492. |
| [15] | Raz C, Shemesh M, Argov-Argaman N. The role of milk fat globule size in modulating the composition of postbiotics produced by Bacillus subtilis and their effect on mammary epithelial cells[J]. Food Chem, 2023, 427: 136730. |
| [16] |
Woodley JM. Protein engineering of enzymes for process applications[J]. Curr Opin Chem Biol, 2013, 17(2): 310-316.
doi: 10.1016/j.cbpa.2013.03.017 pmid: 23562542 |
| [17] | Rath H, Reder A, Hoffmann T, et al. Management of osmoprotectant uptake hierarchy in Bacillus subtilis via a SigB-dependent antisense RNA[J]. Front Microbiol, 2020, 11: 622. |
| [18] | Kashef N, Hamblin MR. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation?[J]. Drug Resist Updat, 2017, 31: 31-42. |
| [19] | Tian HY, Liu JW, Zhang YX, et al. Stress response and signalling of a low-temperature bioaugmentation system in decentralized wastewater treatment: degradation characteristics, community structure, and bioaugmented mechanisms[J]. J Environ Manage, 2023, 342: 118257. |
| [20] |
Garre A, Egea JA, Iguaz A, et al. Relevance of the induced stress resistance when identifying the critical microorganism for microbial risk assessment[J]. Front Microbiol, 2018, 9: 1663.
doi: 10.3389/fmicb.2018.01663 pmid: 30087669 |
| [21] | Bombaywala S, Purohit HJ, Dafale NA. Mobility of antibiotic resistance and its co-occurrence with metal resistance in pathogens under oxidative stress[J]. J Environ Manage, 2021, 297: 113315. |
| [22] |
Garre A, Huertas JP, González-Tejedor GA, et al. Mathematical quantification of the induced stress resistance of microbial populations during non-isothermal stresses[J]. Int J Food Microbiol, 2018, 266: 133-141.
doi: S0168-1605(17)30517-2 pmid: 29216553 |
| [23] | Shuryak I. Review of microbial resistance to chronic ionizing radiation exposure under environmental conditions[J]. J Environ Radioact, 2019, 196: 50-63. |
| [24] |
Jia HY, Fan YS, Feng XD, et al. Enhancing stress-resistance for efficient microbial biotransformations by synthetic biology[J]. Front Bioeng Biotechnol, 2014, 2: 44.
doi: 10.3389/fbioe.2014.00044 pmid: 25368869 |
| [25] |
Tibocha-Bonilla JD, Zuñiga C, Lekbua A, et al. Predicting stress response and improved protein overproduction in Bacillus subtilis[J]. NPJ Syst Biol Appl, 2022, 8(1): 50.
doi: 10.1038/s41540-022-00259-0 pmid: 36575180 |
| [26] |
Davidson JF, Whyte B, Bissinger PH, et al. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae[J]. Proc Natl Acad Sci USA, 1996, 93(10): 5116-5121.
pmid: 8643537 |
| [27] | Campos Guillen J, Jones GH, Saldaña Gutiérrez C, et al. Critical minireview: the fate of tRNACys during oxidative stress in Bacillus subtilis[J]. Biomolecules, 2017, 7(1): 6. |
| [28] | Zhang QQ, Qian H, Li PY, et al. Insight into the evolution of microbial community and antibiotic resistance genes in anammox process induced by copper after recovery from oxytetracycline stress[J]. Bioresour Technol, 2021, 330: 124945. |
| [29] | Angelini LL, Dos Santos RAC, Fox G, et al. Pulcherrimin protects Bacillus subtilis against oxidative stress during biofilm development[J]. NPJ Biofilms Microbiomes, 2023, 9(1): 50. |
| [30] |
Kikuchi K, Galera-Laporta L, Weatherwax C, et al. Electrochemical potential enables dormant spores to integrate environmental signals[J]. Science, 2022, 378(6615): 43-49.
doi: 10.1126/science.abl7484 pmid: 36201591 |
| [31] | Sun YJ, Hürlimann S, Garner E. Growth rate is modulated by monitoring cell wall precursors in Bacillus subtilis[J]. Nat Microbiol, 2023, 8(3): 469-480. |
| [32] |
Arnaouteli S, Bamford NC, Stanley-Wall NR, et al. Bacillus subtilis biofilm formation and social interactions[J]. Nat Rev Microbiol, 2021, 19(9): 600-614.
doi: 10.1038/s41579-021-00540-9 pmid: 33824496 |
| [33] |
Giammarinaro PI, Young MKM, Steinchen W, et al. Diadenosine tetraphosphate regulates biosynthesis of GTP in Bacillus subtilis[J]. Nat Microbiol, 2022, 7(9): 1442-1452.
doi: 10.1038/s41564-022-01193-x pmid: 35953658 |
| [34] | Kei A. Anti-sigma factor-mediated cell surface stress responses in Bacillus subtilis[J]. Genes Genet Syst, 2018, 92(5): 223-234. |
| [35] | Jia ZX, Zhou JW, Han JZ, et al. Proteomics-based analysis of the stress response of Bacillus cereus spores under ultrasound and electrolyzed water treatment[J]. Ultrason Sonochem, 2023, 98: 106523. |
| [36] | Castro-Cerritos KV, Yasbin RE, Robleto EA, et al. Role of ribonucleotide reductase in Bacillus subtilis stress-associated mutagenesis[J]. J Bacteriol, 2017, 199(4): e00715-e00716. |
| [37] | Zhao H, Sachla AJ, Helmann JD. Mutations of the Bacillus subtilis YidC1(SpoIIIJ)insertase alleviate stress associated with σM-dependent membrane protein overproduction[J]. PLoS Genet, 2019, 15(10): e1008263. |
| [38] | Korza G, Camilleri E, Green J, et al. Analysis of the mRNAs in spores of Bacillus subtilis[J]. J Bacteriol, 2019, 201(9): e00007-e00019. |
| [39] |
Zhang YP, Zhu Y, Zhu Y, et al. The importance of engineering physiological functionality into microbes[J]. Trends Biotechnol, 2009, 27(12): 664-672.
doi: 10.1016/j.tibtech.2009.08.006 pmid: 19793618 |
| [40] |
Hecker M, Völker U. General stress response of Bacillus subtilis and other bacteria[J]. Adv Microb Physiol, 2001, 44: 35-91.
pmid: 11407115 |
| [41] | Yeak KYC, Tempelaars M, Wu JL, et al. SigB modulates expression of novel SigB regulon members via Bc1009 in non-stressed and heat-stressed cells revealing its alternative roles in Bacillus cereus[J]. BMC Microbiol, 2023, 23(1): 37. |
| [42] | Krajnc M, Stefanic P, Kostanjšek R, et al. Systems view of Bacillus subtilis pellicle development[J]. NPJ Biofilms Microbiomes, 2022, 8(1): 25. |
| [43] | Dervyn E, Planson AG, Tanaka K, et al. Greedy reduction of Bacillus subtilis genome yields emergent phenotypes of high resistance to a DNA damaging agent and low evolvability[J]. Nucleic Acids Res, 2023, 51(6): 2974-2992. |
| [44] | Lenz P, Bakkes PJ, Müller C, et al. Analysis of protein secretion in Bacillus subtilis by combining a secretion stress biosensor strain with an in vivo split GFP assay[J]. Microb Cell Fact, 2023, 22(1): 203. |
| [45] | Matavacas J, Anand D, von Wachenfeldt C. New insights into the disulfide stress response by the Bacillus subtilis Spx system at a single-cell level[J]. Mol Microbiol, 2023, 120(1): 75-90. |
| [46] | Ho TD, Hastie JL, Intile PJ, et al. The Bacillus subtilis extracytoplasmic function σ factor σ(V)is induced by lysozyme and provides resistance to lysozyme[J]. J Bacteriol, 2011, 193(22): 6215-6222. |
| [47] |
Helmann JD. Bacillus subtilis extracytoplasmic function(ECF)sigma factors and defense of the cell envelope[J]. Curr Opin Microbiol, 2016, 30: 122-132.
doi: S1369-5274(16)30002-9 pmid: 26901131 |
| [48] | Kessenikh AG, Novoyatlova US, Bazhenov SV, et al. Constructing of Bacillus subtilis-based lux-biosensors with the use of stress-inducible promoters[J]. Int J Mol Sci, 2021, 22(17): 9571. |
| [49] |
Radeck J, Fritz G, Mascher T. The cell envelope stress response of Bacillus subtilis: from static signaling devices to dynamic regulatory network[J]. Curr Genet, 2017, 63(1): 79-90.
doi: 10.1007/s00294-016-0624-0 pmid: 27344142 |
| [50] | Gingichashvili S, Duanis-Assaf D, Shemesh M, et al. The adaptive morphology of Bacillus subtilis biofilms: a defense mechanism against bacterial starvation[J]. Microorganisms, 2019, 8(1): 62. |
| [51] | Piepenbreier H, Sim A, Kobras CM, et al. From modules to networks: a systems-level analysis of the bacitracin stress response in Bacillus subtilis[J]. mSystems, 2020, 5(1): e00687-19. |
| [52] | Losick RM. Bacillus subtilis: a bacterium for all seasons[J]. Curr Biol, 2020, 30(19): R1146-R1150. |
| [53] | Yaraguppi DA, Bagewadi ZK, Patil NR, et al. Iturin: a promising cyclic lipopeptide with diverse applications[J]. Biomolecules, 2023, 13(10): 1515. |
| [54] | Guo Y, Liu YY, Yang ZJ, et al. Enhanced production of poly-γ-glutamic acid by Bacillus subtilis using stage-controlled fermentation and viscosity reduction strategy[J]. Appl Biochem Biotechnol, 2024, 196(3): 1527-1543. |
| [55] | Wu YZ, Kawabata H, Kita K, et al. Constitutive glucose dehydrogenase elevates intracellular NADPH levels and luciferase luminescence in Bacillus subtilis[J]. Microb Cell Fact, 2022, 21(1): 266. |
| [56] | Amjad Zanjani FS, Afrasiabi S, Norouzian D, et al. Hyaluronic acid production and characterization by novel Bacillus subtilis harboring truncated Hyaluronan Synthase[J]. AMB Express, 2022, 12(1): 88. |
| [57] | Xu F, Liu C, Xia MM, et al. Characterization of a riboflavin-producing mutant of Bacillus subtilis isolated by droplet-based microfluidics screening[J]. Microorganisms, 2023, 11(4): 1070. |
| [58] | Tian JH, Xing BW, Li MY, et al. Efficient large-scale and scarless genome engineering enables the construction and screening of Bacillus subtilis biofuel overproducers[J]. Int J Mol Sci, 2022, 23(9): 4853. |
| [59] |
Sánchez-Morán H, Kaar JL, Schwartz DK. Supra-biological performance of immobilized enzymes enabled by chaperone-like specific non-covalent interactions[J]. Nat Commun, 2024, 15(1): 2299.
doi: 10.1038/s41467-024-46719-5 pmid: 38485940 |
| [60] | Cui SX, Xia HZ, Chen TC, et al. Cell membrane and electron transfer engineering for improved synthesis of menaquinone-7 in Bacillus subtilis[J]. iScience, 2020, 23(3): 100918. |
| [61] | Shi L, Lin Y, Song JA, et al. Engineered Bacillus subtilis for the production of tetramethylpyrazine,(R, R)-2, 3-butanediol and acetoin[J]. Fermentation, 2023, 9(5): 488. |
| [62] | Wu YK, Chen TC, Liu YF, et al. Design of a programmable biosensor-CRISPRi genetic circuits for dynamic and autonomous dual-control of metabolic flux in Bacillus subtilis[J]. Nucleic Acids Res, 2020, 48(2): 996-1009. |
| [63] | 宁雪伟. 地衣芽胞杆菌厌氧基础生理代谢特性研究[D]. 无锡: 江南大学, 2023. |
| Ning XW. Study on anaerobic basic physiological and metabolic characteristics of Bacillus licheniformis[D]. Wuxi: Jiangnan University, 2023. | |
| [64] | Voigt B, Schroeter R, Jürgen B, et al. The response of Bacillus licheniformis to heat and ethanol stress and the role of the SigB regulon[J]. Proteomics, 2013, 13(14): 2140-2161. |
| [65] | Dong ZX, Chen XL, Cai K, et al. Exploring the metabolomic responses of Bacillus licheniformis to temperature stress by gas chromatography/mass spectrometry[J]. J Microbiol Biotechnol, 2018, 28(3): 473-481. |
| [66] | Hoi LT, Voigt B, Jürgen B, et al. The phosphate-starvation response of Bacillus licheniformis[J]. Proteomics, 2006, 6(12): 3582-3601. |
| [67] | Guo LF, Lu L, Wang HC, et al. Effects of Fe2+ addition to sugarcane molasses on poly-γ-glutamic acid production in Bacillus licheniformis CGMCC NO. 23967[J]. Microb Cell Fact, 2023, 22(1): 37. |
| [68] | Zhang Y, Hu JM, Zhang Q, et al. Enhancement of alkaline protease production in recombinant Bacillus licheniformis by response surface methodology[J]. Bioresour Bioprocess, 2023, 10(1): 27. |
| [69] | Zhang AD, Ma YD, Deng Y, et al. Enhancing protease and amylase activities in Bacillus licheniformis XS-4 for traditional soy sauce fermentation using ARTP mutagenesis[J]. Foods, 2023, 12(12): 2381. |
| [70] | Moon JH, Ajuna HB, Won SJ, et al. The anti-termite activity of Bacillus licheniformis PR2 against the subterranean termite, Reticulitermes speratus kyushuensis morimoto(Isoptera: Rhinotermitidae)[J]. Forests, 2023, 14(5): 1000. |
| [71] | Rahimnahal S, Meimandipour A, Fayazi J, et al. Biochemical and molecular characterization of novel keratinolytic protease from Bacillus licheniformis(KRLr1)[J]. Front Microbiol, 2023, 14: 1132760. |
| [72] | Muff JL, Sokolovski F, Walsh-Korb Z, et al. Surgical treatment of short bowel syndrome-the past, the present and the future, a descriptive review of the literature[J]. Children, 2022, 9(7): 1024. |
| [73] | Boutonnet C, Ginies C, Alpha-Bazin B, et al. S-layer is a key element in metabolic response and entry into the stationary phase in Bacillus cereus AH187[J]. J Proteom, 2023, 289: 105007. |
| [74] | Jana A, Kakkar N, Halder SK, et al. Efficient valorization of feather waste by Bacillus cereus IIPK35 for concomitant production of antioxidant keratin hydrolysate and milk-clotting metallo-serine keratinase[J]. J Environ Manage, 2022, 324: 116380. |
| [75] | Wang XM, Han MN, Jiang JP, et al. Isolation of a Bacillus cereus strain HBL-AI and its application for production of Trans-4-hydroxy-l-proline[J]. Lett Appl Microbiol, 2021, 72(1): 53-59. |
| [76] | M LBK, Muni Kumar D, Sowjanya M, et al. Industrial applications of alkaline protease with novel properties from Bacillus cereus strain S8[J]. J Adv Zool, 2023, 44(S-3): 1314-1322. |
| [77] | Li WC, Huang XX, Liu H, et al. Improvement in bacterial cellulose production by co-culturing Bacillus cereus and Komagataeibacter xylinus[J]. Carbohydr Polym, 2023, 313: 120892. |
| [78] | Abdelkader I, Ben Mabrouk S, Hadrich B, et al. Optimization using response surface methodology of phospholipase C production from Bacillus cereus suitable for soybean oil degumming[J]. Prep Biochem Biotechnol, 2023, 53(10): 1165-1175. |
| [79] | Hyder S, Gondal AS, Sehar A, et al. Use of ginger extract and bacterial inoculants for the suppression of Alternaria solani causing early blight disease in tomato[J]. BMC Plant Biol, 2024, 24(1): 131. |
| [80] | Gomis-Cebolla J, Berry C. Bacillus thuringiensis as a biofertilizer in crops and their implications in the control of phytopathogens and insect pests[J]. Pest Manag Sci, 2023, 79(9): 2992-3001. |
| [81] | Guo HY, Yu XL, Liu ZY, et al. Deltamethrin transformation by Bacillus thuringiensis and the associated metabolic pathways[J]. Environ Int, 2020, 145: 106167. |
| [82] | Glenwright H, Pohl S, Navarro F, et al. The identification of intrinsic chloramphenicol and tetracycline resistance genes in members of the Bacillus cereus group(sensu lato)[J]. Front Microbiol, 2017, 7: 2122. |
| [83] |
Banerjee S, Misra A, Chaudhury S, et al. A Bacillus strain TCL isolated from Jharia coalmine with remarkable stress responses, chromium reduction capability and bioremediation potential[J]. J Hazard Mater, 2019, 367: 215-223.
doi: S0304-3894(18)31178-6 pmid: 30594722 |
| [84] | Driessen JLSP, Johnsen J, Pogrebnyakov I, et al. Adaptive laboratory evolution of Bacillus subtilis to overcome toxicity of lignocellulosic hydrolysate derived from Distiller's dried grains with solubles(DDGS)[J]. Metab Eng Commun, 2023, 16: e00223. |
| [85] |
Kataoka N, Tajima T, Kato J, et al. Development of butanol-tolerant Bacillus subtilis strain GRSW2-B1 as a potential bioproduction host[J]. AMB Express, 2011, 1(1): 10.
doi: 10.1186/2191-0855-1-10 pmid: 21906347 |
| [86] | Yan SM, Wu G. Bottleneck in secretion of α-amylase in Bacillus subtilis[J]. Microb Cell Fact, 2017, 16(1): 124. |
| [87] | Yamamoto J, Chumsakul O, Toya Y, et al. Constitutive expression of the global regulator AbrB restores the growth defect of a genome-reduced Bacillus subtilis strain and improves its metabolite production[J]. DNA Res, 2022, 29(3): dsac015. |
| [88] |
Repar J, Šućurović S, Zahradka K, et al. Stress resistance of Escherichia coli and Bacillus subtilis is modulated by auxins[J]. Can J Microbiol, 2013, 59(11): 766-770.
doi: 10.1139/cjm-2013-0266 pmid: 24206360 |
| [89] |
Ragul K, Syiem I, Sundar K, et al. Characterization of probiotic potential of Bacillus species isolated from a traditional brine pickle[J]. J Food Sci Technol, 2017, 54(13): 4473-4483.
doi: 10.1007/s13197-017-2928-6 pmid: 29184254 |
| [90] | Deka P, Goswami G, Das P, et al. Bacterial exopolysaccharide promotes acid tolerance in Bacillus amyloliquefaciens and improves soil aggregation[J]. Mol Biol Rep, 2019, 46(1): 1079-1091. |
| [91] | Zeng ZB, Zhang JB, Li Y, et al. Probiotic potential of Bacillus licheniformis and Bacillus pumilus isolated from Tibetan yaks, China[J]. Probiotics Antimicrob Proteins, 2022, 14(3): 579-594. |
| [92] | Xia Y, Sun LC, Liang ZY, et al. Chromosome segment scanning for gain- or loss-of-function screening(CHASING)and its application in metabolic engineering[J]. bioRxiv, 2024. DOI: 10.1101/2024.01.31.578163. |
| [93] |
Vinayavekhin N, Vangnai AS. The effects of disruption in membrane lipid biosynthetic genes on 1-butanol tolerance of Bacillus subtilis[J]. Appl Microbiol Biotechnol, 2018, 102(21): 9279-9289.
doi: 10.1007/s00253-018-9298-5 pmid: 30141082 |
| [94] | Zhang CL, Wang C, Zhao S, et al. Role of c-di-GMP in improving stress resistance of alginate-chitosan microencapsulated Bacillus subtilis cells in simulated digestive fluids[J]. Biotechnol Lett, 2021, 43(3): 677-690. |
| [95] | Wang JY, Wang WS, Wang HZ, et al. Improvement of stress tolerance and riboflavin production of Bacillus subtilis by introduction of heat shock proteins from thermophilic Bacillus strains[J]. Appl Microbiol Biotechnol, 2019, 103(11): 4455-4465. |
| [96] |
Avila-Pérez M, Vreede J, Tang YF, et al. In vivo mutational analysis of YtvA from Bacillus subtilis: mechanism of light activation of the general stress response[J]. J Biol Chem, 2009, 284(37): 24958-24964.
doi: 10.1074/jbc.M109.033316 pmid: 19581299 |
| [97] | He K, Xin YP, Shan Y, et al. Phosphorylation residue T175 in RsbR protein is required for efficient induction of sigma B factor and survival of Listeria monocytogenes under acidic stress[J]. J Zhejiang Univ Sci B, 2019, 20(8): 660-669. |
| [98] | Morawska LP, Detert Oude Weme RGJ, Frenzel E, et al. Stress-induced activation of the proline biosynthetic pathway in Bacillus subtilis: a population-wide and single-cell study of the osmotically controlled proHJ promoter[J]. Microb Biotechnol, 2022, 15(9): 2411-2425. |
| [99] |
El-Khoury T, Nguyen HA, Candusso MP, et al. UbK is involved in the resistance of Bacillus subtilis to oxidative stress[J]. Curr Microbiol, 2020, 77(12): 4063-4071.
doi: 10.1007/s00284-020-02239-1 pmid: 33044618 |
| [100] |
Van Beilen J, Blohmke CJ, Folkerts H, et al. RodZ and PgsA play intertwined roles in membrane homeostasis of Bacillus subtilis and resistance to weak organic acid stress[J]. Front Microbiol, 2016, 7: 1633.
pmid: 27818647 |
| [101] | Shen J, Liu ZY, Yu HN, et al. Systematic stress adaptation of Bacillus subtilis to tetracycline exposure[J]. Ecotoxicol Environ Saf, 2020, 188: 109910. |
| [102] | Niu TF, Lv XQ, Liu YF, et al. The elucidation of phosphosugar stress response in Bacillus subtilis guides strain engineering for high N-acetylglucosamine production[J]. Biotechnol Bioeng, 2021, 118(1): 383-396. |
| [103] | Yang W, Yan HX, Dong GH, et al. Comparative transcriptomics reveal different genetic adaptations of biofilm formation in Bacillus subtilis isolate 1JN2 in response to Cd2+ treatment[J]. Front Microbiol, 2022, 13: 1002482. |
| [104] |
Muto A, Fujihara A, Ito KI, et al. Requirement of transfer-messenger RNA for the growth of Bacillus subtilis under stresses[J]. Genes Cells, 2000, 5(8): 627-635.
pmid: 10947848 |
| [105] |
Wu CD, Huang J, Zhou RQ. Progress in engineering acid stress resistance of lactic acid bacteria[J]. Appl Microbiol Biotechnol, 2014, 98(3): 1055-1063.
doi: 10.1007/s00253-013-5435-3 pmid: 24337395 |
| [1] | 王伟宸, 赵进, 黄薇颐, 郭芯竹, 李婉颖, 张卓. 芽胞杆菌代谢产物防治三种常见植物病原真菌的研究进展[J]. 生物技术通报, 2023, 39(3): 59-68. |
| [2] | 李昕悦, 周明海, 樊亚超, 廖莎, 张风丽, 刘晨光, 孙悦, 张霖, 赵心清. 基于转运蛋白工程提升微生物菌株耐受性和生物制造效率的研究进展[J]. 生物技术通报, 2023, 39(11): 123-136. |
| [3] | 晏雄鹰, 王振, 王霞, 杨世辉. 微生物硫代谢与抗逆性[J]. 生物技术通报, 2023, 39(11): 150-167. |
| [4] | 唐瑞琪, 赵心清, 朱笃, 汪涯. 大肠杆菌对木质纤维素水解液抑制物的胁迫耐受性[J]. 生物技术通报, 2023, 39(11): 205-216. |
| [5] | 孙言秋, 谢采芸, 汤岳琴. 耐高温酿酒酵母的构建与高温耐受机制解析[J]. 生物技术通报, 2023, 39(11): 226-237. |
| [6] | 王文韬, 冯颀, 刘晨光, 白凤武, 赵心清. 氧化还原敏感型基因元件增强酵母木质纤维素水解液抑制物胁迫耐受性[J]. 生物技术通报, 2023, 39(11): 360-372. |
| [7] | 张昊鑫, 王中华, 牛兵, 郭慷, 刘璐, 姜瑛, 张仕祥. 产IAA兼具溶磷解钾高效促生菌的筛选、鉴定及其广谱性应用[J]. 生物技术通报, 2022, 38(5): 100-111. |
| [8] | 牛馨, 张莹, 王茂军, 刘文龙, 路福平, 李玉. 解淀粉芽胞杆菌不同整合位点对外源碱性蛋白酶表达的影响[J]. 生物技术通报, 2022, 38(4): 253-260. |
| [9] | 杨瑞先, 刘萍, 王祖华, 阮宝硕, 汪智达. 牡丹根腐病原菌拮抗细菌抑菌活性物质分析[J]. 生物技术通报, 2022, 38(2): 57-66. |
| [10] | 张洁, 夏明聪, 朱文倩, 梁娟, 孙润红, 徐文, 武超, 杨丽荣. 蔬菜根结线虫生防芽胞杆菌的筛选及作用机理研究[J]. 生物技术通报, 2021, 37(7): 175-182. |
| [11] | 翟旭航, 李霞, 元英进. 木质纤维素预处理及高值化技术研究进展[J]. 生物技术通报, 2021, 37(3): 162-174. |
| [12] | 李瑾, 彭可为, 潘求一, 朱哲远, 彭迪. 解淀粉芽胞杆菌HR-2的分离鉴定及对水稻稻瘟病菌的拮抗效果[J]. 生物技术通报, 2021, 37(3): 27-34. |
| [13] | 李昕悦, 张金方, 徐小健, 路福平, 李玉. 芽胞形成相关基因缺失对解淀粉芽胞杆菌生物量及胞外酶表达的影响[J]. 生物技术通报, 2021, 37(3): 35-43. |
| [14] | 要雅倩, 成娜娜, 李培根, 卢毅, 王彦刚, 林榕姗, 周波, 王冰. 解淀粉芽胞杆菌Bacillus amyloliquefaciens T-6的分离鉴定及抗病促生潜力[J]. 生物技术通报, 2020, 36(9): 202-210. |
| [15] | 宋本超, 赵冬梅, 杨志辉, 张岱, 赵志, 朱杰华. 马铃薯黑痣病菌拮抗菌的筛选鉴定及生防因子分析[J]. 生物技术通报, 2019, 35(8): 9-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||