








生物技术通报 ›› 2020, Vol. 36 ›› Issue (11): 164-172.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0065
收稿日期:2020-01-17
出版日期:2020-11-26
发布日期:2020-11-20
作者简介:赵璐瑶,女,硕士研究生,研究方向:酶工程;E-mail: 基金资助:
ZHAO Lu-yao1( ), CHEN Zhen-ya1, HUO Yi-Xin1,2(
), CHEN Zhen-ya1, HUO Yi-Xin1,2( )
)
Received:2020-01-17
Published:2020-11-26
Online:2020-11-20
摘要:
有机化合物尤其是芳香族化合物的硝化反应在化工生产中具有重要作用,芳香族硝基化合物以其能量高、结构稳定的优势成为一种高附加值的化工中间体及工业产品。目前,化学合成法由于操作简便、投资小、生产条件可控成为最常用的硝化方法,但该生产方法转化效率低,废水、废酸产量大,造成环境污染,这些问题限制了硝基化合物的生产及应用。因此,需要寻找更加高效环保的合成方法。生物酶催化的应用日益广泛,且自然界中有许多微生物可通过转氨或氧化作用进行含氮基团的转化,为高能材料的酶促合成提供了资源库。本文总结了硝基化合物的种类和主要功能,归纳了当前已有硝基化合物合成方法以及存在的问题,综述了P450超家族、过氧化物酶和N-加氧酶中主要硝化酶的种类及特点,并对硝化酶的蛋白结构、催化机理及作用方式进行了深入分析,列举了硝化酶的定向进化研究,最后对利用硝化酶制备硝基化合物的研究方向进行了展望。
赵璐瑶, 陈振娅, 霍毅欣. 硝化酶的研究进展[J]. 生物技术通报, 2020, 36(11): 164-172.
ZHAO Lu-yao, CHEN Zhen-ya, HUO Yi-Xin. Advances in Nitrifying Enzymes[J]. Biotechnology Bulletin, 2020, 36(11): 164-172.
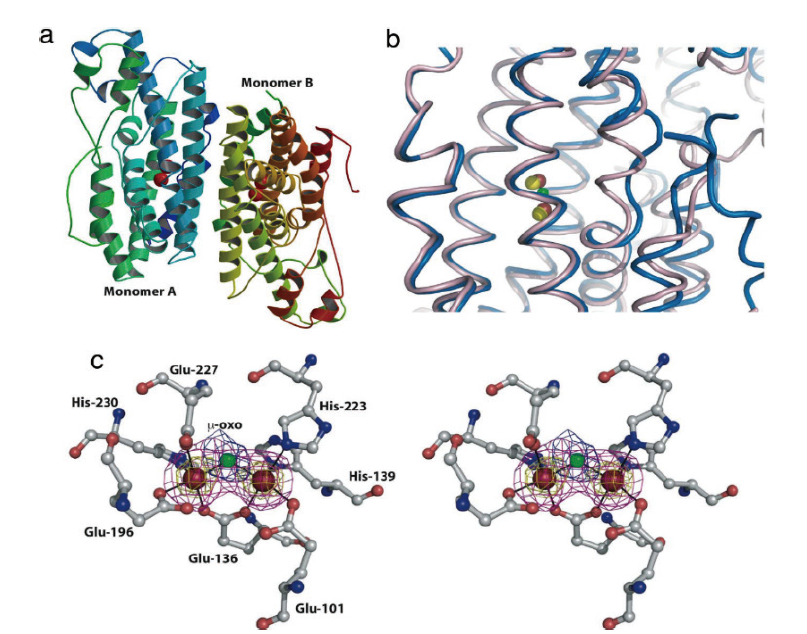
图3 AurF结构分析[46] (a)由蓝到红(氨基)表示的二铁AurF同源二聚体的完整结构图,红色球体为铁原子;(b)二铁AurF(蓝色)及二锰AurF(粉色)活性部位的示意图,红色球体为铁原子,绿色球体为桥联的μ-氧原子,黄色球体为锰原子;(c)AurF活性中心,红球为铁离子,绿球为桥接的氧原子
| 蛋白 | 氧化还原结构 | 原始产物 | 突变位点 | 效果 | 参考文献 |
|---|---|---|---|---|---|
| PrnD | 2Fe-2S | 吡咯尼特林 | F312A L277A | 底物转化效率提高4.89倍 底物转化效率提高3.28倍 | [53] [53] |
| TxtE | 血红素 | 4-硝基色氨酸 | 融合BM3R和14氨基酸连接肽 R59X H176F/H176Y | 增加C5催化位点 产量提高3.5倍 催化位点由C4转换为C5 | [54] [55] [55-56] |
| AurF | 2Fe-2S | p-硝基苯甲酸 | T100L L202F | 相对活性提高3.14倍 相对活性提高3.57倍 | [57] [57] |
表1 硝化酶的改造及效果
| 蛋白 | 氧化还原结构 | 原始产物 | 突变位点 | 效果 | 参考文献 |
|---|---|---|---|---|---|
| PrnD | 2Fe-2S | 吡咯尼特林 | F312A L277A | 底物转化效率提高4.89倍 底物转化效率提高3.28倍 | [53] [53] |
| TxtE | 血红素 | 4-硝基色氨酸 | 融合BM3R和14氨基酸连接肽 R59X H176F/H176Y | 增加C5催化位点 产量提高3.5倍 催化位点由C4转换为C5 | [54] [55] [55-56] |
| AurF | 2Fe-2S | p-硝基苯甲酸 | T100L L202F | 相对活性提高3.14倍 相对活性提高3.57倍 | [57] [57] |
| [1] | Exner O, Krygowski TM. The nitro group as substituent[J]. Chemical Society Reviews, 1996,25(25):71-75. |
| [2] | Ono N. The nitro group in organic synbook[M]. New York:Wiley-VCH, 2002. |
| [3] | Abdallah M, Asghar BH, Zaafarany I. Synjournal of some aromatic nitro compounds and its applications as inhibitors for corrosion of carbon steel inhydrochloric acid solution[J]. Protection of Metals & Physical Chemistry of Surfaces, 2013,49(4):485-491. |
| [4] | Booth G. Nitro compounds, aromatic[M] // Ullmann. Ullmann's encyclopedia of industrial chemistry. New York:Wiley-VCH, 2000: 411-456. |
| [5] |
Tan B, Long X, Peng R, et al. Two important factors influencing shock sensitivity of nitro compounds:Bond dissociation energy of X-NO2(X=C, N, O)and Mulliken charges of nitro group[J]. Journal of Hazardous Materials, 2010,183(1):908-912.
doi: 10.1016/j.jhazmat.2010.07.115 URL |
| [6] | 崔建海. 第十七届全国金属有机化学学术讨论会论文摘要集(2)[C]. 北京:中国化学会, 2012. |
| Cui JH. The 17th national symposium on organometallic chemistry abstracts(2)[C]. Beijing:Chinese Chemical Society, 2012. | |
| [7] | Akhavan J. The chemistry of explosives[M]. 2nded. Great Britain:Royal Society of Chemistry, 2011: 3917-3918. |
| [8] | Ritter H, Licht H. Synjournal and reactions of dinitrated amino and diaminopyridines[J]. J Heterocycl Chem, 1995,32(2):585-590. |
| [9] |
Lancaster NL, Llopis MV. Aromatic nitrations in ionic liquids:the importance of cation choice[J]. Chemical Communications, 2003(22):2812-2813.
URL pmid: 14651117 |
| [10] | 杨超飞, 石磊, 谯娟, 等. 镧系金属磺酸盐催化剂在CL-20合成中的应用[J]. 火炸药学报, 2017(5):22-26. |
| Yang CF, Shi L, Qiao J, et al. Application of lanthanide metal sulfonate catalysts in the synjournal of CL-20[J]. Chinese Journal of Explosives and Propellants, 2017(5):22-26. | |
| [11] | 吕早生, 吕春绪. 一种新的绿色硝化技术[J]. 火炸药学报, 2000,9(4):9-12. |
| Lü ZS, Lü CX. A kind of new green nitration technology[J]. Chinese Journal of Explosives and Propellants, 2000,9(4):9-12. | |
| [12] | Budde CL, Beyer A, Munir IZ, et al. Enzymatic nitration of phenols[J]. J Mol Catal, 2001,15(1-3):55-64. |
| [13] | Muller WE. The benzodiazepine receptor[J]. International Clinical Psychopharmacology, 1990,5(1):72. |
| [14] | Mulla SI, Bharagava RN, Belhaj D, et al. An overview of nitro group-containing compounds and herbicides degradation in microorganisms[M]. Microbial Metabolism of Xenobiotic Compounds, 2019: 319-335. |
| [15] |
Ono N, Kawamura H, Bougauchi M, et al. Porphyrin synjournal from nitrocompounds[J]. Tetrahedron, 1990,46(21):7483-7496.
doi: 10.1016/S0040-4020(01)89062-1 URL |
| [16] |
Dryzhakov M, Hellal M, Wolf E, et al. Nitro-assisted brønsted acid catalysis:application to a challenging catalytic azidation[J]. J Am Chem Soc, 2015,137(30):9555-9558.
doi: 10.1021/jacs.5b06055 URL pmid: 26196521 |
| [17] | Polinski LM. Process for the preparation of nitro-substituted arylamines:America, US5612483A[P]. 1996-02-07. |
| [18] | Lejarazo GSE, et al. Reduction of nitro compounds, through different reaction conditions combinatory chemistry[J]. Chemistry and Chemical Engineering, 2018,2:8. |
| [19] | Pivina TS, Shlyapochnikov VA, Molchanova MS, et al. Molecular screening of high-energy nitrocompounds[J]. Mendeleev Communications, 1991,1(4):122-124. |
| [20] | 邓人杰, 游奎一, 周忠仓, 等. NO2催化硝化萘制备二硝基萘[J]. 中国科技论文, 2015(12):1435-1438. |
| Deng RJ, You KY, Zhou ZC, et al. Preparation of dinitronaphthalene compounds from the nitration reaction of naphthalene with NO2 as nitration reagent[J]. China Science Paper, 2015(12):1435-1438. | |
| [21] |
Wu D, Zhang J, Cui J, et al. AgNO2-mediated direct nitration of the quinoxaline tertiary benzylic C-H bond and direct conversion of 2-methyl quinoxalines into related nitriles[J]. Chemical Communications, 2014,50(74):10857-10860.
doi: 10.1039/c4cc01327a URL pmid: 25089911 |
| [22] | Yang H, Li Y, Wu M, et al. Plant community responses to nitrogen addition and increased precipitation:the importance of water availability and species traits[J]. Global Change Biology, 2011,17(9):2936-2944. |
| [23] | Mellor JM, Mittoo S, Parkes R, et al. Improved nitrations using metal nitrate:sulfuric acid systems[J]. Cheminform, 2010,56(40):8019-8024. |
| [24] | 方东, 施群荣, 巩凯, 等. 芳香族化合物绿色硝化反应研究进展[J]. 含能材料, 2008,16(1):103-112, 120. |
| Fang D, Shi QR, Gong K, et al. Research progress of clean nitration of aromatic compounds[J]. Chinese Journal of Energenic Materials, 2008,16(1):103-112, 120. | |
| [25] |
He J, Hertweck C. Biosynthetic origin of the rare nitroaryl moiety of the polyketide antibiotic aureothin:involvement of an unprecedented N-oxygenase[J]. Journal of the American Chemical Society, 2004,126(12):3694-3695.
doi: 10.1021/ja039328t URL pmid: 15038705 |
| [26] | 庾弘朗. BnCmN3nm微团簇结构和红外光谱的DFT研究[J]. 原子与分子物理学报, 2007,24(5):1028-1034. |
| Yu HL. A DFT study on structures and infrared spectrum of BnCmN3nm mircoclusters[J]. Journal of Atomic and Molecular Physics, 2007,24(5):1028-1034. | |
| [27] | Dunford HB, Stillman JS. On the function and mechanism of action of peroxidases[J]. Coord Chem Rev, 1976,19(3):187-251. |
| [28] | Welinder KG. Superfamily of plant, fungal and bacterial peroxidases[J]. Current Opinion Structure Biology, 1992,2(3):388-393. |
| [29] | Veillard A, Dedieu A, Rohmer M. The oretical studies of the structure of heme models[M]. Springer Netherlands, 1980: 197-225. |
| [30] | Balkus KJ, Pisklak TJ, Huang R. Microperoxidase-11 immobilized in a metal organic framework[J]. ACS Symposium, 2015,986:76-98. |
| [31] |
Low DW, Yang G, Winkler JR, et al. Modification of heme peptides by reverse proteolysis:Spectroscopy of microperoxidase-10 with C-terminal histidine, tyrosine, and methionine residues[J]. J Am Chem Soc, 1997,119(17):4094-4095.
doi: 10.1021/ja970103q URL |
| [32] | Prieto T, Nantes IL, Nascimento OR. Microperoxidase-9 cycle in the presence of cetyltrimethylammonium bromide micelles:tert-butyl hydroperoxide as both an oxidizing and a reducing agent[M]. Nylandore T. Surface and Colloid Science. Berlin:Springer Verlag, 2004: 193-198. |
| [33] | Low DW, Abedin S, Yang G, et al. Manganese microperoxidase-8[J]. Chemtracts, 1998,11(12):923-927. |
| [34] |
Aron J, Baldwin DA, Marques HM, et al. Preparation and analysis of the heme-containing octapeptide(microperoxidase-8)and identification of the monomeric form in aqueous solution[J]. Journal of Inorganic Biochemistry, 1986,27(4):227-243.
URL pmid: 3018151 |
| [35] |
Marques HM. Cyclic voltammetry of imidazole microperoxidase-8:modeling the control of the redox potential of the cytochromes[J]. Inorganic Chemistry, 1990,29(9):1597-1599.
doi: 10.1021/ic00334a002 URL |
| [36] | Ricoux R, Boucher JL, Mansuy D, et al. Microperoxidase-8 catalyzed nitration of phenol by nitrogen dioxide radicals[J]. FEBS Journal, 2010,268(13):3783-3788. |
| [37] |
Lecomte S, Ricoux R, Mahy JP, et al. Microperoxidase-8 adsorbed on a roughened silver electrode as a monomeric high-spin penta-coordinated species:characterization by SERR spectroscopy and electrochemistry[J]. J Biol Inorg Chem, 2004,9(7):850.
doi: 10.1007/s00775-004-0586-4 URL pmid: 15340868 |
| [38] | Budde CL, Beyer A, Munir IZ, et al. Enzymatic nitration of phenols[J]. J Mol Catal, 2001,15(1):55-64. |
| [39] | Mceldoon JP, Dordick JS. Unusual thermal stability of soybean peroxidase[J]. Biotechnol Prog, 1996,12(4):555-558. |
| [40] | Welinder KG, Larsen YB. Covalent structure of soybean seed coat peroxidase[J]. Biothim Biophys Acta, 2004,1698(1):121-126. |
| [41] | 程婕, 杨凌. 细胞色素P450氧化还原酶的研究进展[J]. 中国药理学报, 2006,22(2):129-133. |
| Cheng J, Yang L. New progress in studies on cytochrome P450[J]. Acta Pharmacologica Sinica, 2006,22(2):129-133. | |
| [42] |
Barry SM, Kers JA, Johnson EG, et al. Cytochrome P450-catalyzed L-tryptophan nitration in thaxtomin phytotoxin biosynjournal[J]. Nature Chemical Biology, 2012,8(10):814.
doi: 10.1038/nchembio.1048 URL pmid: 22941045 |
| [43] | Yu F, Li M, Xu C, et al. Structural insights into the mechanism for recognizing substrate of the cytochrome P450 enzyme TxtE[J]. PLoS One, 2013,8(11):81526. |
| [44] |
Dodani SC, Cahn JK, Heinisch T, et al. Structural, functional, and spectroscopic characterization of the substrate scope of the novel nitrating cytochrome P450 TxtE[J]. ChemBioChem, 2014,15(15):2259-2267.
URL pmid: 25182183 |
| [45] |
Simurdiak M, Lee J, Zhao H. A new class of arylamine oxygenases:evidence that p-aminobenzoate N-oxygenase(AurF)is a di-iron enzyme and further mechanistic studies[J]. ChemBioChem, 2006,7(8):1169-1172.
URL pmid: 16927313 |
| [46] | Choi YS, Zhang H, Brunzelle JS, et al. In vitro reconstitution and crystal structure of p-aminobenzoate N-oxygenase(AurF)involved in aureothin biosynthesis[J]. Proceedings of the National Academy of Sciences, 2008,105(19):6858-6863. |
| [47] |
Korboukh VK, Li N, Barr EW, et al. A long-lived, substrate-hydroxylating peroxodiiron III intermediate in the amine oxygenase, AurF, from Streptomyces thioluteus[J]. Journal of the American Chemical Society, 2009,131(38):13608-13609.
URL pmid: 19731912 |
| [48] |
Li N, Korboukh VK, Krebs C, et al. Four-electron oxidation of p-hydroxylaminobenzoate to p-nitrobenzoate by a peroxodiferric complex in AurF from Streptomyces thioluteus[J]. Proc Natl Acad Sci USA, 2010,107(36):15722-15727.
doi: 10.1073/pnas.1002785107 URL pmid: 20798054 |
| [49] |
Wang C, Chen H. Convergent theoretical prediction of reactive oxidant structures in diiron arylamine oxygenases AurF and CmlI:peroxo or hydroperoxo?[J]. Journal of the American Chemical Society. 2017,139(37):13038-13046.
doi: 10.1021/jacs.7b06343 URL pmid: 28844144 |
| [50] | Lu H, Chanco E, Zhao H. CmlI is an N-oxygenase in the biosynjournal of chloramphenicol[J]. Tetrahedron, 2012,68(37):7651-7654. |
| [51] | Knoot CJ, Kovaleva EG, Lipscomb JD. Crystal structure of CmlI, the arylamine oxygenase from the chloramphenicol biosynthetic pathway[J]. J Biol Inorg Chem, 2016,21(56):589-603. |
| [52] | Zhu X, Van KH, Naismith JH. The ternary complex of PrnB(the second enzyme in the pyrrolnitrin biosynjournal pathway), tryptophan, and cyanide yields new mechanistic insights into the indolamine dioxygenase superfamily[J]. Journal of Biological Chemistry, 2010,285(27):21126-21133. |
| [53] |
Lee JK, Ang EL, Zhao H. Probing the substrate specificity of aminopyrrolnitrin oxygenase(PrnD)by mutational analysis[J]. Journal of Bacteriology, 2006,188(17):6179-6183.
URL pmid: 16923884 |
| [54] |
Zuo R, Zhang Y, Huguet-Tapia JC, et al. An artificial self-sufficient cytochrome P450 directly nitrates fluorinated tryptophan analogs with a different regio-selectivity[J]. Biotechnology Journal, 2016,11(5):624-632.
URL pmid: 26743860 |
| [55] | Saroay R. Engineering self-sufficiency and broadened substrate scope into indole-nitrating cytochrome P450 TxtE[D]. New England:University of Warwick, 2018. |
| [56] |
Gober JG, Rydeen AE, Gibson EJ, et al. Mutating a highly conserved residue in diverse cytochrome P450s facilitates diastereoselective olefin cyclopropanation[J]. ChemBioChem, 2016,17(5):394-397.
URL pmid: 26690878 |
| [57] |
Zocher G, Winkler R, Hertweck C, et al. Structure and action of the N-oxygenase AurF from Streptomyces thioluteus[J]. Journal of Molecular Biology, 2007,373(1):65-74.
URL pmid: 17765264 |
| [58] |
Lee J. Reconstitution and characterization of aminopyrrolnitrin oxygenase, a rieske N-oxygenase catalyzes unusual arylamine oxidation[J]. J Biol Chem, 2005,280(44):36719-36727.
doi: 10.1074/jbc.M505334200 URL pmid: 16150698 |
| [59] |
Hammer PE, Hill DS, Lam ST, et al. Four genes from Pseudomonas fluorescens that encode the biosynjournal of pyrrolnitrin[J]. Appl Environ Microbiol, 1997,63(6):2147-2154.
doi: 10.1128/AEM.63.6.2147-2154.1997 URL pmid: 9172332 |
| [60] |
Pée KHV, Salcher O, Lingens F. Formation of pyrrolnitrin and 3-(2-amino-3-chlorophenyl)pyrrole from 7-chlorotryptophan[J]. Angew Chem Int Ed Engl, 1980,19(10):828-829.
doi: 10.1002/anie.198008281 URL |
| [61] |
He J, Hertweck C. Biosynthetic origin of the rare nitroaryl moiety of the polyketide antibiotic aureothin:Involvement of an unprecedented N-oxygenase[J]. Journal of the American Chemical Society, 2004,126(12):3694-3695.
doi: 10.1021/ja039328t URL pmid: 15038705 |
| [1] | 叶云芳, 田清尹, 施婷婷, 王亮, 岳远征, 杨秀莲, 王良桂. 植物中β-紫罗兰酮生物合成及调控研究进展[J]. 生物技术通报, 2023, 39(8): 91-105. |
| [2] | 王玲, 卓燊, 付学森, 刘紫璇, 刘笑蓉, 王志辉, 周日宝, 刘湘丹. 莲生物碱生物合成途径及相关基因研究进展[J]. 生物技术通报, 2023, 39(7): 56-66. |
| [3] | 姜晴春, 杜洁, 王嘉诚, 余知和, 王允, 柳忠玉. 虎杖转录因子PcMYB2的表达特性和功能分析[J]. 生物技术通报, 2023, 39(5): 217-223. |
| [4] | 周定定, 李辉虎, 汤兴涌, 余发新, 孔丹宇, 刘毅. 甘草酸和甘草苷生物合成与调控的研究进展[J]. 生物技术通报, 2023, 39(5): 44-53. |
| [5] | 郁慧丽, 李爱涛. 细胞色素P450酶在香精香料绿色生物合成中的应用[J]. 生物技术通报, 2023, 39(4): 24-37. |
| [6] | 李毅丹, 单晓辉. 赤霉素代谢调控与绿色革命[J]. 生物技术通报, 2022, 38(2): 195-204. |
| [7] | 姚宇, 顾佳珺, 孙超, 申国安, 郭宝林. 植物类黄酮UDP-糖基转移酶研究进展[J]. 生物技术通报, 2022, 38(12): 47-57. |
| [8] | 赵玉雪, 王芸, 余璐瑶, 刘京晶, 斯金平, 张新凤, 张磊. 植物中C-糖基转移酶的结构与应用[J]. 生物技术通报, 2022, 38(10): 18-28. |
| [9] | 徐圆圆, 赵国春, 郝颖颖, 翁学煌, 陈仲, 贾黎明. 无患子RT-qPCR内参基因的筛选与验证[J]. 生物技术通报, 2022, 38(10): 80-89. |
| [10] | 刘雪丹, 杨萌, 张静, 赵东旭. 葡萄糖-木糖共利用对重组大肠杆菌合成D-1,2,4-丁三醇的影响[J]. 生物技术通报, 2021, 37(9): 171-179. |
| [11] | 周正, 李卿, 陈万生, 张磊. 药用植物天然产物生物合成途径及关键催化酶的研究策略[J]. 生物技术通报, 2021, 37(8): 25-34. |
| [12] | 梁振霆, 唐婷. 内生菌对植物次生代谢产物的生物合成影响和抗逆功能研究[J]. 生物技术通报, 2021, 37(8): 35-45. |
| [13] | 陶宇丞, 吕旭冰, 程圣杰, 王彦雯, 王文峰, 焦朕, 王鹏超. 大肠杆菌高效合成L-苯甘氨酸的研究进展[J]. 生物技术通报, 2021, 37(3): 175-184. |
| [14] | 乔自鹏, 王奇志, 杨道茂, 阮丽萍. 真菌介导纳米银生物合成的研究进展[J]. 生物技术通报, 2021, 37(3): 185-197. |
| [15] | 任思羽, 程新宽, 张宇辉, 庄建文, 马龙. 两种新型L-苏氨酸醛缩酶的鉴定及活性检测方法[J]. 生物技术通报, 2021, 37(3): 233-240. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||